Clinical Significance and Patterns of Potential Drug–Drug Interactions in Cardiovascular Patients: Focus on Low-Dose Aspirin and Angiotensin-Converting Enzyme Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Inclusion Criteria
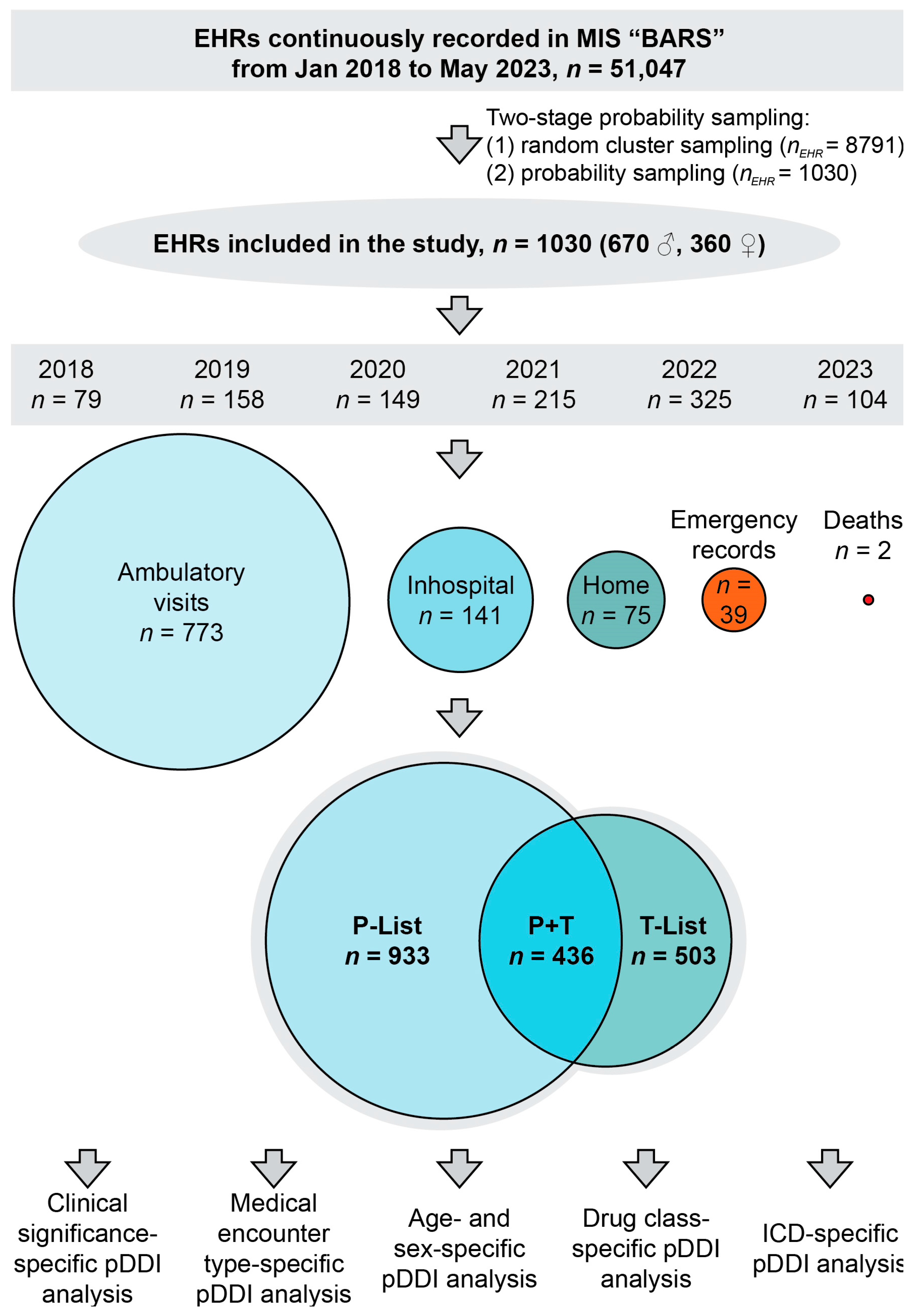
2.3. Characteristics of Sample and Study Design
2.4. Medication Lists
2.5. Polypharmacy, pDDIs, and pDDI Index
2.6. Statistics
2.6.1. Calculation of Sample Size
2.6.2. Statistical Data Analysis
3. Results
3.1. Sample Characteristics
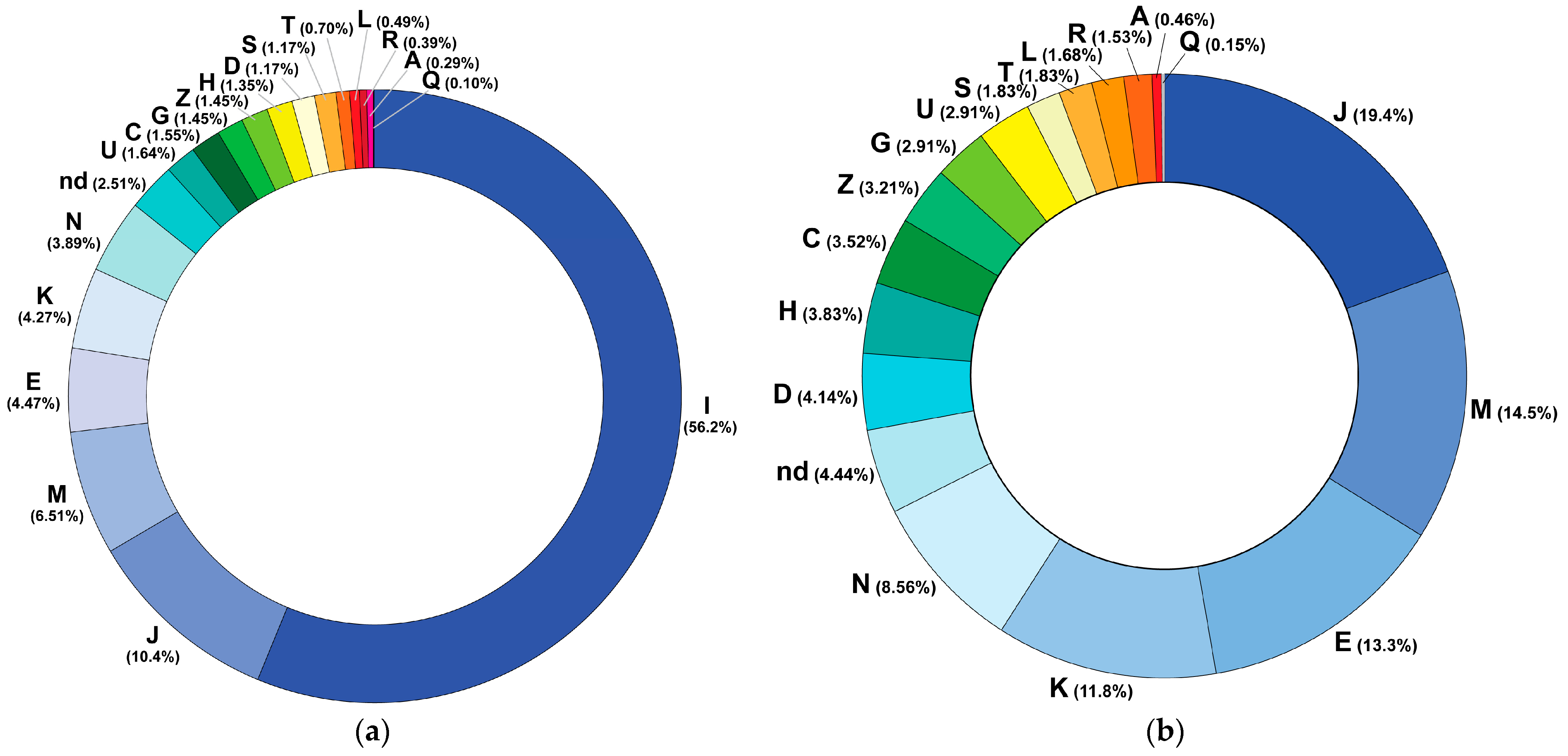
3.2. Pharmacotherapy Patterns
3.3. Polypharmacy
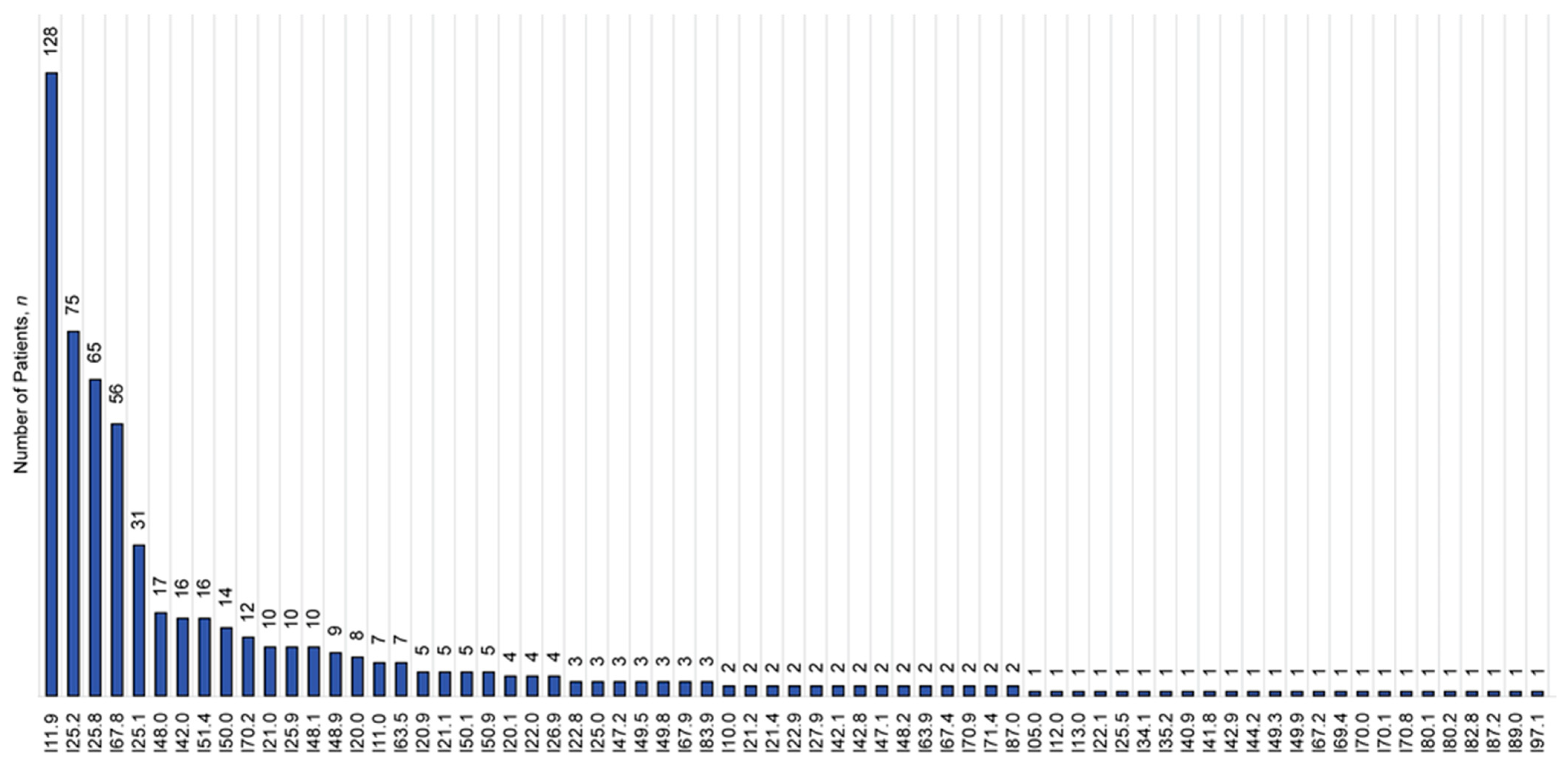
3.4. pDDIs and pDDI Index Values
3.5. In-Hospital versus Ambulatory Patterns of Polypharmacy and pDDIs
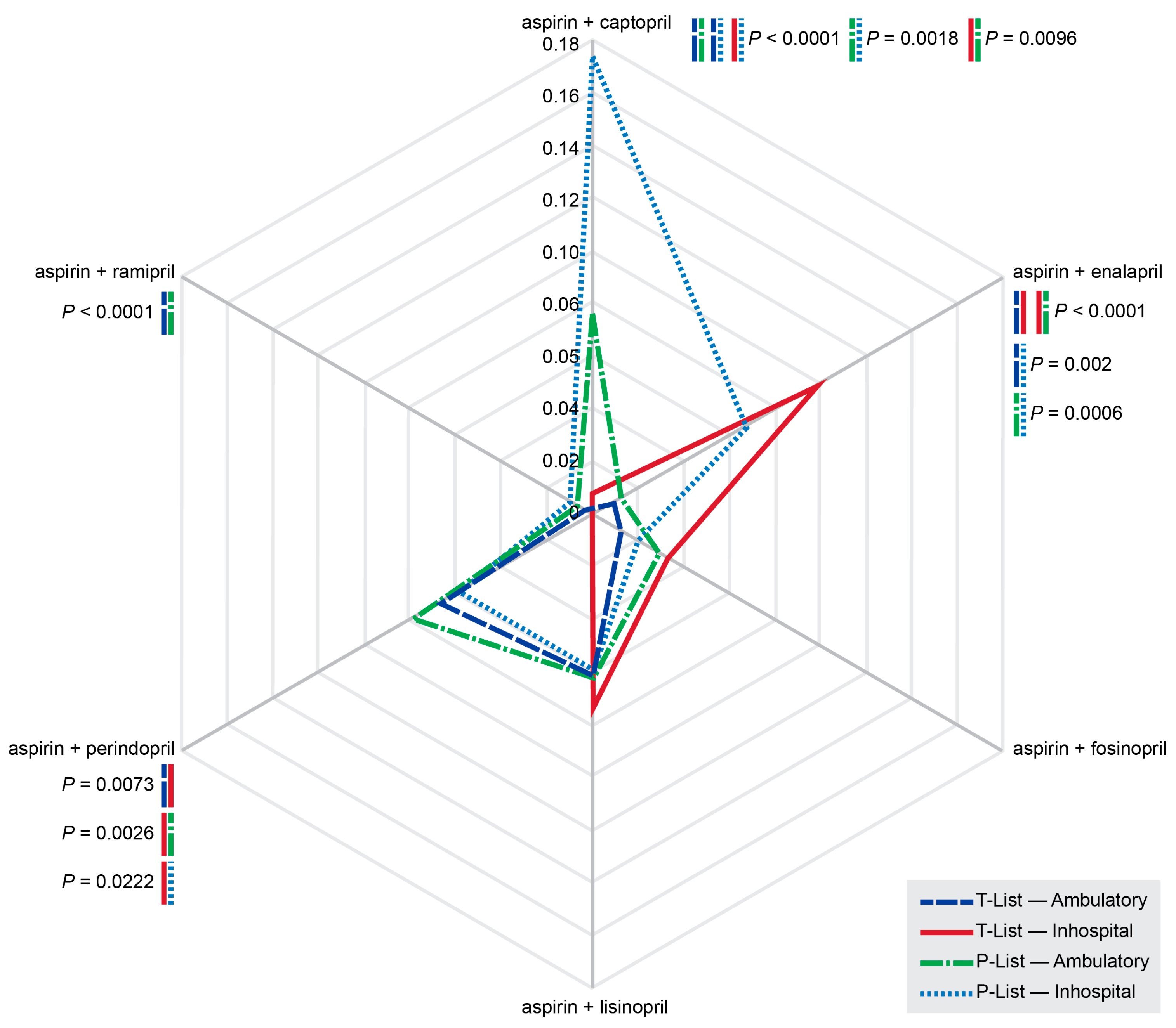
3.6. ‘Aspirin + ACE Inhibitor’ Combinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chowdhury, S.R.; Chandra Das, D.; Sunna, T.C.; Beyene, J.; Hossain, A. Global and regional prevalence of multimorbidity in the adult population in community settings: A systematic review and meta-analysis. EClinicalMedicine 2023, 57, 101860. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Daniel, M. Medical error-the third leading cause of death in the US. BMJ 2016, 353, i2139. [Google Scholar] [CrossRef] [PubMed]
- Anfinogenova, N.D.; Novikova, O.M.; Trubacheva, I.A.; Efimova, E.V.; Chesalov, N.P.; Ussov, W.Y.; Maksimova, A.S.; Shelkovnikova, T.A.; Ryumshina, N.I.; Stepanov, V.A.; et al. Prescribed versus taken polypharmacy and drug-drug interactions in older cardiovascular patients during the COVID-19 pandemic: Observational cross-sectional analytical study. J. Clin. Med. 2023, 12, 5061. [Google Scholar] [CrossRef] [PubMed]
- Dornquast, C.; Dombrowski, M.; Zabel, M.; Willich, S.N.; Reinhold, T. Potential drug-drug interactions in patients with indication for prophylactic implantation of a cardioverter defibrillator: A cross-sectional analysis. BMC Health Serv. Res. 2020, 20, 271. [Google Scholar] [CrossRef] [PubMed]
- Kalash, A.; Abdelrahman, A.; Al-Zakwani, I.; Al Suleimani, Y. Potentially Harmful Drug-Drug Interactions and Their Associated Factors Among Hospitalized Cardiac Patients: A Cross-Sectional Study. Drugs-Real World Outcomes 2023, 10, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Igho-Osagie, E.; Brzozowski, K.; Jin, H.; Brown, J.; Williams, M.G.; Puenpatom, A. Prevalence of potential drug-drug interactions with ritonavir-containing COVID-19 therapy in the United States: An Analysis of the National Health and Nutrition Examination Survey. Clin. Ther. 2023, 45, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Niriayo, Y.L.; Kifle, R.; Asgedom, S.W.; Gidey, K. Drug therapy problems among hospitalized patients with cardiovascular disease. BMC Cardiovasc. Disord. 2024, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Rangraz Jeddi, F.; Nabovati, E.; Peykani, F.; Anvari, S.; Bagheri Toolaroud, P. Potential Drug-Drug Interactions in a Cardiac Center: Development of Simple Software for Pattern Identification. J. Tehran Heart Cent. 2022, 17, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Kilit, T.P.; Özyiğit, F.; Erarslan, S.; Onbaşı, K. Evaluation of potential drug-drug interactions and polypharmacy in hospitalized COVID-19 patients. Afr. Health Sci. 2022, 22, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Cao, D.; Spirito, A.; Nicolas, J.; Pileggi, B.; Kamaleldin, K.; Vogel, B.; Mehran, R. Personalized Approaches to Antiplatelet Treatment for Cardiovascular Diseases: An Umbrella Review. Pharmgenom. Pers. Med. 2023, 16, 973–990. [Google Scholar] [CrossRef]
