Evaluating Factor VIII Concentrates Using Clot Waveform Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. APTT Reagents
2.2. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mannucci, P.M.; Franchini, M. Haematology clinic: Haemophilia A. Hematology 2014, 19, 181–182. [Google Scholar] [CrossRef]
- Mannucci, P.M. Hemophilia treatment innovation: 50 years of progress and more to come. J. Thromb. Haemost. 2023, 21, 403–412. [Google Scholar] [CrossRef]
- Hay, C.R.M.; Nissen, F.; Pipe, S.W. Mortality in congenital hemophilia A—A systematic literature review. J. Thromb. Haemost. 2021, 19 (Suppl. S1), 6–20. [Google Scholar] [CrossRef]
- National Hemophilia Foundation. MASAC Recommendation Concerning Prophylaxis for Hemophilia A and B with and without Inhibitors. Available at Hemophilia A and B Prophylaxis Recommendations|NBDF. Available online: https://www.bleeding.org/healthcare-professionals/guidelines-on-care/masac-documents/masac-document-267-masac-recommendation-concerning-prophylaxis-for-hemophilia-a-and-b-with-and-without-inhibitors?contentid=1007&menuid=57 (accessed on 28 March 2024).
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007, 357, 535–544. [Google Scholar] [CrossRef]
- Oldenburg, J. Optimal treatment strategies for hemophilia: Achievements and limitations of current prophylactic regimens. Blood 2015, 125, 2038–2044. [Google Scholar] [CrossRef]
- Dolan, G.; Benson, G.; Bowyer, A.; Eichler, H.; Hermans, C.; Jiménez-Yuste, V.; Ljung, R.; Pollard, D.; Santagostino, E.; Šalek, S.Z. Principles of care for acquired hemophilia. Eur. J. Haematol. 2021, 106, 762–773. [Google Scholar] [CrossRef]
- Malec, L.; Matino, D. Targeting higher factor VIII levels for prophylaxis in haemophilia A: A narrative review. Haemophilia 2023, 29, 1419–1429. [Google Scholar] [CrossRef]
- Oldenburg, J.; Mahlangu, J.N.; Kim, B.; Schmitt, C.; Callaghan, M.U.; Young, G.; Santagostino, E.; Kruse-Jarres, R.; Negrier, C.; Kessler, C.; et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. N. Engl. J. Med. 2017, 377, 809–818. [Google Scholar] [CrossRef]
