Asymptomatic Chronic Large Pericardial Effusions: To Drain or to Observe?
Abstract
1. Introduction
2. Outlines of Pericardial Anatomy and Physiology
3. Epidemiology and Causes
4. Classification Diagnosis and Symptoms
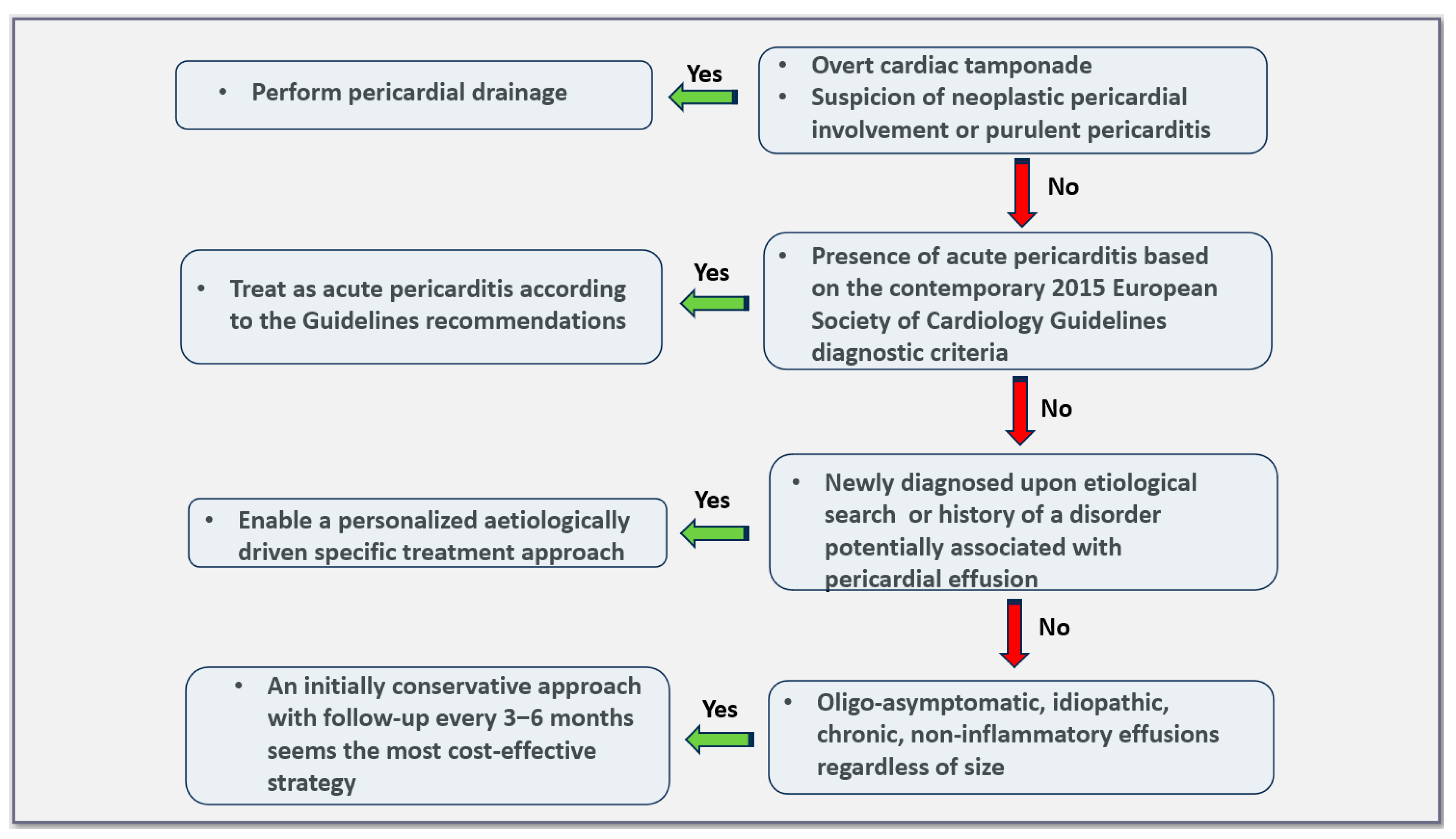
5. Management of Pericardial Effusions
6. Asymptomatic, Large, Idiopathic, Non-Inflammatory Effusions in Light of Current Evidence
7. Prognosis
8. Specific Considerations—Gaps in Knowledge
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luk, A.; Clarke, B.; Dahdah, N.; Ducharme, A.; Krahn, A.; McCrindle, B.; Mizzi, T.; Naus, M.; Udell, J.A.; Virani, S.; et al. Myocarditis and Pericarditis after COVID-19 mRNA Vaccination: Practical Considerations for Care Providers. Can. J. Cardiol. 2021, 37, 1629–1634. [Google Scholar] [CrossRef]
- Alami, A.; Villeneuve, P.J.; Farrell, P.J.; Mattison, D.; Farhat, N.; Haddad, N.; Wilson, K.; Gravel, C.A.; Crispo, J.A.G.; Perez-Lloret, S.; et al. Myocarditis and Pericarditis Post-mRNA COVID-19 Vaccination: Insights from a Pharmacovigilance Perspective. J. Clin. Med. 2023, 12, 4971. [Google Scholar] [CrossRef]
- Montag, K.; Kampf, G. Hospitalised Myocarditis and Pericarditis Cases in Germany Indicate a Higher Post-Vaccination Risk for Young People Mainly after COVID-19 Vaccination. J. Clin. Med. 2022, 11, 6073. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef]
- Lazaros, G.; Imazio, M.; Tsioufis, P.; Lazarou, E.; Vlachopoulos, C.; Tsioufis, C. Chronic Pericardial Effusion: Causes and Management. Can. J. Cardiol. 2023, 39, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef] [PubMed]
- Sagristà-Sauleda, J.; Angel, J.; Permanyer-Miralda, G.; Soler-Soler, J. Long-term follow-up of idiopathic chronic pericardial effusion. N. Engl. J. Med. 1999, 341, 2054–2059. [Google Scholar] [CrossRef]
- Imazio, M.; Lazaros, G.; Valenti, A.; De Carlini, C.C.; Maggiolini, S.; Pivetta, E.; Giustetto, C.; Tousoulis, D.; Adler, Y.; Rinaldi, M.; et al. Outcomes of idiopathic chronic large pericardial effusion. Heart Br. Card. Soc. 2019, 105, 477–481. [Google Scholar] [CrossRef]
