Impact of Concurrent Exercise Training on Cardiac Autonomic Modulation, Metabolic Profile, Body Composition, Cardiorespiratory Fitness, and Quality of Life in Type 2 Diabetes with Cardiac Autonomic Neuropathy: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Settings
2.2. Experiment and Procedure
2.3. Evaluation
2.3.1. Assessment of Cardiac Autonomic Function
- Resting Heart Rate and Heart Rate Variability Assessment
- Heart Rate Recovery Assessment
2.3.2. Blood Pressure
2.3.3. Body Composition Assessment
2.3.4. Biochemical Assessments
2.3.5. Quality of Life
2.3.6. Cardiorespiratory Fitness
2.4. Training Program
2.4.1. Concurrent Exercise Training
- Aerobic Training
- Resistance Training
2.4.2. Control Group
2.5. Statistical Analyses
3. Results
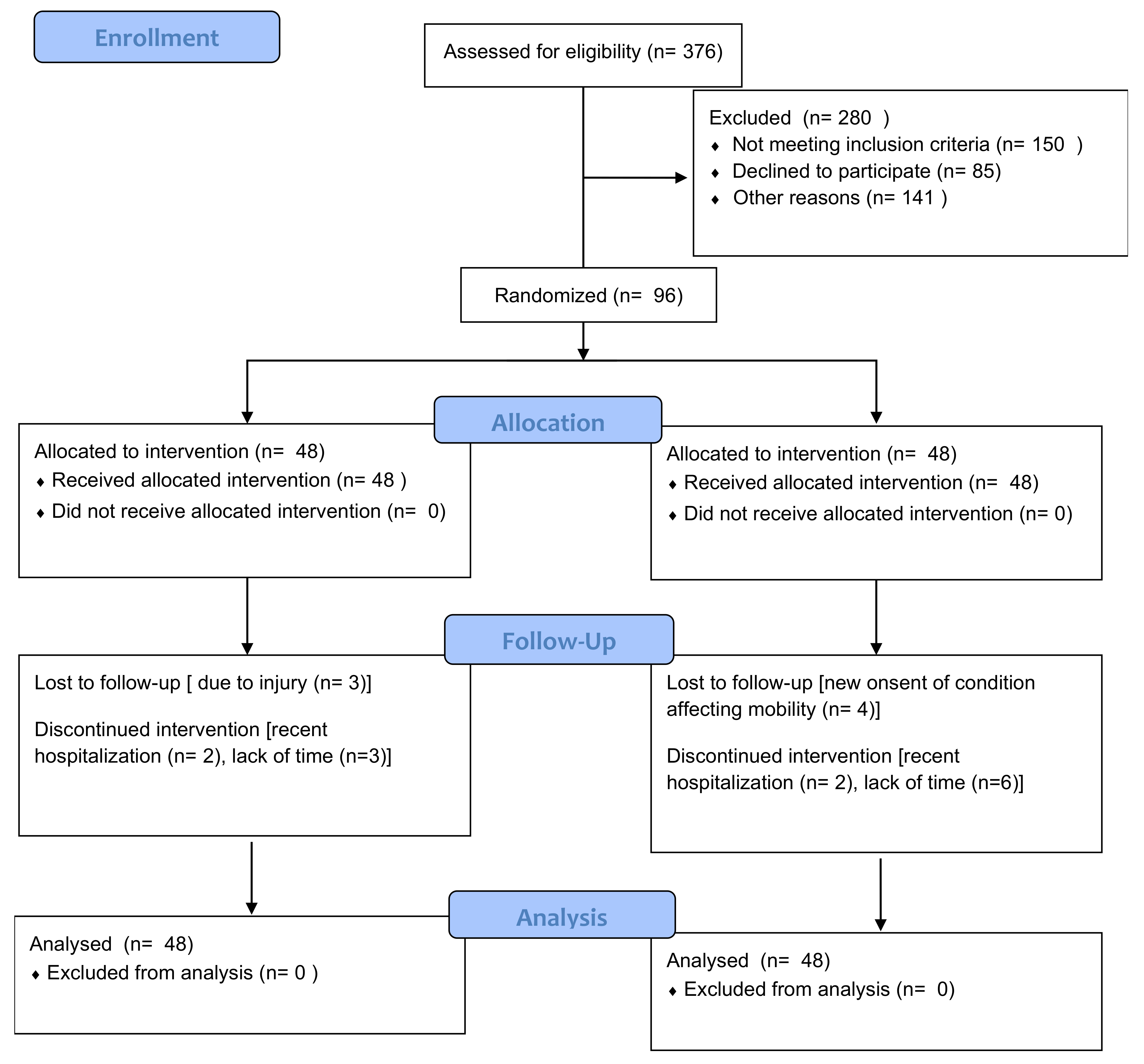
3.1. Participant Flow
3.2. Baseline Characteristics
3.3. ANOVA Findings
4. Discussion
4.1. Cardiac Autonomic Modulation
4.2. Metabolic Profile
4.3. Body Composition
4.4. Cardiorespiratory Fitness
4.5. Quality of Life
4.6. Clinical Utility in Daily Practice
5. Strength
6. Limitations
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.; Mediano, M.F.F.; De Lorenzo, A.; Rodrigues, L.F., Jr. Cardiovascular autonomic neuropathy in diabetes: Pathophysiology, clinical assessment and implications. World J. Diabetes 2021, 12, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [PubMed]
- Ambelu, T.; Teferi, G. The impact of exercise modalities on blood glucose, blood pressure and body composition in patients with type 2 diabetes mellitus. BMC Sports Sci. Med. Rehabil. 2023, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Syeda, U.S.A.; Battillo, D.; Visaria, A.; Malin, S.K. The importance of exercise for glycemic control in type 2 diabetes. Am. J. Med. Open 2023, 9, 100031. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin. J. Med. 2017, 84, S15–S21. [Google Scholar] [CrossRef]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Currier, B.S.; McLeod, J.C.; Banfield, L.; Beyene, J.; Welton, N.J.; D’Souza, A.C.; Keogh, J.A.J.; Lin, L.; Coletta, G.; Yang, A.; et al. Resistance training prescription for muscle strength and hypertrophy in healthy adults: A systematic review and Bayesian network meta-analysis. Br. J. Sports Med. 2023, 57, 1211–1220. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Stauffer, B.L. Effects of resistance training on body weight and body composition in older adults: An inter-individual response difference meta-analysis of randomized controlled trials. Sci. Prog. 2023, 106, 368504231179062. [Google Scholar] [CrossRef]
- Coffey, V.G.; Hawley, J.A. Concurrent exercise training: Do opposites distract? J. Physiol. 2017, 595, 2883–2896. [Google Scholar] [CrossRef]
- Zaki, S.; Sharma, S.; Vats, H. Effectiveness of concurrent exercise training in people with type 2 diabetes: A systematic review and meta-analysis. Physiother. Theory Pract. 2023, 1–22. [Google Scholar] [CrossRef]
- Gordan, R.; Gwathmey, J.K.; Xie, L.H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef]
- Zaki, S.; Alam, F.; Faizan, M.; Sharma, S.; Naqvi, I.H. Association between heart rate variability and cardiorespiratory fitness in individuals with type 2 diabetes mellitus: A cross-sectional study. J. Human. Sport. Exerc. 2024, 19, 779–791. [Google Scholar] [CrossRef]
- Kaze, A.D.; Agoons, D.D.; Santhanam, P.; Erqou, S.; Ahima, R.S.; Echouffo-Tcheugui, J.B. Correlates of cardiorespiratory fitness among overweight or obese individuals with type 2 diabetes. BMJ Open Diabetes Res. Care 2022, 10, e002446. [Google Scholar] [CrossRef]
- Bouamra, M.; Zouhal, H.; Ratel, S.; Makhlouf, I.; Bezrati, I.; Chtara, M.; Behm, D.G.; Granacher, U.; Chaouachi, A. Concurrent Training Promotes Greater Gains on Body Composition and Components of Physical Fitness Than Single-Mode Training (Endurance or Resistance) in Youth With Obesity. Front. Physiol. 2022, 13, 869063. [Google Scholar] [CrossRef]
- Wang, S.; Ji, X.; Zhang, Z.; Xue, F. Relationship between Lipid Profiles and Glycemic Control Among Patients with Type 2 Diabetes in Qingdao, China. Int. J. Environ. Res. Public Health 2020, 17, 5317. [Google Scholar] [CrossRef]
- Sabag, A.; Chang, C.R.; Francois, M.E.; Keating, S.E.; Coombes, J.S.; Johnson, N.A.; Pastor-Valero, M.; Rey Lopez, J.P. The Effect of Exercise on Quality of Life in Type 2 Diabetes: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2023, 55, 1353–1365. [Google Scholar] [CrossRef]
- Silva, L.R.; Gentil, P.; Seguro, C.S.; de Oliveira, J.C.; Silva, M.S.; Marques, V.A.; Beltrame, T.; Rebelo, A.C. High-Intensity Interval Training Improves Cardiac Autonomic Function in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Biology 2022, 11, 66. [Google Scholar] [CrossRef]