- Mahlangu, J.; Oldenburg, J.; Paz-Priel, I.; Negrier, C.; Niggli, M.; Mancuso, M.E.; Schmitt, C.; Jiménez-Yuste, V.; Kempton, C.; Dhalluin, C.; et al. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. N. Engl. J. Med. 2018, 379, 811–822. [Google Scholar] [CrossRef]
- Matsushita, T.; Shapiro, A.; Abraham, A.; Angchaisuksiri, P.; Castaman, G.; Cepo, K.; d’Oiron, R.; Frei-Jones, M.; Goh, A.S.; Haaning, J.; et al. Phase 3 Trial of Concizumab in Hemophilia with Inhibitors. N. Engl. J. Med. 2023, 389, 783–794. [Google Scholar] [CrossRef]
- Keam, S.J. Concizumab: First Approval. Drugs 2023, 83, 1053–1059. [Google Scholar] [CrossRef]
- Leebeek, F.W.G.; Miesbach, W. Gene therapy for hemophilia: A review on clinical benefit, limitations, and remaining issues. Blood 2021, 138, 923–931. [Google Scholar] [CrossRef]
- Baas, L.; van der Graaf, R.; van Hoorn, E.S.; Bredenoord, A.L.; Meijer, K.; SYMPHONY Consortium. The ethics of gene therapy for hemophilia: A narrative review. J. Thromb. Haemost. 2023, 21, 413–420. [Google Scholar] [CrossRef]
- Hermans, C.; Lambert, C. Therapeutic innovations for hemophilia. Rev. Med. Suisse 2020, 16, 47–52. [Google Scholar]
- von Drygalski, A.; Chowdary, P.; Kulkarni, R.; Susen, S.; Konkle, B.A.; Oldenburg, J.; Matino, D.; Klamroth, R.; Weyand, A.C.; Jimenez-Yuste, V.; et al. Efanesoctocog Alfa Prophylaxis for Patients with Severe Hemophilia A. N. Engl. J. Med. 2023, 388, 310–318. [Google Scholar] [CrossRef]
- Dargaud, Y.; Leuci, A.; Ruiz, A.R.; Lacroix-Desmazes, S. Efanesoctocog alfa: The renaissance of Factor VIII replacement therapy. Haematologica 2024. online ahead of print. [Google Scholar]
- Matsumoto, T.; Nogami, K.; Shima, M. A combined approach using global coagulation assays quickly differentiates coagulation disorders with prolonged aPTT and low levels of FVIII activity. Int. J. Hematol. 2017, 105, 174–183. [Google Scholar] [CrossRef]