- Ramste, M.; Ritvos, M.; Häyrynen, S.; Kiiski, J.I.; Niemi, M.; Sinisalo, J. CYP2C19 loss-of-function alleles and use of omeprazole or esomeprazole increase the risk of cardiovascular outcomes in patients using clopidogrel. Clin. Transl. Sci. 2023, 16, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, N.; Tarnow, L.; Vermehren, C. Use of Clopidogrel and Proton Pump Inhibitors Alone or in Combinations in Persons with Diabetes in Denmark; Potential for CYP2C19 Genotype-Guided Drug Therapy. Metabolites 2021, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Khaled, A.; Almaghaslah, D.; Nagib, R.; Makki, S.; Siddiqua, A. Detection and analysis of potential drug-drug interactions among patients admitted to the cardiac care unit in a tertiary care hospital. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.G.; Lauer, M.S. Using aspirin and ACE inhibitors in combination: Why the hullabaloo? Cleve Clin. J. Med. 2001, 68, 569–574. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harężlak, T.; Religioni, U.; Szymański, F.M.; Hering, D.; Barańska, A.; Neumann-Podczaska, A.; Allan, M.; Merks, P. Drug Interactions Affecting Kidney Function: Beware of Health Threats from Triple Whammy. Adv. Ther. 2022, 39, 140–147. [Google Scholar] [CrossRef]
- Perić, A.; Udilović, A.; Dobrić, S.; Vezmar Kovačević, S. The impact of treatment choices on potential drug-drug interactions in hypertensive patients. Br. J. Clin. Pharmacol. 2022, 88, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Kjeldsen, K.P.; Delpón, E.; Semb, A.G.; Cerbai, E.; Dobrev, D.; Savarese, G.; Sulzgruber, P.; Rosano, G.; Borghi, C.; et al. Facing the challenge of polypharmacy when prescribing for older people with cardiovascular disease. A review by the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 406–419. [Google Scholar] [CrossRef]
- Rochon, P.A.; Petrovic, M.; Cherubini, A.; Onder, G.; O’Mahony, D.; Sternberg, S.A.; Stall, N.M.; Gurwitz, J.H. Polypharmacy, inappropriate prescribing, and deprescribing in older people: Through a sex and gender lens. Lancet Healthy Longev. 2021, 2, e290–e300. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Villacastín, J.; Caballero, R.; Delpón, E. Drug-induced atrial fibrillation. A narrative review of a forgotten adverse effect. Pharmacol. Res. 2024, 200, 107077. [Google Scholar] [CrossRef]
- Anfinogenova, N.D.; Trubacheva, I.A.; Popov, S.V.; Efimova, E.V.; Ussov, W.Y. Trends and concerns of potentially inappropriate medication use in patients with cardiovascular diseases. Expert Opin. Drug Saf. 2021, 20, 1191–1206. [Google Scholar] [CrossRef]
- Anfinogenova, Y.; Grakova, E.V.; Shvedova, M.; Kopieva, K.V.; Teplyakov, A.T.; Popov, S.V. Interdisciplinary approach to compensation of hypoglycemia in diabetic patients with chronic heart failure. Heart Fail. Rev. 2018, 23, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Anfinogenova, Y.; Trubacheva, I.A.; Serebryakova, V.N.; Popov, S.V. New trends and challenges of population cardiology. Sib. Med. J. 2019, 34, 24–38. (In Russian) [Google Scholar] [CrossRef]
- Amkreutz, J.; Koch, A.; Buendgens, L.; Trautwein, C.; Eisert, A. Clinical decision support systems differ in their ability to identify clinically relevant drug interactions of immunosuppressants in kidney transplant patients. J. Clin. Pharm. Ther. 2017, 42, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Frahm, N.; Bachmann, P.; Debus, J.L.; Haker, M.C.; Mashhadiakbar, P.; Langhorst, S.E.; Baldt, J.; Streckenbach, B.; Heidler, F.; et al. Screening for severe drug-drug interactions in patients with multiple sclerosis: A comparison of three drug interaction databases. Front. Pharmacol. 2022, 13, 946351. [Google Scholar] [CrossRef] [PubMed]