- Lazaros, G.; Antonopoulos, A.S.; Lazarou, E.; Vlachopoulos, C.; Foukarakis, E.; Androulakis, A.; Manginas, A.; Theodoros, K.; Karavidas, A.; Tousoulis, D. Long-Term Outcome of Pericardial Drainage in Cases of Chronic, Large, Hemodynamically Insignificant, C-Reactive Protein Negative, Idiopathic Pericardial Effusions. Am. J. Cardiol. 2020, 126, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Tamanini, S.; Bizzi, E.; Maestroni, S.; Cumetti, D.; Novembre, M.L.; Lauri, G.; Agalbato, C.; Cia, A.D.; Paolisso, P.; et al. Post-cardiac injury syndrome and pericardial effusion recurrence after pericardial effusion drainage in chronic idiopathic pericardial effusion. Eur. J. Intern. Med. 2024, 123, 132–137. [Google Scholar] [CrossRef]
- Peebles, C.R.; Shambrook, J.S.; Harden, S.P. Pericardial disease—Anatomy and function. Br. J. Radiol. 2011, 84, S324–S337. [Google Scholar] [CrossRef]
- Vogiatzidis, K.; Zarogiannis, S.G.; Aidonidis, I.; Solenov, E.I.; Molyvdas, P.-A.; Gourgoulianis, K.I.; Hatzoglou, C. Physiology of pericardial fluid production and drainage. Front. Physiol. 2015, 6. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2015.00062 (accessed on 10 October 2022). [CrossRef]
- Khayata, M.; Alkharabsheh, S.; Shah, N.P.; Verma, B.R.; Gentry, J.L.; Summers, M.; Xu, B.; Asher, C.; Klein, A.L. Case series, contemporary review and imaging guided diagnostic and management approach of congenital pericardial defects. Open Heart 2020, 7, e001103. [Google Scholar] [CrossRef]
- Lopez, D.; Asher, C.R. Congenital Absence of the Pericardium. Prog. Cardiovasc. Dis. 2017, 59, 398–406. [Google Scholar] [CrossRef]
- Adler, Y.; Ristić, A.D.; Imazio, M.; Brucato, A.; Pankuweit, S.; Burazor, I.; Seferović, P.M.; Oh, J.K. Cardiac tamponade. Nat. Rev. Dis. Primer 2023, 9, 36. [Google Scholar] [CrossRef]
- Spodick, D.H. Acute cardiac tamponade. N. Engl. J. Med. 2003, 349, 684–690. [Google Scholar] [CrossRef]
- Mitiku, T.Y.; Heidenreich, P.A. A small pericardial effusion is a marker of increased mortality. Am. Heart J. 2011, 161, 152–157. [Google Scholar] [CrossRef]
- Imazio, M.; Spodick, D.H.; Brucato, A.; Trinchero, R.; Adler, Y. Controversial issues in the management of pericardial diseases. Circulation 2010, 121, 916–928. [Google Scholar] [CrossRef]