- Tokutake, T.; Baba, H.; Shimada, Y.; Takeda, W.; Sato, K.; Hiroshima, Y.; Kirihara, T.; Shimizu, I.; Nakazawa, H.; Kobayashi, H.; et al. Exogenous Magnesium Chloride Reduces the Activated Partial Thromboplastin Times of Lupus Anticoagulant-Positive Patients. PLoS ONE 2016, 11, e0157835. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wada, H.; Nishioka, Y.; Nishio, M.; Abe, Y.; Nishioka, J.; Kamikura, Y.; Sase, T.; Kaneko, T.; Houdijk, W.P.; et al. Frequency of Abnormal Biphasic aPTT Clot Waveforms in Patients with Underlying disorders Associated with Disseminated Intravascular Coagulation. Clin. Appl. Thromb./Hemost. 2006, 12, 185–192. [Google Scholar] [CrossRef]
- Byun, J.H.; Jang, I.S.; Kim, J.W.; Koh, E.H. Establishing the heparin therapeutic range using aPTT and anti-Xa measurements for monitoring unfractionated heparin therapy. Blood Res. 2016, 51, 171–174. [Google Scholar] [CrossRef]
- Bertaggia Calderara, D.; Marchi Cappelletti, R.; Batista Mesquita Sauvage, A.P.; Durual, S.; Gomez, F.J.; Zermatten, M.G.; Aliotta, A.; Casini, A.; Alberio, L. Pharmacodynamics Monitoring of Emicizumab in Patients with Hemophilia A. Thromb Haemost. 2023, 123, 955–965. [Google Scholar] [CrossRef]
- Bakajima, Y.; Mizumachi, K.; Shimonishi, N.; Furukawa, S.; Yada, K.; Ogiwara, K.; Takeyama, M.; Shima, M.; Nogami, K. Comparisons of global coagulation potential and bleeding episodes in emicizumab-treated hemophilia A patients and mild hemophilia A patients. Int. J. Hematol. 2022, 115, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.M.; Ramsey, P.; Evans, V.; Tang, L.; Apeler, H.; Leong, L.; Murphy, J.E.; Laux, V.; Myles, T. Evaluation of the activated partial thromboplastin time assay for clinical monitoring of PEGylated recombinant factor VIII (BAY 94-9027) for haemophilia A. Haemophilia 2014, 20, 593–600. [Google Scholar] [CrossRef]
- Wada, H.; Shiraki, K.; Matsumoto, T.; Ohishi, K.; Shimpo, H.; Sakano, Y.; Nishii, H.; Shimaoka, M. The Evaluation of APTT Reagents in Reference Plasma, Recombinant FVIII Products; Kovaltry and Jivi Using CWA, including sTF/7FIX Assay. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029620976913. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wada, H.; Shiraki, K.; Suzuki, K.; Yamashita, Y.; Tawara, I.; Shimpo, H.; Shimaoka, M. The Evaluation of Clot Waveform Analyses for Assessing Hypercoagulability in Patients Treated with Factor VIII Concentrate. J. Clin. Med. 2023, 12, 6320. [Google Scholar] [CrossRef]