- Kheshti, R.; Aalipour, M.; Namazi, S. A comparison of five common drug-drug interaction software programs regarding accuracy and comprehensiveness. J. Res. Pharm. Pract. 2016, 5, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Marcath, L.A.; Xi, J.; Hoylman, E.K.; Kidwell, K.M.; Kraft, S.L.; Hertz, D.L. Comparison of nine tools for screening drug-drug interactions of oral oncolytics. J. Oncol. Pract. 2018, 14, e368–e374. [Google Scholar] [CrossRef] [PubMed]
- Vonbach, P.; Dubied, A.; Krähenbühl, S.; Beer, J.H. Evaluation of frequently used drug interaction screening programs. Pharm. World Sci. 2008, 30, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Assefa, Y.A.; Kedir, A.; Kahaliw, W. Survey on polypharmacy and drug-drug interactions among elderly people with cardiovascular diseases at Yekatit 12 Hospital, Addis Ababa, Ethiopia. Integr. Pharm. Res. Pract. 2020, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Ramasubbu, S.K.; Agnihotri, A.; Kumar, B.; Rawat, V.S. Leading 20 drug-drug interactions, polypharmacy, and analysis of the nature of risk factors due to QT interval prolonging drug use and potentially inappropriate psychotropic use in elderly psychiatry outpatients. Ther. Adv. Cardiovasc. Dis. 2021, 15, 7539447211058892. [Google Scholar] [CrossRef]
- Jain, S.; Jain, P.; Sharma, K.; Saraswat, P. A prospective analysis of drug interactions in patients of intensive cardiac care unit. J. Clin. Diagn. Res. 2017, 11, FC01–FC04. [Google Scholar] [CrossRef]
- Roca, B.; Roca, M. Assessment of drug interactions with online electronic checkers in multi-pathological patients. Pharmacology 2022, 107, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Şen, S.; Karahan, E.; Büyükulaş, C.; Polat, Y.O.; Üresin, A.Y. Colchicine for cardiovascular therapy: A drug interaction perspective and a safety meta-analysis. Anatol. J. Cardiol. 2021, 25, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Kolbin, A.S.; Niyazov, R.R.; Kalinichenko, V.V.; Glagolev, S.V. Use of real-world data for drug development. Klin. Farmakol. Ter. Clin. Pharmacol. Ther. 2023, 32, 16–23. [Google Scholar] [CrossRef]
- Knevel, R.; Liao, K.P. From real-world electronic health record data to real-world results using artificial intelligence. Ann. Rheum. Dis. 2023, 82, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Galluppi, G.R.; Ahamadi, M.; Bhattacharya, S.; Budha, N.; Gheyas, F.; Li, C.C.; Chen, Y.; Dosne, A.G.; Kristensen, N.R.; Magee, M.; et al. Considerations for industry-preparing for the FDA model-informed drug development (MIDD) paired meeting program. Clin. Pharmacol. Ther. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Dagenais, S.; Lee, C.; Cronenberger, C.; Wang, E.; Sahasrabudhe, V. Proposing a framework to quantify the potential impact of pharmacokinetic drug-drug interactions caused by a new drug candidate by using real world data about the target patient population. Clin. Transl. Sci. 2024, 17, e13741. [Google Scholar] [CrossRef] [PubMed]
- Mikl, V.; Baltic, D.; Czypionka, T.; Degelsegger-Márquez, A.; Forgó, N.; Gouya-Lechner, G.; Herzog, A.; Klimek, P.; Lumenta, D.B.; Mraz, B.; et al. A national evaluation analysis and expert interview study of real-world data sources for research and healthcare decision-making. Sci. Rep. 2024, 14, 9751. [Google Scholar] [CrossRef]
- Dumbreck, S.; Flynn, A.; Nairn, M.; Wilson, M.; Treweek, S.; Mercer, S.W.; Alderson, P.; Thompson, A.; Payne, K.; Guthrie, B. Drug-disease and drug-drug interactions: Systematic examination of recommendations in 12 UK national clinical guidelines. BMJ 2015, 350, h949. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, J.E.; Meo, N.; Kassebaum, N.J.; Collison, M.L.; Mokdad, A.H.; Naghavi, M. Association of adverse effects of medical treatment with mortality in the United States: A secondary analysis of the global burden of diseases, injuries, and risk factors study. JAMA Netw. Open 2019, 2, e187041. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ahn, I.; Cho, H.N.; Gwon, H.; Kang, H.J.; Seo, H.; Choi, H.; Kim, K.P.; Jun, T.J.; Kim, Y.H. RIDAB: Electronic medical record-integrated real world data platform for predicting and summarizing interactions in biomedical research from heterogeneous data resources. Comput. Methods Programs Biomed. 2022, 221, 106866. [Google Scholar] [CrossRef]