- Mayosi, B.M.; Wiysonge, C.S.; Ntsekhe, M.; Gumedze, F.; Volmink, J.A.; Maartens, G.; Aje, A.; Thomas, B.M.; Thomas, K.M.; Awotedu, A.A.; et al. Mortality in patients treated for tuberculous pericarditis in sub-Saharan Africa. S. Afr. Med. J. Suid Afr. Tydskr. Vir Geneeskd. 2008, 98, 36–40. [Google Scholar]
- Furqan, M.M.; Chetrit, M.; Cremer, P.; Klein, A.L. Abstract 15826: Misclassification of Pericardial Disease Diagnosis; International Classification of Diseases-10 Coding Requires Validation. Circulation 2019, 140, A15826. [Google Scholar]
- Cosyns, B.; Plein, S.; Nihoyanopoulos, P.; Smiseth, O.; Achenbach, S.; Andrade, M.J.; Pepi, M.; Ristic, A.; Imazio, M.; Paelinck, B.; et al. European Association of Cardiovascular Imaging (EACVI) position paper: Multimodality imaging in pericardial disease. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 12–31. [Google Scholar] [CrossRef]
- Chetrit, M.; Xu, B.; Kwon, D.H.; Ramchand, J.; Rodriguez, R.E.; Tan, C.D.; Jellis, C.L.; Johnston, D.R.; Renapurkar, R.D.; Cremer, P.C.; et al. Imaging-Guided Therapies for Pericardial Diseases. JACC Cardiovasc. Imaging 2020, 13, 1422–1437. [Google Scholar] [CrossRef]
- Klein, A.L.; Abbara, S.; Agler, D.A.; Appleton, C.P.; Asher, C.R.; Hoit, B.; Hung, J.; Garcia, M.J.; Kronzon, I.; Oh, J.K.; et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: Endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2013, 26, 965–1012.e15. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Vrettos, A.; Androulakis, E.; Kamperou, C.; Vlachopoulos, C.; Tsioufis, K.; Mohiaddin, R.; Lazaros, G. Cardiac magnetic resonance imaging of pericardial diseases: A comprehensive guide. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 983–998. [Google Scholar] [CrossRef]
- Little, W.C.; Freeman, G.L. Pericardial Disease. Circulation 2006, 113, 1622–1632. [Google Scholar] [CrossRef]
- LeWinter, M.M. Clinical practice. Acute pericarditis. N. Engl. J. Med. 2014, 371, 2410–2416. [Google Scholar] [CrossRef]