- Toh, C.H.; Samis, J.; Downey, C.; Walker, J.; Becker, L.; Brufatto, N.; Tejidor, L.; Jones, G.; Houdijk, W.; Giles, A.; et al. Biphasic transmittance waveform in the APTT coagulation assay is due to the formation of a Ca(++)-dependent complex of C-reactive protein with very-low-density lipoprotein and is a novel marker of impending disseminated intravascular coagulation. Blood 2002, 100, 2522–2529. [Google Scholar] [CrossRef]
- Shima, M.; Matsumoto, T.; Fukuda, K.; Kubota, Y.; Tanaka, I.; Nishiya, K.; Giles, A.R.; Yoshioka, A. The utility of activated partial thromboplastin time (aPTT) clot waveform analysis in the investigation of hemophilia A patients with very low levels of factor VIII activity (FVIII:C). Thromb. Haemost. 2002, 87, 436–441. [Google Scholar]
- Shima, M. Understanding the hemostatic effects of recombinant factor VIIa by clot wave form analysis. Semin. Hematol. 2004, 41 (Suppl. S1), 125–131. [Google Scholar] [CrossRef]
- Wada, H.; Ichikawa, Y.; Ezaki, M.; Shiraki, K.; Moritani, I.; Yamashita, Y.; Matsumoto, T.; Masuya, M.; Tawara, I.; Shimpo, H.; et al. Clot Waveform Analysis Demonstrates Low Blood Coagulation Ability in Patients with Idiopathic Thrombocytopenic Purpura. J. Clin. Med. 2021, 10, 5987. [Google Scholar] [CrossRef]
- Wada, H.; Ichikawa, Y.; Ezaki, E.; Matsumoto, T.; Yamashita, Y.; Shiraki, K.; Shimaoka, M.; Shimpo, H. The reevaluation of thrombin time using a clot waveform analysis. J. Clin. Med. 2021, 10, 4840. [Google Scholar] [CrossRef]
- Nogami, K.; Soeda, T.; Matsumoto, T.; Kawabe, Y.; Kitazawa, T.; Shima, M. Routine measurements of factor VIII activity and inhibitor titer in the presence of emicizumab utilizing anti-idiotype monoclonal antibodies. J. Thromb. Haemost. 2018, 16, 1383–1390. [Google Scholar] [CrossRef]