- Shelkovnikova, T.A.; Maksimova, A.S.; Ryumshina, N.I.; Mochula, O.V.; Vaizov, V.K.; Ussov, W.Y.; Anfinogenova, N.D. Transformative effect of COVID-19 pandemic on magnetic resonance imaging services in one tertiary cardiovascular center. J. Imaging 2023, 9, 108. [Google Scholar] [CrossRef]
- Shelkovnikova, T.A.; Maksimova, A.S.; Ryumshina, N.I.; Mochula, O.V.; Vaizov, V.K.; Ussov, W.Y.; Anfinogenova, N.D. Magnetic resonance imaging-derived portrait of cardiac patients in one specialized cardiovascular center during COVID-19 pandemic. Sib. J. Clin. Exp. Med. 2022, 37, 105–113. [Google Scholar] [CrossRef]
- Medscape Drug Interaction Checker. Available online: https://reference.medscape.com/drug-interactionchecker (accessed on 11 June 2024).
- Falconi, G.; Kashan, S. Drug Interactions in Palliative Care. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Lion, S.; Evrard, P.; Foulon, V.; Spinewine, A. Drug-drug interactions in nursing home residents: Analysis from the COME-ON trial. Age Ageing 2023, 52, afac278. [Google Scholar] [CrossRef] [PubMed]
- Reinhild Haerig, T.; Krause, D.; Klaassen-Mielke, R.; Rudolf, H.; Trampisch, H.J.; Thuermann, P. Potentially inappropriate medication including drug-drug interaction and the risk of frequent falling, hospital admission, and death in older adults—Results of a large cohort study (getABI). Front. Pharmacol. 2023, 14, 1062290. [Google Scholar] [CrossRef] [PubMed]
- Chazova, I.E.; Zhernakova, Y.V. Diagnosis and treatment of arterial hypertension [Guidelines]. Syst. Hypertens. 2019, 16, 6–31. [Google Scholar] [CrossRef]
- Alhumaidi, R.M.; Bamagous, G.A.; Alsanosi, S.M.; Alqashqari, H.S.; Qadhi, R.S.; Alhindi, Y.Z.; Ayoub, N.; Falemban, A.H. Risk of polypharmacy and its outcome in terms of drug interaction in an elderly population: A retrospective cross-sectional study. J. Clin. Med. 2023, 12, 3960. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Sha, S.; Brenner, H.; Schöttker, B. Longitudinal associations of polypharmacy and frailty with major cardiovascular events and mortality among more than half a million middle-aged participants of the UK Biobank. Maturitas 2024, 185, 107998. [Google Scholar] [CrossRef]
- Domiati, S.; Poushuju, R. Clinical pharmacist evaluation of medication inappropriateness in a geriatric hospital. Ann. Pharm. Fr. 2022, 80, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Sugimoto, M.; Kodaira, C.; Nishino, M.; Yamade, M.; Uotani, T.; Sahara, S.; Ichikawa, H.; Kagami, T.; Iwaizumi, M.; et al. Influence of low-dose proton pump inhibitors administered concomitantly or separately on the anti-platelet function of clopidogrel. J. Thromb. Thrombolysis 2017, 43, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Grymonprez, M.; Capiau, A.; Steurbaut, S.; Boussery, K.; Mehuys, E.; Somers, A.; Petrovic, M.; De Backer, T.L.; Lahousse, L. Pharmacodynamic drug-drug interactions and bleeding outcomes in patients with atrial fibrillation using non-vitamin K antagonist oral anticoagulants: A nationwide cohort study. Cardiovasc. Drugs Ther. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Hall, K.T.; Kessler, T.; Buring, J.E.; Passow, D.; Sesso, H.D.; Zee, R.Y.L.; Ridker, P.M.; Chasman, D.I.; Schunkert, H. Genetic variation at the coronary artery disease risk locus GUCY1A3 modifies cardiovascular disease prevention effects of aspirin. Eur. Heart J. 2019, 40, 3385–3392. [Google Scholar] [CrossRef]