- Pennacchioni, A.; Nanni, G.; Sgura, F.A.; Imberti, J.F.; Monopoli, D.E.; Rossi, R.; Longo, G.; Arrotti, S.; Vitolo, M.; Boriani, G. Percutaneous pericardiocentesis for pericardial effusion: Predictors of mortality and outcomes. Intern. Emerg. Med. 2021, 16, 1771–1777. [Google Scholar] [CrossRef]
- Mayosi, B.M. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-Saharan Africa. Heart Br. Card. Soc. 2007, 93, 1176–1183. [Google Scholar] [CrossRef]
- Mercé, J.; Sagristà-Sauleda, J.; Permanyer-Miralda, G.; Soler-Soler, J. Should pericardial drainage be performed routinely in patients who have a large pericardial effusion without tamponade? Am. J. Med. 1998, 105, 106–109. [Google Scholar] [CrossRef]
- Kermani-Alghoraishi, M.; Pouramini, A.; Kafi, F.; Khosravi, A. Coronavirus Disease 2019 (COVID-19) and Severe Pericardial Effusion: From Pathogenesis to Management: A Case Report Based Systematic Review. Curr. Probl. Cardiol. 2022, 47, 100933. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Hatziantoniou, S.; Vlachopoulos, C.; Spanakis, N.; Tsioufis, C.; Tsakris, A.; Lazaros, G. Temporal relationship of myocarditis and pericarditis following COVID-19 vaccination: A pragmatic approach. Int. J. Cardiol. 2022, 358, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, S.A.; Halvorsen, S.; Sinnaeve, P.R.; Sambola, A.; Gulati, G.; Lancellotti, P.; Van Der Meer, P.; Lyon, A.R.; Farmakis, D.; Lee, G.; et al. Evaluation and management of cancer patients presenting with acute cardiovascular disease: A Consensus Document of the Acute CardioVascular Care (ACVC) association and the ESC council of Cardio-Oncology—Part 1: Acute coronary syndromes and acute pericardial diseases. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 947–959. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Adler, Y. Management of pericardial effusion. Eur. Heart J. 2013, 34, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Pericardial Effusion and Cardiac Tamponade in the New Millennium. Curr. Cardiol. Rep. 2017, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, J.G.; Bonaventura, A.; Vecchié, A.; Wohlford, G.F.; Mauro, A.G.; Jordan, J.H.; Grizzard, J.D.; Montecucco, F.; Berrocal, D.H.; Brucato, A.; et al. Management of Acute and Recurrent Pericarditis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 76–92. [Google Scholar] [CrossRef]