- Wada, H.; Shiraki, K.; Matsumoto, T.; Suzuki, K.; Yamashita, Y.; Tawara, I.; Shimpo, H.; Shimaoka, M. A Clot Waveform Analysis of Thrombin Time Using a Small Amount of Thrombin Is Useful for Evaluating the Clotting Activity of Plasma Independent of the Presence of Emicizumab. J. Clin. Med. 2022, 11, 6142. [Google Scholar] [CrossRef]
- Berntorp, E.; Salvagno, G.L. Standardization and clinical utility of thrombin-generation assays. Semin. Thromb. Hemost. 2008, 34, 670–682. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Berntorp, E. Thrombin generation testing for monitoring hemophilia treatment: A clinical perspective. Semin. Thromb. Hemost. 2010, 36, 780–790. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wada, H.; Toyoda, H.; Hirayama, M.; Yamashita, Y.; Katayama, N. Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti-factor IXa/factor X bispecific antibody emicizumab: Comment. Thromb. Haemost. 2018, 16, 1665–1666. [Google Scholar] [CrossRef]
- Wada, H.; Shiraki, K.; Matsumoto, T.; Shimpo, H.; Shimaoka, M. Clot waveform analysis for hemostatic abnormalities. Ann. Lab. Med. 2023, 43, 531–538. [Google Scholar] [CrossRef]
- Hegemann, I.; Koch, K.; Clausen, W.H.O.; Ezban, M.; Brand-Staufer, B. Evaluation of N8-GP Activity Using a One-Stage Clotting Assay: A Single-Center Experience. Acta Haematol. 2020, 143, 504–508. [Google Scholar] [CrossRef]
- Bowyer, A.; Kitchen, S.; Maclean, R. Effects of emicizumab on APTT, one-stage and chromogenic assays of factor VIII in artificially spiked plasma and in samples from haemophilia A patients with inhibitors. Haemophilia 2020, 26, 536–542. [Google Scholar] [CrossRef]
- Jeanpierre, E.; Pouplard, C.; Lasne, D.; Le Cam Duchez, V.; Eschwege, V.; Flaujac, C.; Galinat, H.; Harzallah, I.; Proulle, V.; Smahi, M.; et al. Factor VIII and IX assays for post-infusion monitoring in hemophilia patients: Guidelines from the French BIMHO group (GFHT). Eur. J. Haematol. 2020, 105, 103–115. [Google Scholar] [CrossRef]