- Kessler, T.; Wolf, B.; Eriksson, N.; Kofink, D.; Mahmoodi, B.K.; Rai, H.; Tragante, V.; Åkerblom, A.; Becker, R.C.; Bernlochner, I.; et al. Association of the coronary artery disease risk gene GUCY1A3 with ischaemic events after coronary intervention. Cardiovasc. Res. 2019, 115, 1512–1518. [Google Scholar] [CrossRef]
- Mehuys, E.; De Backer, T.; De Keyser, F.; Christiaens, T.; Van Hees, T.; Demarche, S.; Van Tongelen, I.; Boussery, K. Prevalence and management of drug interactions between nonsteroidal anti-inflammatory drugs and antithrombotics in ambulatory care. Br. J. Clin. Pharmacol. 2022, 88, 3896–3902. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.W.; Petrucci, G.; Rocca, B. Aspirin, stroke and drug-drug interactions. Vasc. Pharmacol. 2016, 87, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Schrör, K.; Verheugt, F.W.A.; Trenk, D. Drug-drug interaction between antiplatelet therapy and lipid-lowering agents (statins and PCSK9 inhibitors). Thromb. Haemost. 2023, 123, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.J.; Pagidipati, N.J.; Bosworth, H.B. Improving medication adherence in cardiovascular disease. Nat. Rev. Cardiol. 2024, 21, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.; Garden, E.; Nanyonga, R.C.; Squires, A.; Nakaggwa, F.; Schwartz, J.I.; Heller, D.J. Causes of medication non-adherence and the acceptability of support strategies for people with hypertension in Uganda: A qualitative study. Int. J. Nurs. Stud. 2022, 126, 104143. [Google Scholar] [CrossRef] [PubMed]
- Muhič, N.; Mrhar, A.; Brvar, M. Comparative analysis of three drug-drug interaction screening systems against probable clinically relevant drug-drug interactions: A prospective cohort study. Eur. J. Clin. Pharmacol. 2017, 73, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, N.; Allegaert, K. COVID-19 and antiepileptic drugs: An approach to guide practices when nirmatrelvir/ritonavir is co-prescribed. Eur. J. Clin. Pharmacol. 2022, 78, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Roll, S.C. The influence of pharmacogenetics on the clinical relevance of pharmacokinetic drug-drug interactions: Drug-gene, drug-gene-gene and drug-drug-gene interactions. Pharmaceuticals 2021, 14, 487. [Google Scholar] [CrossRef]
- Øvretveit, K.; Ingeström, E.M.L.; Spitieris, M.; Tragante, V.; Wade, K.H.; Thomas, L.F.; Wolford, B.N.; Wisløff, U.; Gudbjartsson, D.F.; Holm, H.; et al. Polygenic risk scores associate with blood pressure traits across the lifespan. Eur. J. Prev. Cardiol. 2024, 31, 644–654. [Google Scholar] [CrossRef]
- Cecchin, E.; Posocco, B.; Mezzalira, S.; Appetecchia, M.; Toffoli, G. The role of gender pharmacogenetics in the personalization of drug treatment. J. Pharmacol. Exp. Ther. 2023, 386, 190–197. [Google Scholar] [CrossRef]
- Chernyavsky, A.M. Genetically-mediated aspirin resistance and its impact on thrombotic complications in atherosclerosis. In Proceedings of the Fifth All-Russian Science-and-Education Forum with International Participation “Cardiology of XXI Century: Alliances and Potential”, Tomsk, Russia, 24–26 April 2024. [Google Scholar]
- Hou, X. Epoxidase inhibitor-aspirin resistance and the relationship with genetic polymorphisms: A review. J. Int. Med. Res. 2024, 52, 3000605241230429. [Google Scholar] [CrossRef] [PubMed]
- Forgerini, M.; Lucchetta, R.C.; Urbano, G.; de Nadai, T.R.; de Carvalho Mastroianni, P. Genetic polymorphisms associated with upper gastrointestinal bleeding: A systematic review. Pharmacogenom. J. 2021, 21, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Beaini, H.; Bjorkman, C.; Johnson, K.; Araj, F.G. Sirolimus potentiated angioedema: A case report and review of the literature. Open Med. (Wars) 2024, 19, 20230884. [Google Scholar] [CrossRef]