- Hamasaki, H. The Effect of Exercise on Cardiovascular Autonomic Nervous Function in Patients with Diabetes: A Systematic Review. Healthcare 2023, 11, 2668. [Google Scholar] [CrossRef]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011, 34 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Ewing, D.J.; Martyn, C.N.; Young, R.J.; Clarke, B.F. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985, 8, 491–498. [Google Scholar] [CrossRef]
- ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA; American College of Sports Medicine: Indianapolis, IN, USA, 2013.
- Bassi, D.; Mendes, R.G.; Arakelian, V.M.; Caruso, F.C.R.; Cabiddu, R.; Júnior, J.C.B.; Arena, R.; Borghi-Silva, A. Potential Effects on Cardiorespiratory and Metabolic Status After a Concurrent Strength and Endurance Training Program in Diabetes Patients—A Randomized Controlled Trial. Sports Med. Open 2016, 2, 31. [Google Scholar] [CrossRef]
- Catai, A.M.; Pastre, C.M.; Godoy, M.F.; Silva, E.D.; Takahashi, A.C.M.; Vanderlei, L.C.M. Heart rate variability: Are you using it properly? Standardisation checklist of procedures. Braz. J. Phys. Ther. 2020, 24, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Bhati, P.; Hussain, M.E. Sleep duration is a significant predictor of cardiac autonomic neuropathy in type 2 diabetes mellitus. Prim. Care Diabetes 2019, 13, 452–461. [Google Scholar] [CrossRef]
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381.
- Peltenburg, P.J.; Kallas, D.; Bos, J.M.; Lieve, K.V.; Franciosi, S.; Roston, T.M.; Denjoy, I.; Sorensen, K.B.; Ohno, S.; Roses-Noguer, F. An international multicenter cohort study on β-blockers for the treatment of symptomatic children with catecholaminergic polymorphic ventricular tachycardia. Circulation 2022, 145, 333–344. [Google Scholar] [CrossRef]
- Bhati, P.; Hussain, M.E. Leisure-Time Physical Activity and Glycemic Control Independently Predicts Cardiac Autonomic Neuropathy in Type 2 Diabetes Mellitus. J. Phys. Act. Health 2021, 18, 1393–1403. [Google Scholar] [CrossRef]
- Okutucu, S.; Karakulak, U.N.; Aytemir, K.; Oto, A. Heart rate recovery: A practical clinical indicator of abnormal cardiac autonomic function. Expert. Rev. Cardiovasc. Ther. 2011, 9, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Balady, G.J.; Beasley, J.W.; Bricker, J.T.; Duvernoy, W.F.; Froelicher, V.F.; Mark, D.B.; Marwick, T.H.; McCallister, B.D.; Thompson, P.D., Jr.; et al. ACC/AHA Guidelines for Exercise Testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J. Am. Coll. Cardiol. 1997, 30, 260–311. [Google Scholar] [CrossRef] [PubMed]
- Shetler, K.; Marcus, R.; Froelicher, V.F.; Vora, S.; Kalisetti, D.; Prakash, M.; Do, D.; Myers, J. Heart rate recovery: Validation and methodologic issues. J. Am. Coll. Cardiol. 2001, 38, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Shimbo, D.; Carey, R.M.; Charleston, J.B.; Gaillard, T.; Misra, S.; Myers, M.G.; Ogedegbe, G.; Schwartz, J.E.; Townsend, R.R. Measurement of blood pressure in humans: A scientific statement from the American Heart Association. Hypertension 2019, 73, e35–e66. [Google Scholar] [CrossRef]
- Ogedegbe, G.; Pickering, T. Principles and techniques of blood pressure measurement. Cardiol. Clin. 2010, 28, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Ma, W.Y.; Yang, C.Y.; Shih, S.R.; Hsieh, H.J.; Hung, C.S.; Chiu, F.C.; Lin, M.S.; Liu, P.H.; Hua, C.H.; Hsein, Y.C.; et al. Measurement of Waist Circumference: Midabdominal or iliac crest? Diabetes Care 2013, 36, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.G.; Stevens, J.; Truesdale, K.P.; Cai, J.; Adair, L.S.; North, K.E. Hip circumference and incident metabolic risk factors in Chinese men and women: The People’s Republic of China study. Metab. Syndr. Relat. Disord. 2011, 9, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Pollock, M.L.; Ward, A. Generalized equations for predicting body density of women. Med. Sci. Sports Exerc. 1980, 12, 175–181. [Google Scholar] [CrossRef]
- Siri, W. Body Composition from Fluid Spaces and Density: Analysis of Methods; National Academy of Sciences: Washington, DC, USA, 1961. [Google Scholar]
- Bradley, C.; Todd, C.; Gorton, T.; Symonds, E.; Martin, A.; Plowright, R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: The ADDQoL. Qual. Life Res. 1999, 8, 79–91. [Google Scholar] [CrossRef]
- Bradley, C. The Audit of Diabetes-Dependent Quality of Life (ADDQoL). User Guidelines. 2017. Available online: https://healthpsychologyresearch.com/ (accessed on 30 June 2024).