- Adcock, D.M.; Strandberg, K.; Shima, M.; Marlar, R.A. Advantages, disadvantages and optimization of one-stage and chromogenic factor activity assays in haemophilia A and B. Int. J. Lab. Hematol. 2018, 40, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, S.; Blakemore, J.; Friedman, K.D.; Hart, D.P.; Ko, R.H.; Perry, D.; Platton, S.; Tan-Castillo, D.; Young, G.; Luddington, R.J. A computer-based model to assess costs associated with the use of factor VIII and factor IX one-stage and chromogenic activity assays. J. Thromb. Haemost. 2016, 14, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, G.; Cerbone, A.M.; Coppola, A.; Cimino, E.; Di Capua, M.; Pamparana, F.; Tufano, A.; Di Minno, M.N. Longer-acting factor VIII to overcome limitations in haemophilia management: The PEGylated liposomes formulation issue. Haemophilia 2010, 16 (Suppl. S1), 2–6. [Google Scholar] [CrossRef]
- Mei, B.; Pan, C.; Jiang, H.; Tjandra, H.; Strauss, J.; Chen, Y.; Liu, T.; Zhang, X.; Severs, J.; Newgren, J.; et al. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood 2010, 116, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, F.; Kenet, G.; Pekrul, I.; Pruthi, R.K.; Ramge, P.; Spannagl, M.J. Laboratory testing in hemophilia: Impact of factor and non-factor replacement therapy on coagulation assays. Thromb. Haemost. 2020, 18, 1242–1255. [Google Scholar] [CrossRef]
- Gray, E.; Kitchen, S.; Bowyer, A.; Chowdary, P.; Jenkins, P.V.; Murphy, P.; Platton, S.; Riddell, A.; Lester, W. Laboratory measurement of factor replacement therapies in the treatment of congenital haemophilia: A United Kingdom Haemophilia Centre Doctors’ Organisation guideline. Haemophilia 2020, 26, 6–16. [Google Scholar] [CrossRef]
- Suzuki, A.; Suzuki, N.; Kanematsu, T.; Okamoto, S.; Tamura, S.; Kikuchi, R.; Katsumi, A.; Kiyoi, H.; Kojima, T.; Matsushita, T. Impact of variation in reagent combinations for one-stage clotting assay on assay discrepancy in nonsevere haemophilia A. Int. J. Lab. Hematol. 2021, 43, 131–138. [Google Scholar] [CrossRef]