- Lepore, N.; Carpenter, T.; Wolff, A. Angioedema from triple therapy: A case report. Cureus 2023, 15, e46247. [Google Scholar] [CrossRef] [PubMed]
- Sychev, I.V.; Denisenko, N.P.; Kachanova, A.A.; Lapshtaeva, A.V.; Abdullaev, S.P.; Goncharova, L.N.; Mirzaev, K.B.; Sychev, D.A. Pharmacogenetic markers of development of angioneurotic edema as a secondary side effect to enalapril in patients with essential arterial hypertension. Int. J. Risk Saf. Med. 2024, 35, 37–47. [Google Scholar] [CrossRef]
- Sychev, I.V.; Denisenko, N.P.; Kachanova, A.A.; Lapshtaeva, A.V.; Goncharova, L.N.; Mirzaev, K.B.; Sychev, D.A. Pharmacogenetic predictors of development of secondary to enalapril dry cough in hypertensive patients. Drug Metab. Pers. Ther. 2023, 38, 247–254. [Google Scholar] [CrossRef]
- Sinitsina, I.I.; Boyarko, A.V.; Temirbulatov, I.I.; Sychev, D.A.; Akmalova, K.A.; Sozaeva, Z.A.; Grishina, E.A.; Mirzaev, K.B.; Asoskova, A.V.; Fisenko, V.P. CYP2C9 gene polymorphisms influence on antihypertensive effectiveness and hypouricemic effect of losartan among patients with arterial hypertension: An observational study. Drug Metab. Pers. Ther. 2022, 38, 163–168. [Google Scholar] [CrossRef]
- Luizon, M.R.; Pereira, D.A.; Sandrim, V.C. Pharmacogenomics of hypertension and preeclampsia: Focus on gene-gene interactions. Front. Pharmacol. 2018, 9, 168. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Pharmacogenomics of hypertension treatment. Int. J. Mol. Sci. 2020, 21, 4709. [Google Scholar] [CrossRef]
- Siest, G.; Marteau, J.B.; Maumus, S.; Berrahmoune, H.; Jeannesson, E.; Samara, A.; Batt, A.M.; Visvikis-Siest, S. Pharmacogenomics and cardiovascular drugs: Need for integrated biological system with phenotypes and proteomic markers. Eur. J. Pharmacol. 2005, 527, 1–22. [Google Scholar] [CrossRef]
- Beitelshees, A.L.; Gong, Y.; Wang, D.; Schork, N.J.; Cooper-Dehoff, R.M.; Langaee, T.Y.; Shriver, M.D.; Sadee, W.; Knot, H.J.; Pepine, C.J.; et al. KCNMB1 genotype influences response to verapamil SR and adverse outcomes in the INternational VErapamil SR/Trandolapril STudy (INVEST). Pharmacogenet. Genom. 2007, 17, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Malki, M.A.; Pearson, E.R. Drug-drug-gene interactions and adverse drug reactions. Pharmacogenom. J. 2020, 20, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Stampe, N.K.; Glinge, C.; Rasmussen, B.S.; Bhardwaj, P.; Linnet, K.; Jabbari, R.; Paludan-Müller, C.; Hassager, C.; Kjærgaard, J.; Tfelt-Hansen, J.; et al. Toxicological profile using mass spectrometry in sudden cardiac arrest survivors admitted to a tertiary centre. Resuscitation 2024, 198, 110197. [Google Scholar] [CrossRef]
- Yao, X.; Tsang, T.; Sun, Q.; Quinney, S.; Zhang, P.; Ning, X.; Li, L.; Shen, L. Mining and visualizing high-order directional drug interaction effects using the FAERS database. BMC Med. Inf. Decis. Mak. 2020, 20, 50. [Google Scholar] [CrossRef]

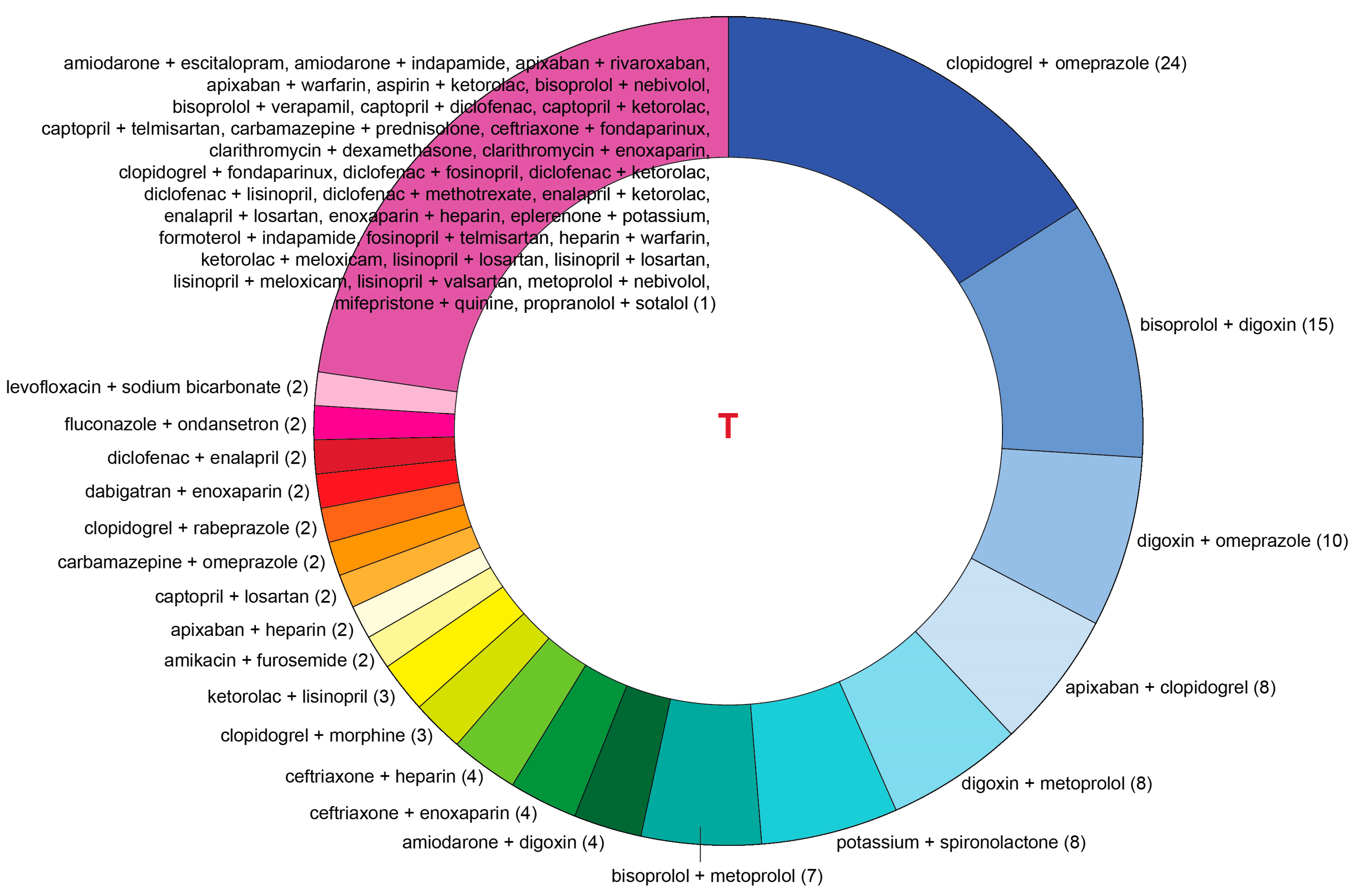
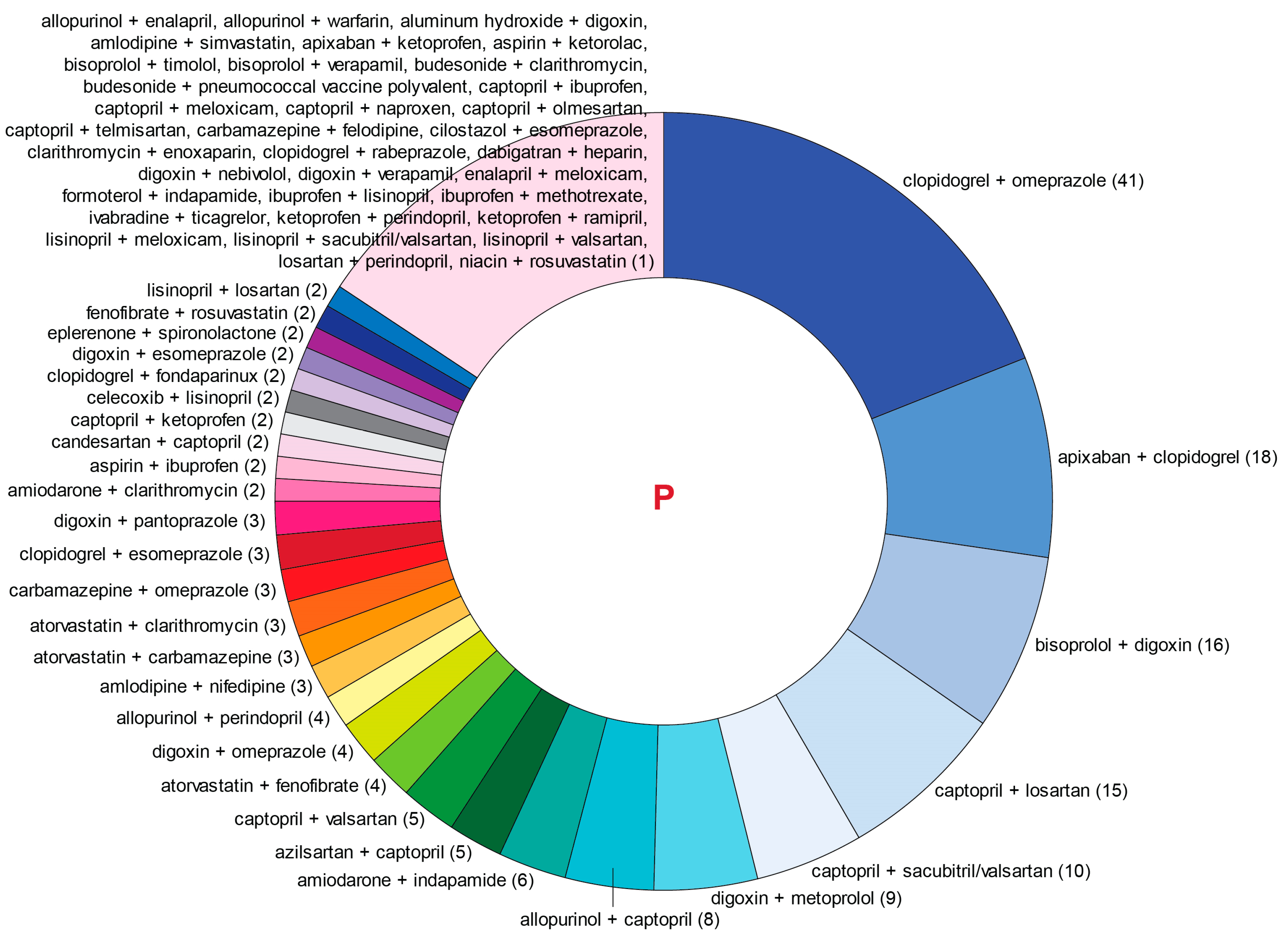
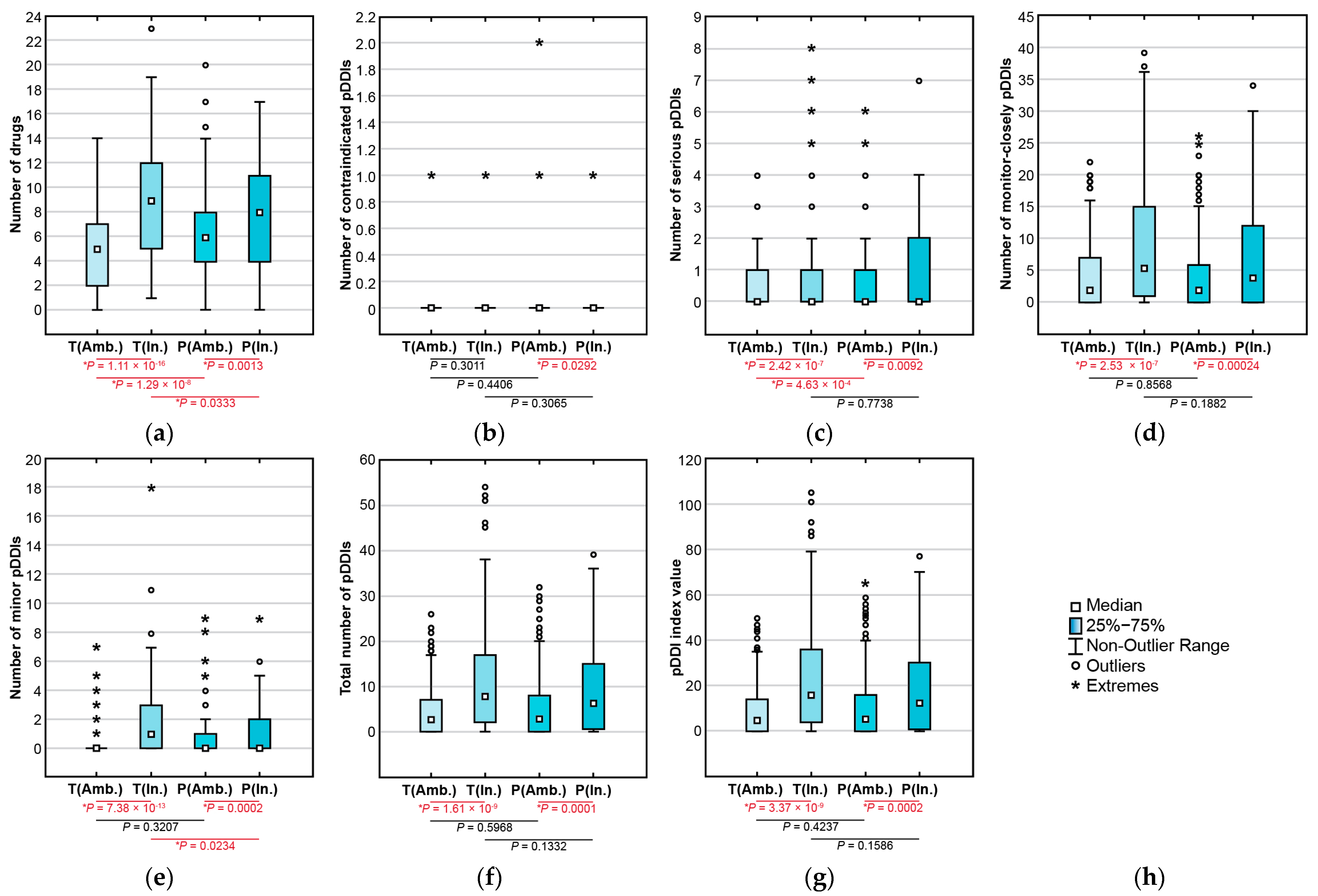
| Pairwise Drug Combination | T-List, n | P-List, n | Pairwise Drug Combination | T-List, n | P-List, n |
|---|---|---|---|---|---|
| Contraindicated pDDIs | |||||
| amitriptyline + indapamide | 1 | 1 | carbamazepine + linezolid | 1 | 0 |
| apixaban + carbamazepine | 0 | 1 | indapamide + sotalol | 2 | 0 |
| captopril + sacubitril/valsartan | 0 | 10 | lisinopril + sacubitril/valsartan | 0 | 1 |
| Serious pDDIs | |||||
| allopurinol + captopril | 0 | 8 | ceftriaxone + heparin | 4 | 0 |