- Buoro, S.; Tombetti, E.; Ceriotti, F.; Simon, C.; Cugola, D.; Seghezzi, M.; Innocente, F.; Maestroni, S.; Del Carmen Baigorria Vaca, M.; Moioli, V.; et al. What is the normal composition of pericardial fluid? Heart Br. Card. Soc. 2021, 107, 1584–1590. [Google Scholar] [CrossRef]
- Lazaros, G.; Oikonomou, V.; Oikonomou, E.; Aznaouridis, K.; Vlachopoulos, C.; Vogiatzi, G.; Lazarou, E.; Imazio, M.; Brucato, A.; Adler, Y.; et al. Recurrence of Pericardial Effusion after Pericardiocentesis: Does Catheter-Induced Acute Pericardial Inflammation Play a Role? Am. J. Med. Sci. 2021, 361, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Verma, B.R.; Brooksbank, J.; Khayata, M.; Klein, A.L. Symptomatic Recurrent Chylopericardium: How to Manage This Rare Entity? JACC Case Rep. 2021, 3, 1318–1321. [Google Scholar] [CrossRef]
- Ariyarajah, V.; Spodick, D.H. Cardiac tamponade revisited: A postmortem look at a cautionary case. Tex. Heart Inst. J. 2007, 34, 347–351. [Google Scholar]
- Imazio, M.; Brucato, A.; Maestroni, S.; Cumetti, D.; Belli, R.; Trinchero, R.; Adler, Y. Risk of constrictive pericarditis after acute pericarditis. Circulation 2011, 124, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Il’Giovine, Z.J.; Gage, A.; Higgins, A. Cardiac Tamponade and Pericardiocentesis: Recognition, Standard Techniques, and Modern Advancements. Cardiol. Clin. 2024, 42, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.H.; Espinosa, R.E.; Nishimura, R.A.; Sinak, L.J.; Hayes, S.N.; Melduni, R.M.; Oh, J.K. Pericardial disease: Diagnosis and management. Mayo Clin. Proc. 2010, 85, 572–593. [Google Scholar] [CrossRef] [PubMed]
- Ristić, A.D.; Imazio, M.; Adler, Y.; Anastasakis, A.; Badano, L.P.; Brucato, A.; Caforio, A.L.P.; Dubourg, O.; Elliott, P.; Gimeno, J.; et al. Triage strategy for urgent management of cardiac tamponade: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2014, 35, 2279–2284. [Google Scholar] [CrossRef]