- Beltz, N.M.; Gibson, A.L.; Janot, J.M.; Kravitz, L.; Mermier, C.M.; Dalleck, L.C. Graded Exercise Testing Protocols for the Determination of VO(2)max: Historical Perspectives, Progress, and Future Considerations. J. Sports Med. 2016, 2016, 3968393. [Google Scholar] [CrossRef] [PubMed]
- Trabulo, M.; Mendes, M.; Mesquita, A.; Seabra-Gomes, R. [Does the modified Bruce protocol induce physiological stress equal to that of the Bruce protocol?]. Rev. Port. Cardiol. 1994, 13, 753–760; 735–756. [Google Scholar] [PubMed]
- Colberg, S.R. Exercise and Diabetes: A Clinician’s Guide to Prescribing Physical Activity; American Diabetes Association: Alexandria, VA, USA, 2013. [Google Scholar]
- Colberg, S.R. Key Points from the Updated Guidelines on Exercise and Diabetes. Front. Endocrinol. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Jorge, M.L.M.P.; de Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.D.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S17–S38. [Google Scholar] [CrossRef]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate; a longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar] [PubMed]
- Annibalini, G.; Lucertini, F.; Agostini, D.; Vallorani, L.; Gioacchini, A.; Barbieri, E.; Guescini, M.; Casadei, L.; Passalia, A.; Del Sal, M.; et al. Concurrent Aerobic and Resistance Training Has Anti-Inflammatory Effects and Increases Both Plasma and Leukocyte Levels of IGF-1 in Late Middle-Aged Type 2 Diabetic Patients. Oxidative Med. Cell. Longev. 2017, 2017, 3937842. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, A.; Soltani, M.; Daraei, A.; Nohbaradar, H.; Haghighi, M.M.; Khosravi, N.; Johnson, K.E.; Laher, I.; Hackney, A.C.; VanDusseldorp, T.A.; et al. The Effects of Aerobic-Resistance Training and Broccoli Supplementation on Plasma Dectin-1 and Insulin Resistance in Males with Type 2 Diabetes. Nutrients 2021, 13, 3144. [Google Scholar] [CrossRef] [PubMed]
- Brzycki, M. Strength Testing—Predicting a One-Rep Max from Reps-to-Fatigue. J. Phys. Educ. Recreat. Danc. 1993, 64, 88–90. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Care in Diabetes—2023 Abridged for Primary Care Providers. Clin. Diabetes 2022, 41, 4–31. [Google Scholar] [CrossRef]
- Gupta, S.K. Intention-to-treat concept: A review. Perspect. Clin. Res. 2011, 2, 109–112. [Google Scholar] [CrossRef]
- Su, X.; He, J.; Cui, J.; Li, H.; Men, J. The effects of aerobic exercise combined with resistance training on inflammatory factors and heart rate variability in middle-aged and elderly women with type 2 diabetes mellitus. Ann. Noninvasive Electrocardiol. 2022, 27, e12996. [Google Scholar] [CrossRef] [PubMed]