| allopurinol + enalapril | 0 | 1 | celecoxib + lisinopril | 0 | 2 |
| allopurinol + perindopril | 0 | 3 | cilostazol + esomeprazole | 0 | 1 |
| allopurinol + warfarin | 0 | 1 | clarithromycin + dexamethasone | 1 | 0 |
| aluminum hydroxide + digoxin | 0 | 1 | clarithromycin + enoxaparin | 1 | 1 |
| amikacin + furosemide | 2 | 0 | clopidogrel + esomeprazole | 0 | 3 |
| amiodarone + clarithromycin | 0 | 2 | clopidogrel + fondaparinux | 1 | 2 |
| amiodarone + digoxin | 4 | 0 | clopidogrel + morphine | 3 | 0 |
| amiodarone + escitalopram | 1 | 0 | clopidogrel + omeprazole | 24 | 41 |
| amiodarone + indapamide | 1 | 6 | clopidogrel + rabeprazole | 2 | 1 |
| amlodipine + nifedipine | 0 | 3 | dabigatran + enoxaparin | 2 | 0 |
| amlodipine + simvastatin | 0 | 1 | dabigatran + heparin | 0 | 1 |
| apixaban + clopidogrel | 8 | 18 | diclofenac + enalapril | 2 | 0 |
| apixaban + heparin | 2 | 0 | diclofenac + fosinopril | 1 | 0 |
| apixaban + ketoprofen | 0 | 1 | diclofenac + ketorolac | 1 | 0 |
| apixaban + rivaroxaban | 1 | 0 | diclofenac + lisinopril | 1 | 0 |
| apixaban + warfarin | 1 | 0 | diclofenac + methotrexate | 1 | 0 |
| aspirin + captopril | 3 | 84 | digoxin + esomeprazole | 0 | 2 |
| aspirin + enalapril | 18 | 20 | digoxin + metoprolol | 8 | 9 |
| aspirin + fosinopril | 10 | 28 | digoxin + nebivolol | 0 | 1 |
| aspirin + ibuprofen | 0 | 2 | digoxin + omeprazole | 10 | 4 |
| aspirin + ketorolac | 1 | 1 | digoxin + pantoprazole | 0 | 3 |
| aspirin + lisinopril | 36 | 58 | digoxin + verapamil | 0 | 1 |
| aspirin + perindopril | 25 | 74 | enalapril + ketorolac | 1 | 0 |
| aspirin + ramipril | 0 | 6 | enalapril + losartan | 1 | 0 |
| atorvastatin + carbamazepine | 0 | 3 | enalapril + meloxicam | 0 | 1 |
| atorvastatin + clarithromycin | 0 | 3 | enoxaparin + heparin | 1 | 0 |
| atorvastatin + fenofibrate | 0 | 4 | eplerenone + potassium | 1 | 0 |
| azilsartan + captopril | 0 | 5 | eplerenone + spironolactone | 0 | 2 |
| bisoprolol + digoxin | 15 | 16 | fenofibrate + rosuvastatin | 0 | 2 |
| bisoprolol + metoprolol | 7 | 0 | fluconazole + ondansetron | 2 | 0 |
| bisoprolol + nebivolol | 1 | 0 | formoterol + indapamide | 1 | 1 |
| bisoprolol + timolol | 0 | 1 | fosinopril + telmisartan | 1 | 0 |
| bisoprolol + verapamil | 1 | 1 | heparin + warfarin | 1 | 0 |
| budesonide + clarithromycin | 0 | 1 | ibuprofen + lisinopril | 0 | 1 |
| budesonide + pneumococcal vaccine polyvalent | 0 | 1 | ibuprofen + methotrexate | 0 | 1 |
| candesartan + captopril | 0 | 2 | ivabradine + ticagrelor | 0 | 1 |
| captopril + diclofenac | 1 | 0 | ketoprofen + perindopril | 0 | 1 |
| captopril + ibuprofen | 0 | 1 | ketoprofen + ramipril | 0 | 1 |
| captopril + ketoprofen | 0 | 2 | ketorolac + lisinopril | 3 | 0 |
| captopril + ketorolac | 1 | 0 | ketorolac + meloxicam | 1 | 0 |
| captopril + losartan | 2 | 15 | levofloxacin + sodium bicarbonate | 2 | 0 |
| captopril + meloxicam | 0 | 1 | lisinopril + losartan | 2 | 0 |
| captopril + naproxen | 0 | 1 | lisinopril + losartan | 0 | 2 |
| captopril + olmesartan | 0 | 1 | lisinopril + meloxicam | 1 | 1 |
| captopril + sacubitril/valsartan | 0 | 10 | lisinopril + sacubitril/valsartan | 0 | 1 |
| captopril + telmisartan | 1 | 1 | lisinopril + valsartan | 1 | 1 |
| captopril + valsartan | 0 | 5 | losartan + perindopril | 0 | 1 |
| carbamazepine + felodipine | 0 | 1 | metoprolol + nebivolol | 1 | 0 |
| carbamazepine + omeprazole | 2 | 3 | mifepristone + quinine | 1 | 0 |
| carbamazepine + prednisolone | 1 | 0 | niacin + rosuvastatin | 0 | 1 |
| ceftriaxone + enoxaparin | 4 | 0 | potassium + spironolactone | 8 | 0 |
| ceftriaxone + fondaparinux | 1 | 0 | propranolol + sotalol | 1 | 0 |
| Characteristic | Value |
|---|---|