- Adi, O.; Fong, C.P.; Ahmad, A.H.; Azil, A.; Ranga, A.; Panebianco, N. Pericardial decompression syndrome: A complication of pericardiocentesis. Am. J. Emerg. Med. 2021, 45, e3–e688. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, Y.; Goyal, A.; Khalid, N.; Sharma, N.; Nayyar, R.; Spodick, D.H.; Chhabra, L. Pericardial decompression syndrome: A comprehensive review. World J. Cardiol. 2019, 11, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Rafique, A.M.; Patel, N.; Biner, S.; Eshaghian, S.; Mendoza, F.; Cercek, B.; Siegel, R.J. Frequency of recurrence of pericardial tamponade in patients with extended versus nonextended pericardial catheter drainage. Am. J. Cardiol. 2011, 108, 1820–1825. [Google Scholar] [CrossRef]
- El Haddad, D.; Iliescu, C.; Yusuf, S.W.; William, W.N.; Khair, T.H.; Song, J.; Mouhayar, E.N. Outcomes of Cancer Patients Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion. J. Am. Coll. Cardiol. 2015, 66, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Strobbe, A.; Adriaenssens, T.; Bennett, J.; Dubois, C.; Desmet, W.; McCutcheon, K.; Van Cleemput, J.; Sinnaeve, P.R. Etiology and Long-Term Outcome of Patients Undergoing Pericardiocentesis. J. Am. Heart Assoc. 2017, 6, e007598. [Google Scholar] [CrossRef]
- Atar, S.; Chiu, J.; Forrester, J.S.; Siegel, R.J. Bloody pericardial effusion in patients with cardiac tamponade: Is the cause cancerous, tuberculous, or iatrogenic in the 1990s? Chest 1999, 116, 1564–1569. [Google Scholar] [CrossRef]
- Chahine, J.; Shekhar, S.; Mahalwar, G.; Imazio, M.; Collier, P.; Klein, A. Pericardial Involvement in Cancer. Am. J. Cardiol. 2021, 145, 151–159. [Google Scholar] [CrossRef]
- Saab, J.; Hoda, R.S.; Narula, N.; Hoda, S.A.; Geraghty, B.E.; Nasar, A.; Alperstein, S.A.; Port, J.L.; Giorgadze, T. Diagnostic yield of cytopathology in evaluating pericardial effusions: Clinicopathologic analysis of 419 specimens. Cancer Cytopathol. 2017, 125, 128–137. [Google Scholar] [CrossRef]
- Wei, W.; Nie, H. Severe purulent pericarditis caused by invasive Eikenella corrodens: Case report and literature review. BMC Infect. Dis. 2019, 19, 657. [Google Scholar] [CrossRef]
- Sagristà-Sauleda, J.; Mercé, J.; Permanyer-Miralda, G.; Soler-Soler, J. Clinical clues to the causes of large pericardial effusions. Am. J. Med. 2000, 109, 95–101. [Google Scholar] [CrossRef]