- Dodt, J.; Hubbard, A.R.; Wicks, S.J.; Gray, E.; Neugebauer, B.; Charton, E.; Silvester, G. Potency determination of factor VIII and factor IX for new product labelling and postinfusion testing: Challenges for caregivers and regulators. Haemophilia 2015, 21, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Hedner, U. NovoSeven as a universal haemostatic agent. Blood Coagul. Fibrinolysis 2000, 11, S107–S111. [Google Scholar] [CrossRef]
- Hedner, U. FVIIa as therapeutic agent in hemophilia and beyond. Front. Biosci. (Elite Ed) 2012, 4, 1210–1223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wada, H.; Shiraki, K.; Matsumoto, T.; Ohishi, K.; Shimpo, H.; Shimaoka, M. Effects of platelet and phospholipids on clot formation activated by a small amount of tissue factor. Thromb. Res. 2020, 193, 146–153. [Google Scholar] [CrossRef]
- Sekar, R.; Mimoun, A.; Bou-Jaoudeh, M.; Loyau, S.; Delignat, S.; Daventure, V.; Bonilla, P.; Bhale, A.S.; Venkataraman, K.; Rayes, J.; et al. High factor VIII concentrations interfere with glycoprotein VI-mediated platelet activation in vitro. J. Thromb. Haemost. 2024, in press. [CrossRef]
- Savage, S.A.; Zarzaur, B.L.; Pohlman, T.H.; Brewer, B.L.; Magnotti, L.J.; Croce, M.A.; Lim, G.H.; Martin, A.C. Clot dynamics and mortality: The MA-R ratio. J. Trauma Acute Care Surg. 2017, 83, 628–634. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Synovitis in hemophilia: Preventing, detecting, and treating joint bleeds. Expert Rev. Hematol. 2023, 16, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Efanesoctocog Alfa: First Approval. Drugs 2023, 83, 633–638. [Google Scholar] [CrossRef]
- Jenkins, P.V.; Rawley, O.; Smith, O.P.; O’Donnell, J.S. Elevated factor VIII levels and risk of venous thrombosis. Br. J. Haematol. 2012, 157, 653–663. [Google Scholar] [CrossRef]
- Kovac, M.; Kovac, Z.; Tomasevic, Z.; Vucicevic, S.; Djordjevic, V.; Pruner, I.; Radojkovic, D. Factor V Leiden mutation and high FVIII are associated with an increased risk of VTE in women with breast cancer during adjuvant tamoxifen-results from a prospective, single center, case control study. Eur. J. Intern. Med. 