| White/Caucasian, n (%) | 1030 (100) |
| Men, n (%) | 670 (65.0) |
| Women, n (%) | 360 (35.0) |
| Age (males), median (IQR), years | 63 (56; 71) |
| Age (females), median (IQR), years | 71 (61; 77) |
| Outpatient visits, n (%) | 773 (75.1) |
| Home consultations, n (%) | 77 (7.5) |
| In-hospital care, n (%) | 141 (13.7) |
| Emergency events, n (%) | 37 (3) |
| Postmortem documentation, n (%) | 2 (0.2) |
| Primary diagnosis of COVID-19, n (%) | 17 (1.7) |
| Estimated history of COVID-19, n (%) | 145 (14.1) |
| Time of creating health records | January 2018–May 2023 |
| Aspirin + ACE Inhibitor * Combination Associated with Serious pDDIs | T-List, n (%) | P-List, n (%) | P-to-T-List Percentage Ratio | p-Value |
|---|---|---|---|---|
| Aspirin + captopril | 3 (1.24) | 84 (17.3) | 13.94 | <0.0001 |
| Aspirin + enalapril | 18 (7.44) | 20 (4.12) | 0.553 | 0.0020 |
| Aspirin + fosinopril | 10 (4.13) | 28 (5.76) | 1.394 | 1 |
| Aspirin + lisinopril | 36 (14.9) | 58 (11.9) | 0.802 | 0.0014 |
| Aspirin + perindopril | 25 (10.3) | 74 (15.2) | 1.474 | 0.9203 |
| Aspirin + ramipril | 0 (0.00) | 6 (1.24) | n.a. ** | 0.3323 |
| Aspirin + ACE inhibitor | 92 (38.0) | 270 (55.6) | 1.461 | <0.0001 |
| Aspirin + ACE Inhibitor Combination Associated with Monitor-Closely pDDIs | T-List, n (%) | P-List, n (%) | P-to-T-List Percentage Ratio | p-Value |
|---|---|---|---|---|
| Aspirin + captopril | 32 (0.95) | 84 (1.99) | 2.105 | 0.0409 |
| Aspirin + enalapril | 22 (0.65) | 22 (0.52) | 0.802 | 0.0574 |
| Aspirin + fosinopril | 14 (0.41) | 30 (0.71) | 1.719 | 0.6714 |
| Aspirin + lisinopril | 48 (1.42) | 63 (1.50) | 1.053 | 0.0769 |
| Aspirin + perindopril | 40 (1.18) | 78 (1.85) | 1.564 | 0.6892 |
| Aspirin + ramipril | 2 (0.06) | 6 (0.14) | 2.406 | 0.7913 |
| Aspirin + ACE inhibitor | 158 (4.68) | 283 (6.72) | 1.437 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anfinogenova, N.D.; Stepanov, V.A.; Chernyavsky, A.M.; Karpov, R.S.; Efimova, E.V.; Novikova, O.M.; Trubacheva, I.A.; Falkovskaya, A.Y.; Maksimova, A.S.; Ryumshina, N.I.; et al. Clinical Significance and Patterns of Potential Drug–Drug Interactions in Cardiovascular Patients: Focus on Low-Dose Aspirin and Angiotensin-Converting Enzyme Inhibitors. J. Clin. Med. 2024, 13, 4289. https://doi.org/10.3390/jcm13154289
Anfinogenova ND, Stepanov VA, Chernyavsky AM, Karpov RS, Efimova EV, Novikova OM, Trubacheva IA, Falkovskaya AY, Maksimova AS, Ryumshina NI, et al. Clinical Significance and Patterns of Potential Drug–Drug Interactions in Cardiovascular Patients: Focus on Low-Dose Aspirin and Angiotensin-Converting Enzyme Inhibitors. Journal of Clinical Medicine. 2024; 13(15):4289. https://doi.org/10.3390/jcm13154289
Chicago/Turabian StyleAnfinogenova, Nina D., Vadim A. Stepanov, Alexander M. Chernyavsky, Rostislav S. Karpov, Elena V. Efimova, Oksana M. Novikova, Irina A. Trubacheva, Alla Y. Falkovskaya, Aleksandra S. Maksimova, Nadezhda I. Ryumshina, and et al. 2024. "Clinical Significance and Patterns of Potential Drug–Drug Interactions in Cardiovascular Patients: Focus on Low-Dose Aspirin and Angiotensin-Converting Enzyme Inhibitors" Journal of Clinical Medicine 13, no. 15: 4289. https://doi.org/10.3390/jcm13154289
APA StyleAnfinogenova, N. D., Stepanov, V. A., Chernyavsky, A. M., Karpov, R. S., Efimova, E. V., Novikova, O. M., Trubacheva, I. A., Falkovskaya, A. Y., Maksimova, A. S., Ryumshina, N. I., Shelkovnikova, T. A., Ussov, W. Y., Vaizova, O. E., Popov, S. V., & Repin, A. N. (2024). Clinical Significance and Patterns of Potential Drug–Drug Interactions in Cardiovascular Patients: Focus on Low-Dose Aspirin and Angiotensin-Converting Enzyme Inhibitors. Journal of Clinical Medicine, 13(15), 4289. https://doi.org/10.3390/jcm13154289