- Das, N.; Feingold, B. Pericardial Decompression Syndrome. N. Engl. J. Med. 2023, 389, e54. [Google Scholar] [CrossRef]
- Altman, E.; Rutsky, O.; Shturman, A.; Yampolsky, Y.; Atar, S. Anterior parasternal approach for creation of a pericardial window. Ann. R. Coll. Surg. Engl. 2015, 97, 375. [Google Scholar] [CrossRef]
- Imazio, M.; Colopi, M.; De Ferrari, G.M. Pericardial diseases in patients with cancer: Contemporary prevalence, management and outcomes. Heart Br. Card. Soc. 2020, 106, 569–574. [Google Scholar] [CrossRef]
- De Filippo, O.; Gatti, P.; Rettegno, S.; Iannaccone, M.; D’Ascenzo, F.; Lazaros, G.; Brucato, A.; Tousoulis, D.; Adler, Y.; Imazio, M. Is pericardial effusion a negative prognostic marker? Meta-analysis of outcomes of pericardial effusion. J. Cardiovasc. Med. Hagerstown Md 2019, 20, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.M.; Barnes, M.E.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Outcomes of clinically significant idiopathic pericardial effusion requiring intervention. Am. J. Cardiol. 2003, 91, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Serati, L.; Mardigyan, V.; Dominioni, C.C.; Agozzino, F.; Bizzi, E.; Trotta, L.; Nivuori, M.; Maestroni, S.; Negro, E.; Imazio, M.; et al. Pericardial Diseases in Pregnancy. Can. J. Cardiol. 2023, 39, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Rammos, A.; Papaioannou, E.; Lazaros, G.; Siminelakis, S.; Naka, K.K. Large pericardial effusion in a woman in the second trimester of pregnancy: A case report. Eur. Heart J. Case Rep. 2024, 8, ytae080. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Conte, E.; Agalbato, C.; Lauri, G.; Mushtaq, S.; Carollo, C.; Bonomi, A.; Zanotto, L.; Melotti, E.; Dalla Cia, A.; Guglielmo, M.; et al. Prevalence and prognosis of pericardial effusion in patients affected by pectus excavatum: A case-control study. Int. J. Cardiol. 2021, 344, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M. Pericardial involvement in systemic inflammatory diseases. Heart Br. Card. Soc. 2011, 97, 1882–1892. [Google Scholar] [CrossRef]