- Routledge, F.S.; Campbell, T.S.; McFetridge-Durdle, J.A.; Bacon, S.L. Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 2010, 26, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Tauveron, I.; Magdasy, S.; Benichou, T.; Bagheri, R.; Ugbolue, U.C.; Navel, V.; Dutheil, F. Effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251863. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Casellini, C.; Parson, H.K.; Colberg, S.R.; Nevoret, M.-L. Cardiac Autonomic Neuropathy in Diabetes: A Predictor of Cardiometabolic Events. Front. Neurosci. 2018, 12, 591. [Google Scholar] [CrossRef] [PubMed]
- Nascimentoa, P.M.; Vieiraa, M.C.; Sperandeib, S.; Serraa, S.M. Supervised exercise improves autonomic modulation in participants in cardiac rehabilitation programs. Rev. Port. Cardiol. 2016, 35, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Dewar, A.; Kass, L.; Stephens, R.C.M.; Tetlow, N.; Desai, T. Heart Rate Recovery Assessed by Cardiopulmonary Exercise Testing in Patients with Cardiovascular Disease: Relationship with Prognosis. Int. J. Environ. Res. Public Health 2023, 20, 4678. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.A.; Minson, C.T.; Halliwill, J.R. The cardiovascular system after exercise. J. Appl. Physiol. 2017, 122, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.; Moiz, J.A.; Bhati, P.; Menon, G.R. Efficacy of high-intensity interval training on cardiac autonomic modulation in cardiovascular diseases and lifestyle disorders: A systematic review and meta-analysis. Comp. Exerc. Physiol. 2022, 18, 37–52. [Google Scholar] [CrossRef]
- Abulmeaty, M.M.A.; Aljuraiban, G.S.; Alaidarous, T.A.; Alkahtani, N.M. Body Composition and the Components of Metabolic Syndrome in Type 2 Diabetes: The Roles of Disease Duration and Glycemic Control. Diabetes Metab. Syndr. Obes. 2020, 13, 1051–1059. [Google Scholar] [CrossRef]
- Pandey, A.; Swift, D.L.; McGuire, D.K.; Ayers, C.R.; Neeland, I.J.; Blair, S.N.; Johannsen, N.; Earnest, C.P.; Berry, J.D.; Church, T.S. Metabolic Effects of Exercise Training Among Fitness-Nonresponsive Patients With Type 2 Diabetes: The HART-D Study. Diabetes Care 2015, 38, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.L.; Neiberg, R.H.; Espeland, M.A.; Johnson, K.C.; Knowler, W.C.; Pi-Sunyer, X.; Staiano, A.E.; Wagenknecht, L.E.; Wing, R.R. Waist Circumference Change During Intensive Lifestyle Intervention and Cardiovascular Morbidity and Mortality in the Look AHEAD Trial. Obesity 2020, 28, 1902–1911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, Q.; Zeng, Y.; Cheng, L. Effectiveness of combined exercise in people with type 2 diabetes and concurrent overweight/obesity: A systematic review and meta-analysis. BMJ Open 2021, 11, e046252. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.L.; Hunter, G.R.; Gower, B.A.; Bamman, M.M.; Windham, S.T.; Moellering, D.R.; Fisher, G. Exercise Effects on Mitochondrial Function and Lipid Metabolism during Energy Balance. Med. Sci. Sports Exerc. 2020, 52, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.H.; Goncalves, R.L.S.; Madiraju, A.K.; Strasser, B.; Sparks, L.M. Resistance training to improve type 2 diabetes: Working toward a prescription for the future. Nutr. Metab. 2017, 14, 24. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xiao, H. Exercise for Mental Well-Being: Exploring Neurobiological Advances and Intervention Effects in Depression. Life 2023, 13, 1505. [Google Scholar] [CrossRef]
- Gacesa, J.Z.; Klasnja, A.V.; Grujic, N.G. Changes in strength, endurance, and fatigue during a resistance-training program for the triceps brachii muscle. J. Athl. Train. 2013, 48, 804–809. [Google Scholar] [CrossRef]



| Variables | CET (n = 48) | Control (n = 48) | p-Value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Age (years) | 46.38 ± 7.55 | 47.56 ± 7.88 | 0.45 | |
| Sex | Male/Female | 29/19 | 33/15 | 0.52 |
| Marital status | Married/Single/Widower/Divorced | 40/4/2/2 | 45/2/1/0 | 0.34 |
| Professional activity | Working/Not working | 40/8 | 33/15 | 0.15 |
| Education | Primary and vocational/Preuniversity/Higher | 8/19/10 | 11/30/18 | 0.07 |
| Place of residence | Rural/Urban | 7/41 | 4/44 | 0.33 |
| Smoking | Never/Past/Present | 40/8/0 | 40/4/4 | 0.06 |
| Alcohol | Drinking/Not drinking | 0/48 | 2/46 | 0.49 |
| Duration of diabetes | 7.14 ± 4.84 | 7.04 ± 4.92 | 0.91 | |
| Drugs | Oral antidiabetic/Insulin/Diet | 45/2/1 | 43/2/3 | 0.59 |
| CARTs | ||||
| Diagnosis, n (n%) | ||||
| Early | 21 (43.8) | 19 (39.6) | 0.83 | |
| Definite | 27 (56.3) | 29 (60.4) | 0.83 | |
| E:I ratio | 0.93 ± 0.12 | 0.86 ± 0.23 | 0.80 | |
| ΔHR | 8.51 ± 3.73 | 7.56 ± 3.78 | 0.22 | |
| VR | 1.06 ± 0.26 | 1.13 ± 0.18 | 0.15 | |
| ΔDBP (mmHg) | 4.06 ± 2.29 | 4.21 ± 1.66 | 0.72 | |
| 30:15 ratio | 0.95 ± 0.09 | 0.97 ± 0.07 | 0.33 | |
| ΔSBP (mmHg) | 5.69 ± 2.57 | 6.02 ± 2.23 | 0.49 |
| Variables | CET (n = 48) | Control (n = 48) | p-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Resting HRV parameters | |||
| SDNN (ms) | 33.55 ± 4.93 | 33.75 ± 4.23 | 0.87 |
| RMSSD (ms) | 28.97 ± 5.99 | 31.28 ± 5.73 | 0.05 |
| pNN50 (%) | 1.76 ± 0.25 | 1.83 ± 0.27 | 0.24 |
| TP (ms2) | 2260.52 ± 1585.84 | 1952.51 ± 1384.43 | 0.31 |
| LF power (ms2) | 279.12 ± 44.64 | 279.05 ± 50.87 | 0.99 |
| HF power (ms2) | 295.67 ± 42.89 | 296.89 ± 51.09 | 0.89 |
| LF/HF ratio | 1.34 ± 0.45 | 1.37 ± 0.48 | 0.70 |
| Heart rate recovery | |||
| Peak HR (bpm) | 167.56 ± 10.33 | 169.56 ± 12.21 | 0.38 |
| HRR30s (bpm) | 3.18 ± 1.57 | 2.89 ± 1.37 | 0.33 |
| HRR1 (bpm) | 18.12 ± 3.80 | 18.66 ± 4.02 | 0.50 |
| HRR2 (bpm) | 34.56 ± 6.50 | 33.64 ± 4.55 | 0.42 |
| HRR3 (bpm) | 60.41 ± 6.88 | 59.43 ± 6.69 | 0.48 |
| Cardiovascular function | |||
| HRrest (bpm) | 77.15 ± 7.57 | 76.35 ± 6.94 | 0.60 |
| SBP (mmHg) | 129.44 ± 16.28 | 130.83 ± 18.18 | 0.69 |