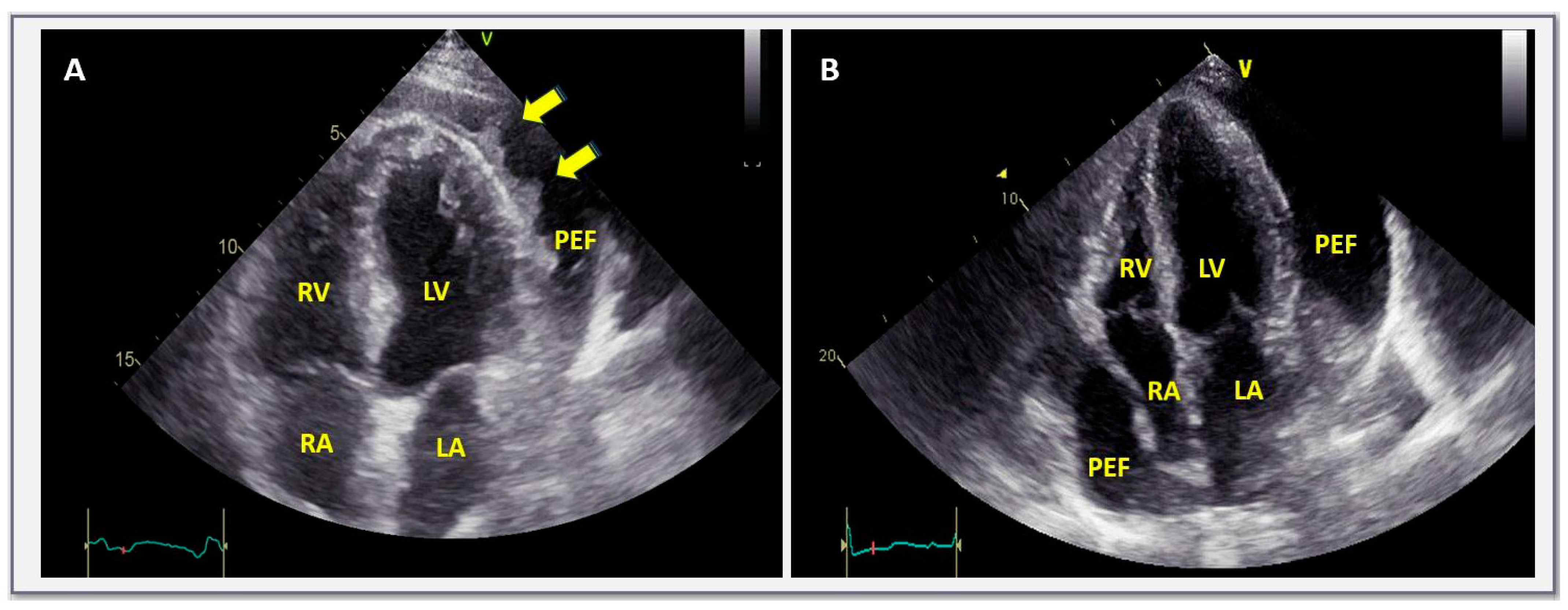
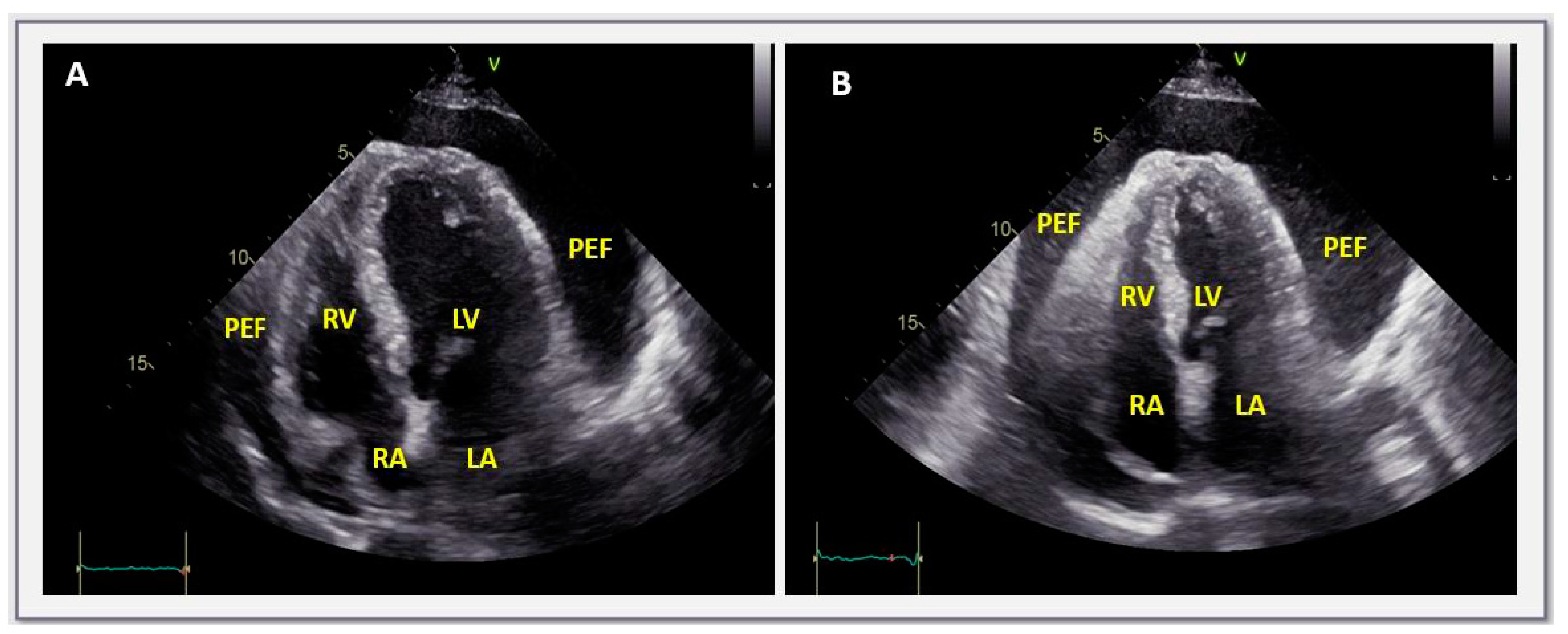
| Size: | Small: <10 mm |
| Moderate: Between 10 and 20 mm | |
| Large: >10 mm | |
| Onset: | Acute: <1 week |
| Subacute: Between 1 week and 3 months | |
| Chronic: >3 months | |
| Distribution: | Circumferential |
| Localized | |
| Composition: | Transudate |
| Exudate inflammatory effusions, hemopericardium, pyopericardium, chylopericardium, pneumopericardium | |
| Hemodynamic effects: | Hemodinamically insignificant |
| Cardiac tamponade | |
| Effusive-constrictive |
| Variable | Specificity | Sensitivity |
|---|---|---|
| Right atrial collapse (inversion) with duration of atrial collapse to cardiac cycle duration >0.34 | 100% | >90% |
| Right ventricular collapse | 72–100% | 48–100% |
| Inferior vena cava enlargement (>20 mm) with blunted respiratory response (<50% with inspiration) | 40% | 97% |
| Swinging heart | N.A. | N.A. |
| Respiratory variation of 25% or more in transmitral early diastolic filling (E) velocity | N.A. | N.A. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarou, E.; Vlachopoulos, C.; Antonopoulos, A.; Imazio, M.; Brucato, A.; Tsioufis, C.; Lazaros, G. Asymptomatic Chronic Large Pericardial Effusions: To Drain or to Observe? J. Clin. Med. 2024, 13, 3887. https://doi.org/10.3390/jcm13133887
Lazarou E, Vlachopoulos C, Antonopoulos A, Imazio M, Brucato A, Tsioufis C, Lazaros G. Asymptomatic Chronic Large Pericardial Effusions: To Drain or to Observe? Journal of Clinical Medicine. 2024; 13(13):3887. https://doi.org/10.3390/jcm13133887
Chicago/Turabian StyleLazarou, Emilia, Charalambos Vlachopoulos, Alexios Antonopoulos, Massimo Imazio, Antonio Brucato, Costas Tsioufis, and George Lazaros. 2024. "Asymptomatic Chronic Large Pericardial Effusions: To Drain or to Observe?" Journal of Clinical Medicine 13, no. 13: 3887. https://doi.org/10.3390/jcm13133887
APA StyleLazarou, E., Vlachopoulos, C., Antonopoulos, A., Imazio, M., Brucato, A., Tsioufis, C., & Lazaros, G. (2024). Asymptomatic Chronic Large Pericardial Effusions: To Drain or to Observe? Journal of Clinical Medicine, 13(13), 3887. https://doi.org/10.3390/jcm13133887