| DBP (mmHg) | 86.33 ± 5.43 | 86.33 ± 7.33 | 0.70 |
| Glycemic control | |||
| FBG (mg/dL) | 133.8 ± 28.73 | 141.67 ± 29.20 | 0.18 |
| PPBG (mg/dL) | 147.44 ± 29.22 | 173.50 ± 42.33 | 0.50 |
| HbA1c (%) | 6.83 ± 0.23 | 6.89 ± 0.15 | 0.11 |
| Body composition | |||
| BMI (kg/m2) | 27.15 ± 2.96 | 26.69 ± 2.72 | 0.43 |
| WC (cm) | 96.79 ± 2.30 | 96.73 ± 2.21 | 0.89 |
| HC (cm) | 103.29 ± 2.75 | 103.42 ± 2.59 | 0.81 |
| WHR | 0.93 ± 0.31 | 0.92 ± 0.04 | 0.23 |
| PBF | 34.27 ± 0.56 | 34.38 ± 0.47 | 0.31 |
| Lipid profile | |||
| TC (mg/dL) | 212.19 ± 14.53 | 214.77 ± 4.16 | 0.38 |
| TG (mg/dL) | 166.88 ± 9.60 | 170.42 ± 7.77 | 0.05 |
| LDL-C (mg/dL) | 133.13 ± 10.75 | 136.56 ± 11.25 | 0.12 |
| HDL-C (mg/dL) | 51.65 ± 3.17 | 52.58 ± 2.80 | 0.12 |
| Cardiorespiratory fitness | |||
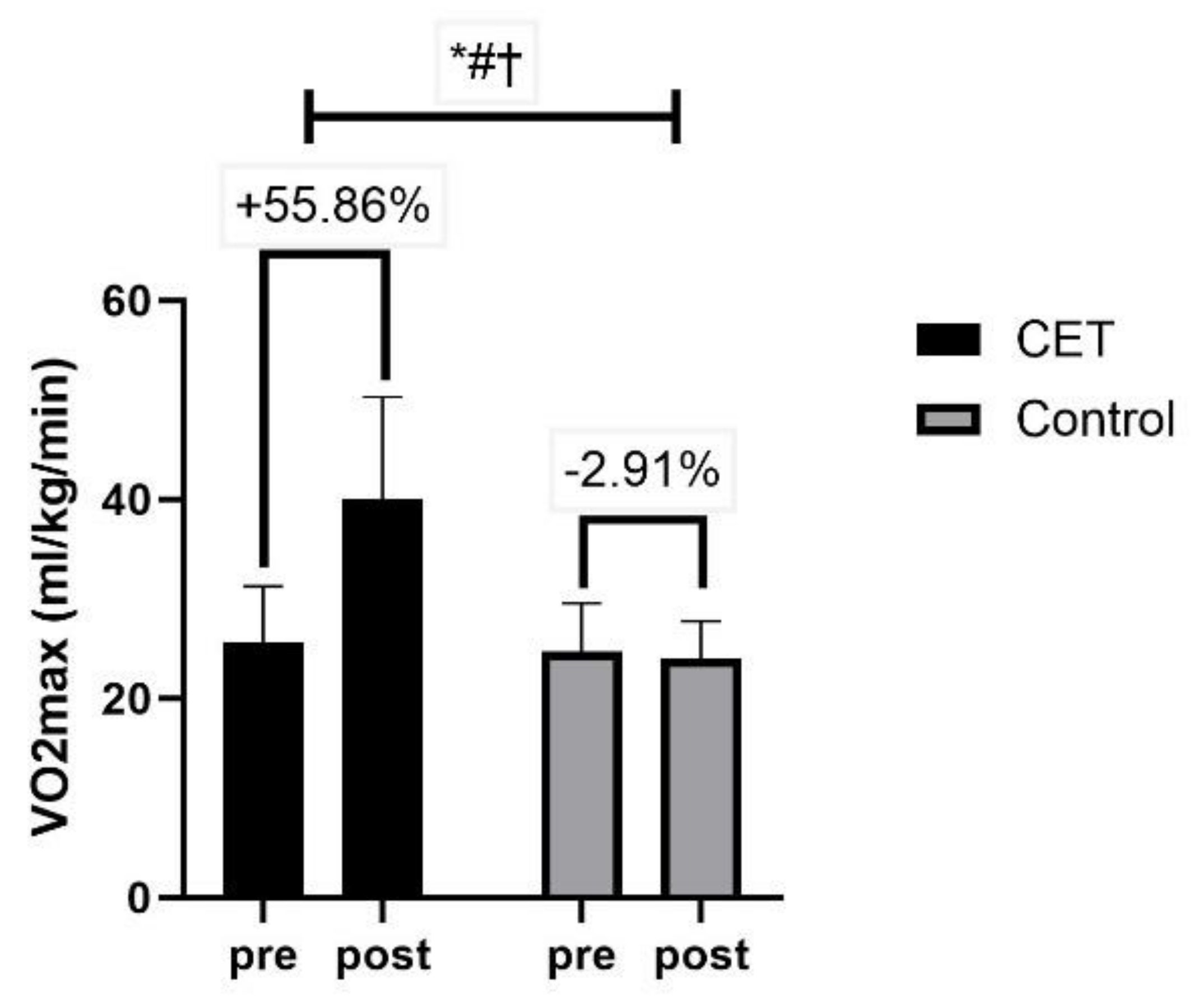
| VO2max (mL/kg/min) | 25.69 ± 5.59 | 24.73 ± 4.83 | 0.37 |
| Diabetes Dependent QoL (ADDQoL-19) | |||
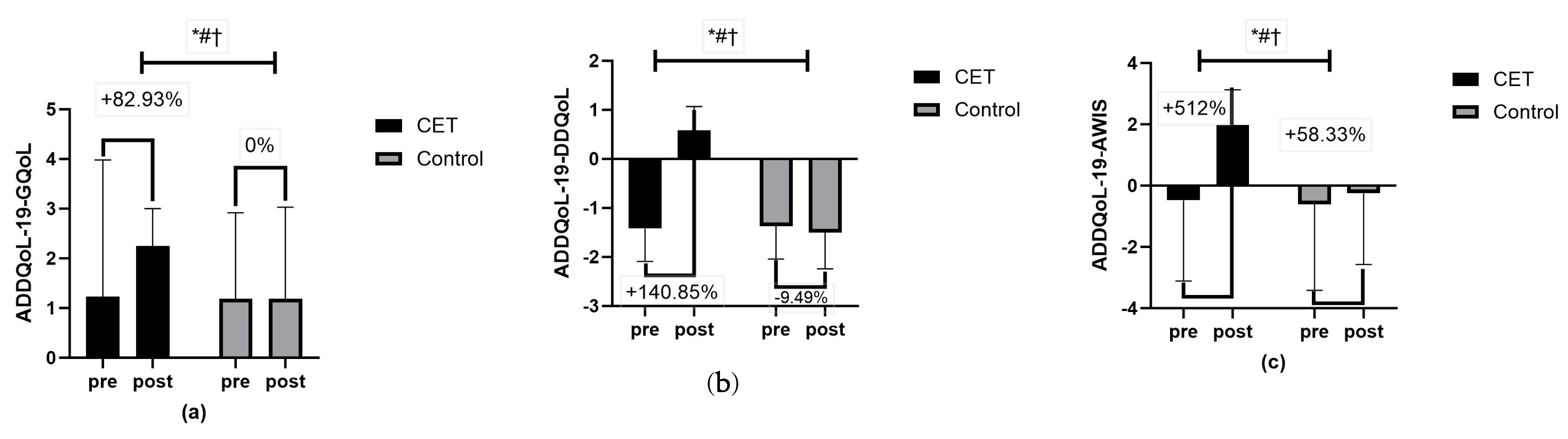
| ADDQoL-19-GQoL | 1.23 ± 2.75 | 1.19 ± 1.73 | 0.90 |
| ADDQoL-19-DDQoL | −1.42 ± 0.67 | −1.37 ± 0.67 | 0.76 |
| ADDQoL-19-AWIS | −0.48 ± 2.63 | −0.60 ± 2.82 | 0.82 |
| Variables | CET (n = 48) (End of 13th Week) | Control (n = 48) (End of 13th Week) | Source of Variation | p-Value | F-Value | pη2 |
|---|---|---|---|---|---|---|
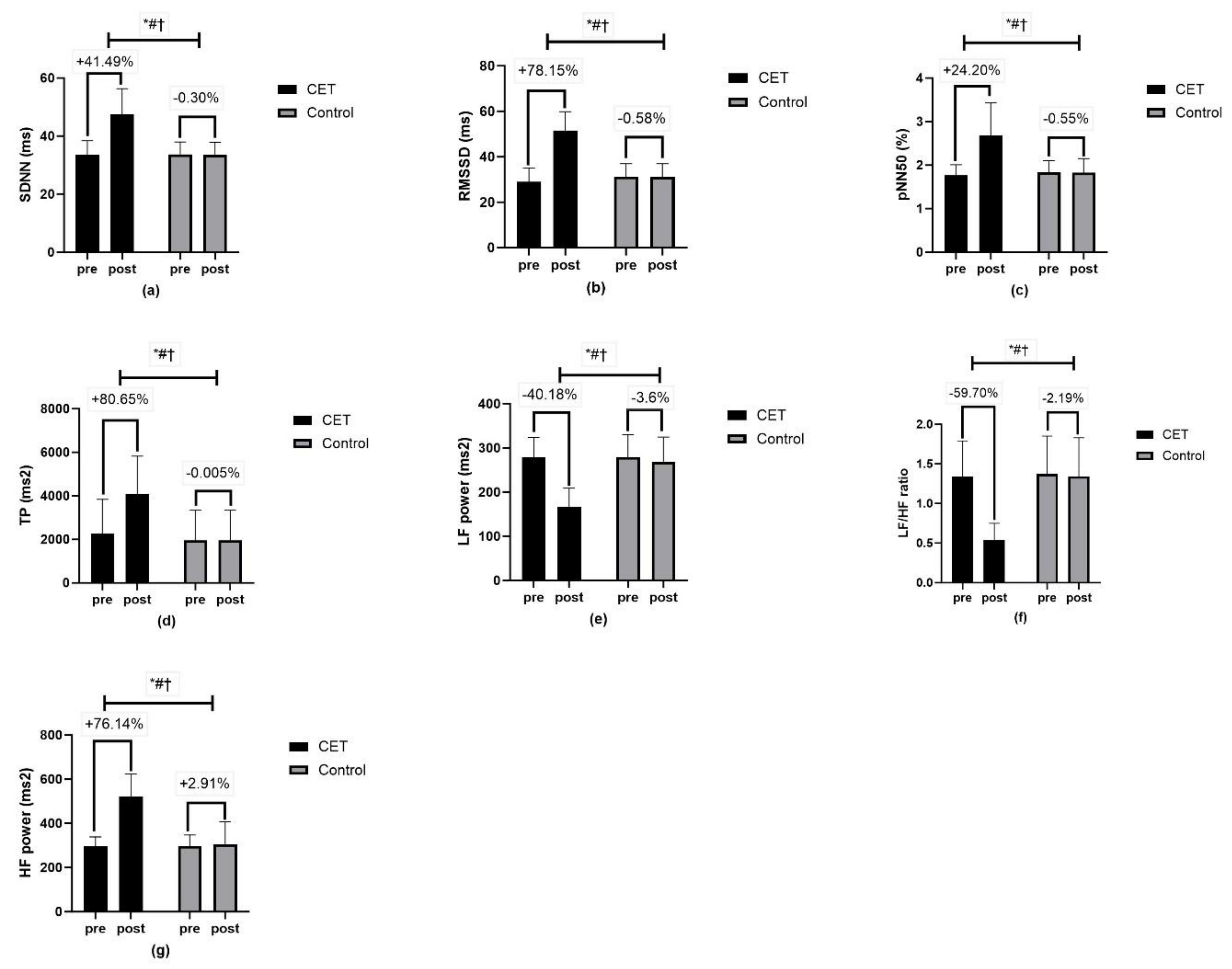
| Heart rate variability | ||||||
| SDNN (ms) | 47.47 ± 8.87 | 33.65 ± 4.26 | Time | <0.01 ** | 127.40 | 0.57 |
| Group × Time | <0.01 ** | 131.43 | 0.58 | |||
| Group | <0.01 ** | 43.12 | 0.31 | |||
| RMSSD (ms) | 51.61 ± 8.22 | 31.10 ± 5.93 | Time | <0.01 ** | 196.23 | 0.67 |
| Group × Time | <0.01 ** | 202.62 | 0.68 | |||
| Group | <0.01 ** | 72.05 | 0.43 | |||
| pNN50 (%) | 2.68 ± 0.75 | 1.82 ± 0.33 | Time | <0.01 ** | 78.69 | 0.45 |
| Group × Time | <0.01 ** | 82.35 | 0.46 | |||
| Group | <0.01 ** | 27.15 | 0.22 | |||
| TP (ms2) | 4084.72 ± 1740.65 | 1952.41 ± 1385.02 | Time | <0.01 ** | 41.61 | 0.30 |
| Group × Time | <0.01 ** | 41.62 | 0.30 | |||
| Group | <0.01 ** | 19.15 | 0.16 | |||
| LF power (ms2) | 166.97 ± 42.45 | 269.01 ± 55.56 | Time | <0.01 ** | 78.51 | 0.45 |
| Group × Time | <0.01 ** | 54.83 | 0.36 | |||
| Group | <0.01 ** | 50.85 | 0.35 | |||
| HF power (ms2) | 520.85 ± 103.46 | 305.54 ± 101.93 | Time | <0.01 ** | 132.09 | 0.58 |
| Group × Time | <0.01 ** | 113.28 | 0.54 | |||
| Group | <0.01 ** | 70.49 | 0.42 | |||
| LF/HF ratio | 0.54 ± 0.21 | 1.34 ± 0.49 | Time | <0.01 ** | 190.84 | 0.67 |
| Group × Time | <0.01 ** | 156.90 | 0.62 | |||
| Group | <0.01 ** | 26.03 | 0.21 | |||
| Heart rate recovery | ||||||
| Peak HR (bpm) | 134.93 ± 10.07 | 167.70 ± 12.38 | Time | <0.01 ** | 226.15 | 0.70 |
| Group × Time | <0.01 ** | 229.92 | 0.71 | |||
| Group | <0.01 ** | 15.05 | 0.38 | |||
| HRR30s (bpm) | 6.43 ± 3.03 | 3.89 ± 4.30 | Time | <0.01 ** | 32.01 | 0.24 |
| Group × Time | <0.01 ** | 8.97 | 0.08 | |||
| Group | <0.01 ** | 10.36 | 0.09 | |||
| HRR1 (bpm) | 34.06 ± 7.77 | 18.87 ± 4.06 | Time | <0.01 ** | 155.63 | 0.62 |
| Group × Time | <0.01 ** | 147.70 | 0.61 | |||
| Group | <0.01 ** | 76.21 | 0.44 | |||
| HRR2 (bpm) | 65.81 ± 11.64 | 32.72 ± 4.36 | Time | <0.01 ** | 224.68 | 0.70 |
| Group × Time | <0.01 ** | 252.66 | 0.72 | |||
| Group | <0.01 ** | 232.42 | 0.71 | |||
| HRR3 (bpm) | 76.41 ± 7.55 | 58.31 ± 6.78 | Time | <0.01 ** | 73.56 | 0.43 |
| Group × Time | <0.01 ** | 97.50 | 0.50 | |||
| Group | 0.57 | 0.31 | 0.00 | |||
| Cardiovascular function | ||||||
| HRrest | 77.15 ± 7.57 | 76.35 ± 6.94 | Time | <0.01 ** | 17.74 | 0.15 |
| Group × Time | <0.01 ** | 12.82 | 0.12 | |||
| Group | 0.06 | 3.46 | 0.03 | |||
| SBP (mmHg) | 129.44 ± 16.28 | 130.83 ± 18.18 | Time | <0.01 ** | 16.44 | 0.14 |
| Group × Time | <0.01 ** | 12.49 | 0.11 | |||
| Group | 0.13 | 2.27 | 0.02 | |||
| DBP (mmHg) | 86.33 ± 5.43 | 86.33 ± 7.33 | Time | <0.01 ** | 19.22 | 0.17 |
| Group × Time | 0.85 | 0.03 | 0.00 | |||
| Group | 0.57 | 0.31 | 0.00 | |||
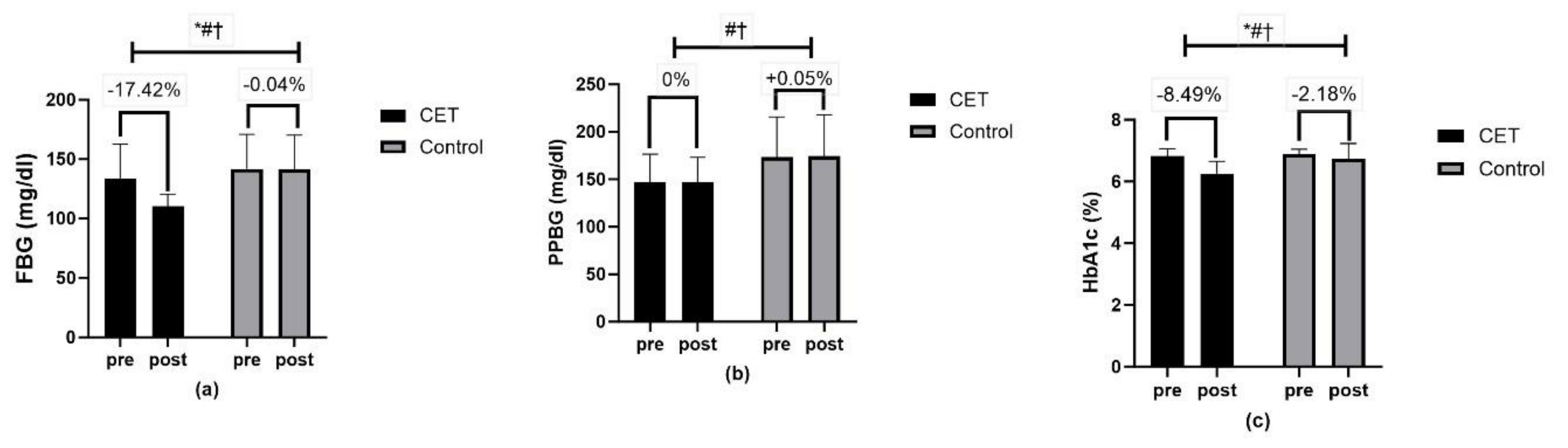
| Glycemic control | ||||||
| FBG (mg/dL) | 110.48 ± 10.11 | 141.60 ± 28.63 | Time | <0.01 ** | 25.89 | 0.21 |
| Group × Time | <0.01 ** | 25.61 | 0.21 | |||
| Group | <0.01 ** | 17.40 | 0.15 | |||
| PPBG (mg/dL) | 147.44 ± 25.72 | 174.38 ± 43.32 | Time | <0.01 ** | 46.27 | 0.33 |
| Group × Time | <0.01 ** | 54.63 | 0.36 | |||
| Group | 0.29 | 4.90 | 0.05 | |||
| HbA1c (%) | 6.25 ± 0.40 | 6.74 ± 0.49 | Time | <0.01 ** | 49.76 | 0.34 |
| Group × Time | <0.01 ** | 17.30 | 0.15 | |||
| Group | <0.01 ** | 30.54 | 0.24 | |||
| Body composition | ||||||
| BMI (kg/m2) | 27.53 ± 2.88 | 26.69 ± 2.72 | Time | <0.01 ** | 0.48 | 0.00 |
| Group × Time | <0.01 ** | 0.48 | 0.00 | |||
| Group | 0.20 | 1.62 | 0.01 | |||
| WC (cm) | 90.71 ± 4.53 | 96.60 ± 2.56 | Time | <0.01 ** | 68.41 | 0.42 |
| Group × Time | <0.01 ** | 63.01 | 0.40 | |||
| Group | <0.01 ** | 34.32 | 0.26 | |||
| HC (cm) | 96.00 ± 7.11 | 102.42 ± 3.54 | Time | <0.01 ** | 51.92 | 0.35 |
| Group × Time | <0.01 ** | 29.53 | 0.23 | |||
| Group | <0.01 ** | 22.64 | 0.19 | |||
| WHR | 0.93 ± 0.03 | 0.93 ± 0.02 | Time | <0.01 ** | 2.64 | 0.02 |
| Group × Time | <0.01 ** | 3.63 | 0.03 | |||
| Group | 0.75 | 0.09 | 0.001 | |||
| PBF | 28.34 ± 3.90 | 34.00 ± 1.30 | Time | <0.01 ** | 113.91 | 0.54 |
| Group × Time | <0.01 ** | 88.18 | 0.48 | |||
| Group | <0.01 ** | 87.45 | 0.48 | |||
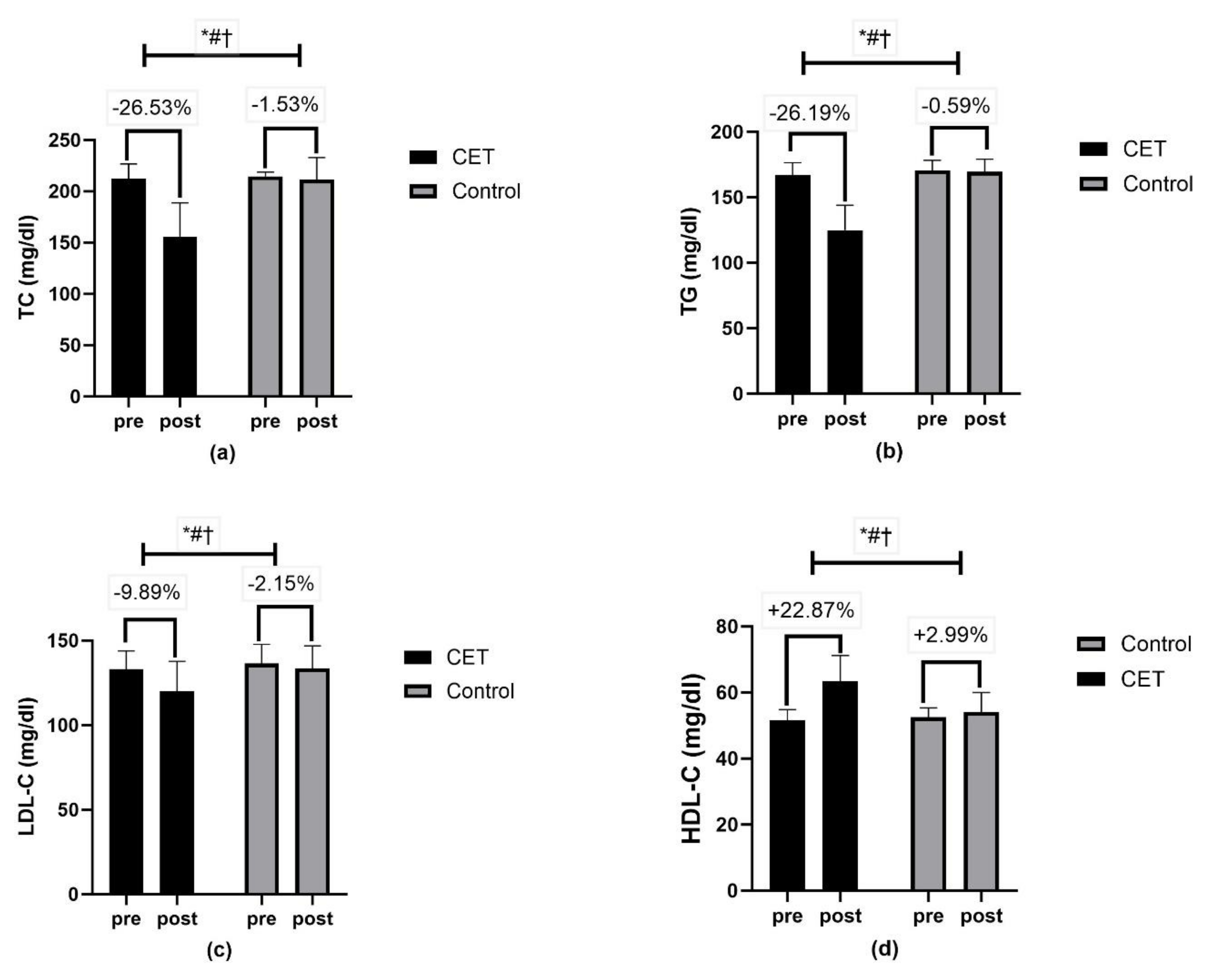
| Lipid profile | ||||||
| TC (mg/dL) | 155.90 ± 32.93 | 211.40 ± 21.70 | Time | <0.01 ** | 114.19 | 0.54 |
| Group × Time | <0.01 ** | 89.81 | 0.48 | |||
| Group | <0.01 ** | 65.62 | 0.41 | |||
| TG (mg/dL) | 124.85 ± 19.03 | 169.42 ± 9.74 | Time | <0.01 ** | 188.61 | 0.66 |
| Group × Time | <0.01 ** | 171.48 | 0.64 | |||
| Group | <0.01 ** | 148.32 | 0.61 | |||
| LDL-C (mg/dL) | 119.96 ± 17.88 | 133.63 ± 13.35 | Time | <0.01 ** | 29.37 | 0.23 |
| Group × Time | <0.01 ** | 11.85 | 0.11 | |||
| Group | <0.01 ** | 13.28 | 0.12 | |||
| HDL-C (mg/dL) | 63.46 ± 7.78 | 54.15 ± 5.87 | Time | <0.01 ** | 96.98 | 0.50 |
| Group × Time | <0.01 ** | 56.96 | 0.37 | |||
| Group | <0.01 ** | 24.43 | 0.20 | |||
| Cardiorespiratory fitness | ||||||
| VO2max (mL/kg/min) | 40.04 ± 10.27 | 24.01 ± 3.76 | Time | <0.01 ** | 69.43 | 0.42 |
| Group × Time | <0.01 ** | 84.80 | 0.47 | |||
| Group | <0.01 ** | 62.78 | 0.40 | |||
| Diabetes Dependent QoL (ADDQoL-19) | ||||||
| ADDQoL-19-GQoL | 2.25 ± 0.75 | 1.19 ± 1.84 | Time | <0.01 ** | 15.78 | 0.14 |
| Group × Time | <0.01 ** | 15.78 | 0.14 | |||
| Group | <0.01 ** | 3.45 | 0.03 | |||
| ADDQoL-19-DDQoL | 0.58 ± 0.49 | −1.50 ± 0.74 | Time | <0.01 ** | 225.00 | 0.70 |
| Group × Time | <0.01 ** | 289.00 | 0.75 | |||
| Group | <0.01 ** | 74.68 | 0.44 | |||
| ADDQoL-19-AWIS | 1.98 ± 1.15 | −0.25 ± 2.32 | Time | <0.01 ** | 18.72 | 0.16 |
| Group × Time | <0.01 ** | 10.48 | 0.10 | |||
| Group | <0.01 ** | 11.51 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaki, S.; Alam, M.F.; Sharma, S.; El-Ashker, S.; Ahsan, M.; Nuhmani, S. Impact of Concurrent Exercise Training on Cardiac Autonomic Modulation, Metabolic Profile, Body Composition, Cardiorespiratory Fitness, and Quality of Life in Type 2 Diabetes with Cardiac Autonomic Neuropathy: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 3910. https://doi.org/10.3390/jcm13133910
Zaki S, Alam MF, Sharma S, El-Ashker S, Ahsan M, Nuhmani S. Impact of Concurrent Exercise Training on Cardiac Autonomic Modulation, Metabolic Profile, Body Composition, Cardiorespiratory Fitness, and Quality of Life in Type 2 Diabetes with Cardiac Autonomic Neuropathy: A Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(13):3910. https://doi.org/10.3390/jcm13133910
Chicago/Turabian StyleZaki, Saima, Md Farhan Alam, Saurabh Sharma, Said El-Ashker, Mohammad Ahsan, and Shibili Nuhmani. 2024. "Impact of Concurrent Exercise Training on Cardiac Autonomic Modulation, Metabolic Profile, Body Composition, Cardiorespiratory Fitness, and Quality of Life in Type 2 Diabetes with Cardiac Autonomic Neuropathy: A Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 13: 3910. https://doi.org/10.3390/jcm13133910
APA StyleZaki, S., Alam, M. F., Sharma, S., El-Ashker, S., Ahsan, M., & Nuhmani, S. (2024). Impact of Concurrent Exercise Training on Cardiac Autonomic Modulation, Metabolic Profile, Body Composition, Cardiorespiratory Fitness, and Quality of Life in Type 2 Diabetes with Cardiac Autonomic Neuropathy: A Randomized Controlled Trial. Journal of Clinical Medicine, 13(13), 3910. https://doi.org/10.3390/jcm13133910