2015, 26, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Badescu, M.C.; Ciocoiu, M.; Rezus, E.; Badulescu, O.V.; Tanase, D.M.; Ouatu, A.; Dima, N.; Ganceanu-Rusu, A.R.; Popescu, D.; Seritean Isac, P.N.; et al. Current Therapeutic Approach to Acute Myocardial Infarction in Patients with Congenital Hemophilia. Life 2021, 11, 1072. [Google Scholar] [CrossRef]
- Faghmous, I.; Nissen, F.; Kuebler, P.; Flores, C.; Patel, A.M.; Pipe, S.W. Estimating the risk of thrombotic events in people with congenital hemophilia A using US claims data. J. Comp. Eff. Res. 2021, 10, 1323–1336. [Google Scholar] [CrossRef] [PubMed]


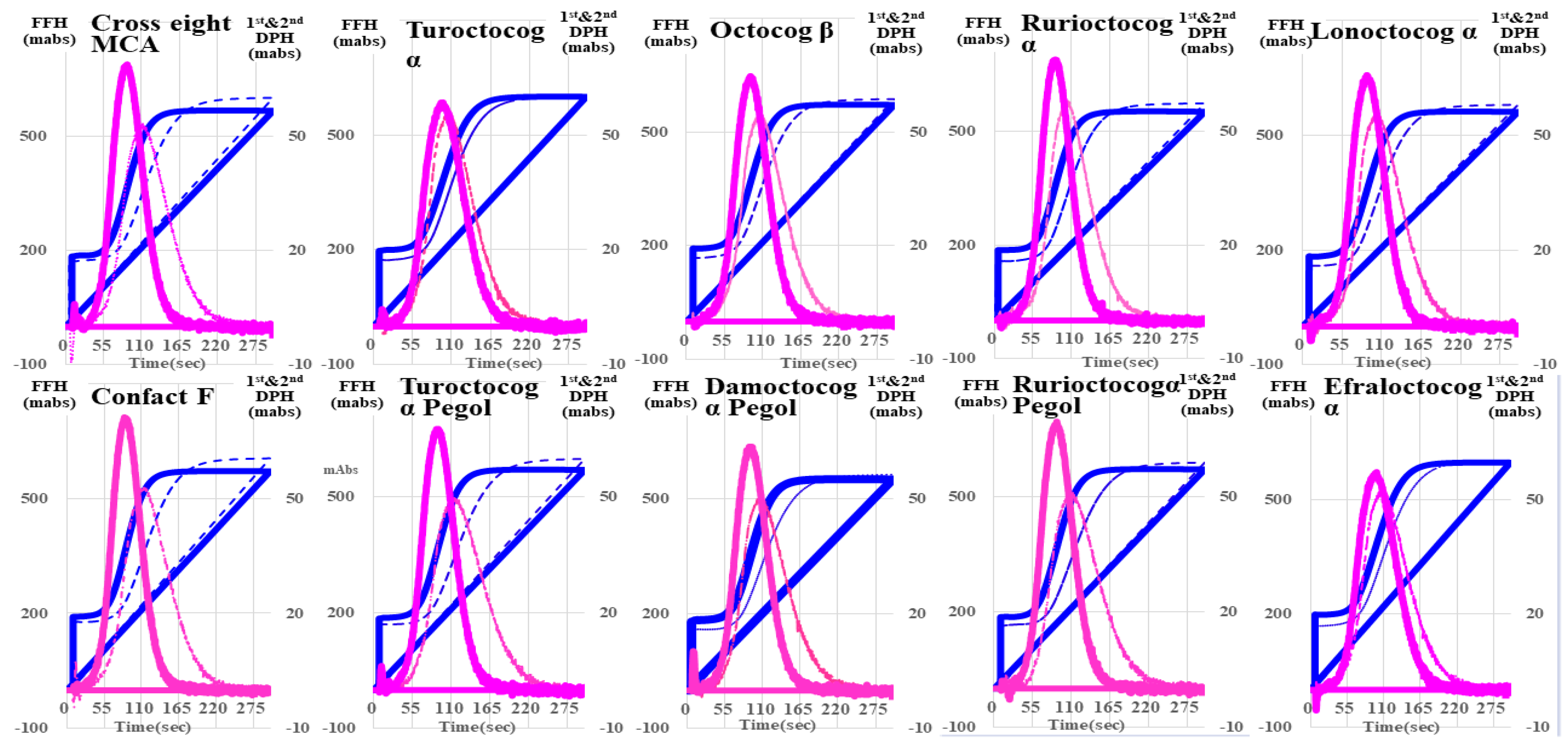
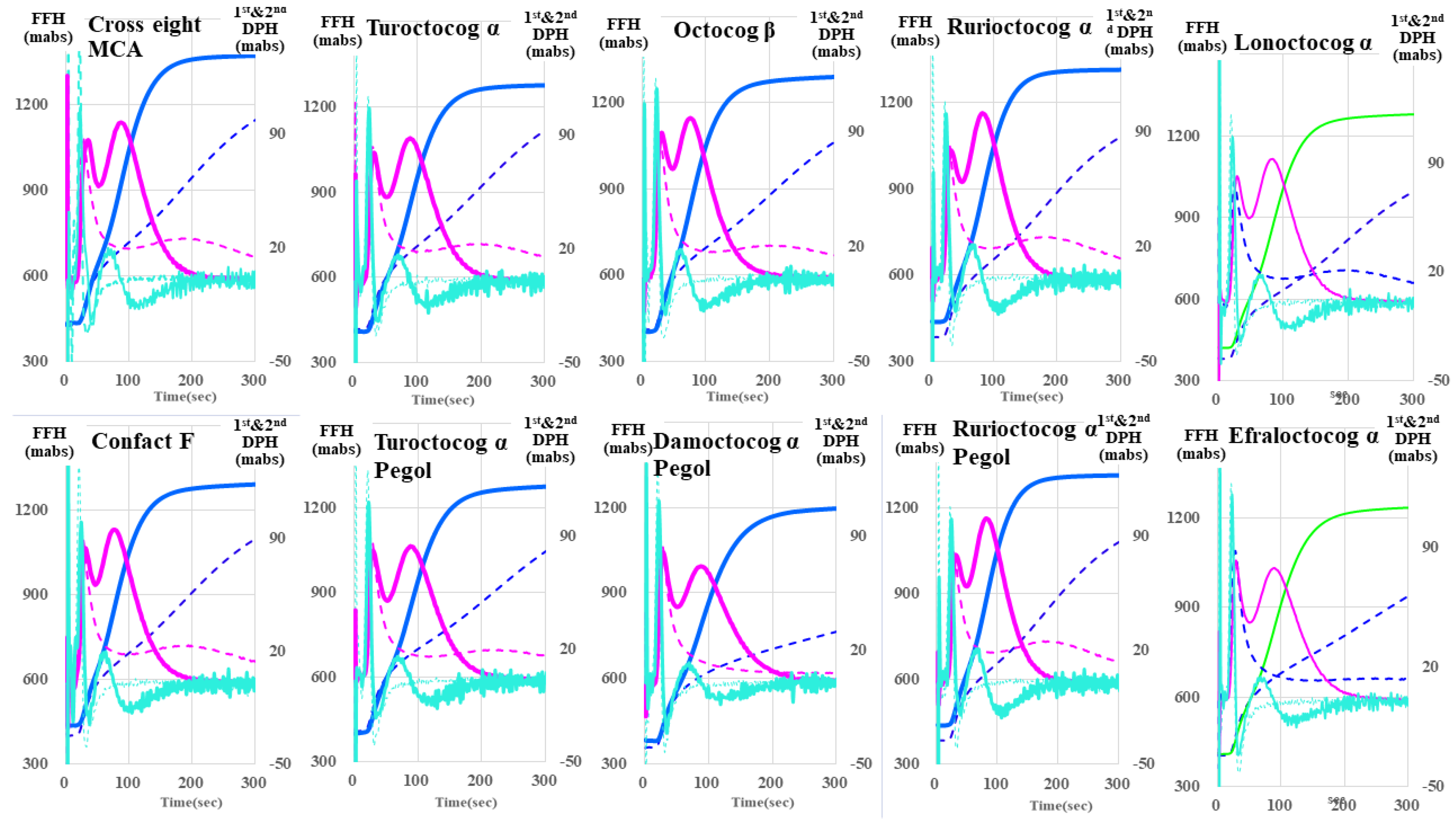
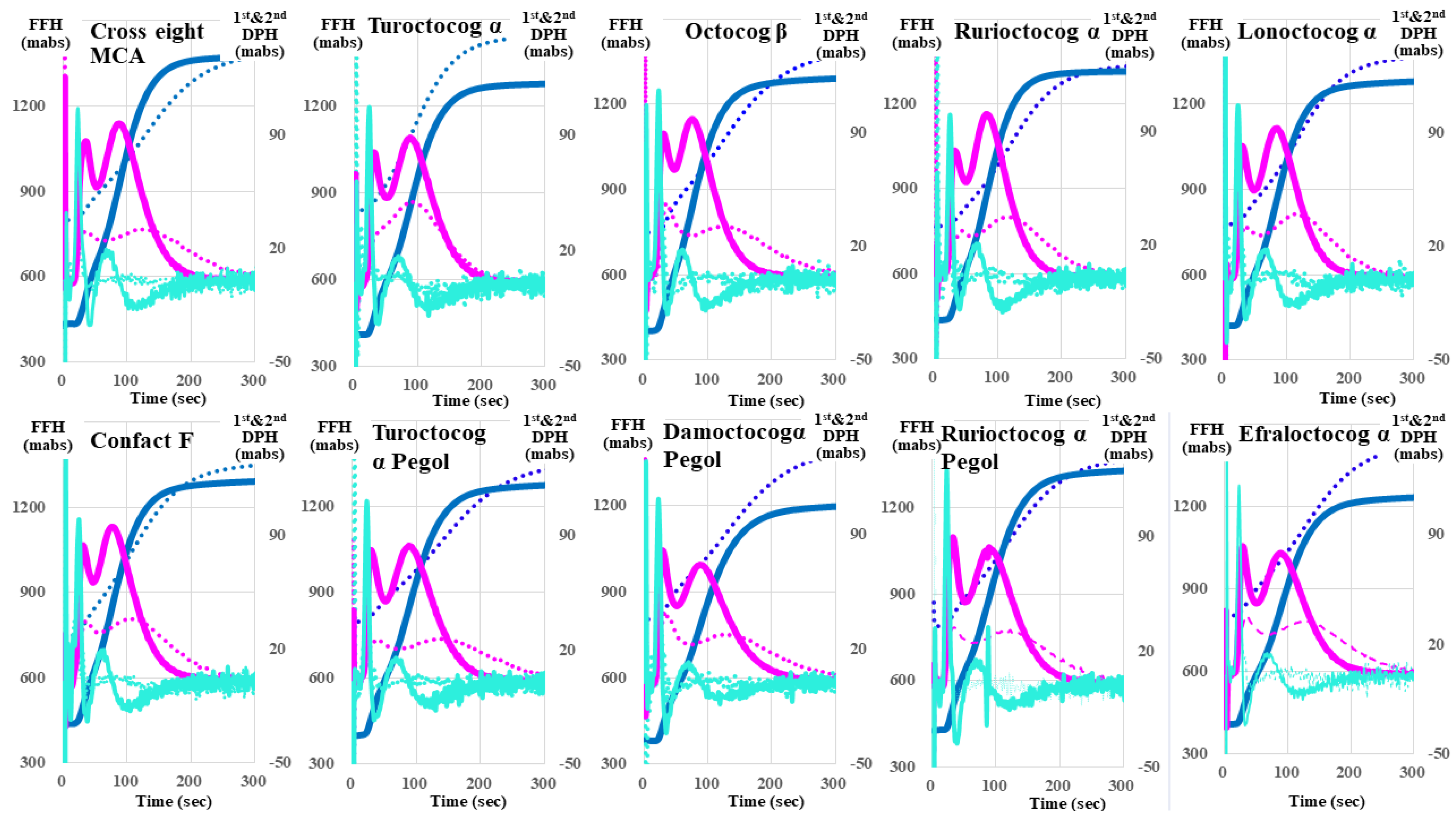
| Second Derivative | First Derivative | Fibrin Formation Curve | ||||
|---|---|---|---|---|---|---|
| PT (s) | PH (mabs) | PT (s) | PH (mabs) | PT (s) | PH (mabs) | |
| Cross eight MC | 34.9 ± 1.0 ** (↓) | 733 ± 97.7 ** (↑) | 38.2 ± 1.14 ** (↓) | 266 ± 10.1 NS | 39.2 ± 1.2 ** (↓) | 427 ± 9.8 *** (↑) |
| Confact F | 35.9 ± 0.6 NS | 798 ± 46.5 *** (↑) | 39.1 ± 0.8 ** (↓) | 271 ± 9.2 NS | 40.1 ± 0.8 * | 574 ± 8.5 *** (↑) |
| Turoctocog α | 36.1 ± 0.6 NS | 621 ± 31.6 * (↑) | 40.3 ± 0.7 NS | 251 ± 2.5 NS | 41.0 ± 0.6 NS | 420 ± 6.6 *** (↑) |
| Turoctocog α Pegol | 43.2 ± 0.9 *** (↑) | 368 ± 15.5 *** (↓) | 50.9 ± 1.1 *** (↑) | 204 ± 7.1 *** (↓) | 51.2 ± 1.0 *** (↑) | 352 ± 47.8 NS |
| Octocog β | 35.0 ± 0.7 ** (↓) | 684 ± 34.9 ** (↑) | 38.4 ± 0.7 *** (↓) | 258 ± 7.2 NS | 39.4 ± 0.7 ** (↓) | 538 ± 17.0 *** (↑) |
| Damoctocog α Pegol | 55.5 ± 7.1 *** (↑) | 93.7 ± 10.1 *** (↓) | 69.3 ± 8.1 *** (↑) | 115 ± 5.5 *** (↓) | 70.2 ± 8.1 *** (↑) | 530 ± 3.4 *** (↑) |
| Rurioctocog α | 33.6 ± 0.6 *** (↓) | 719 ± 23.1 *** (↑) | 36.9 ± 0.7 *** (↓) | 263 ± 3.8 NS | 37.9 ± 0.7 *** (↓) | 406 ± 6.5 *** (↑) |
| Rurioctocog α pegol | 38.3 ± 0.8 NS | 557 ± 16.7 | 43.5 ± 0.9 ** (↑) | 237 ± 5.2 * | 44.1 ± 0.8 NS | 503 ± 10.3 *** (↑) |
| Lonoctocog α | 32.1 ± 0.3 *** (↓) | 697 ± 8.7 ** (↑) | 36.3 ± 0.4 *** (↓) | 254 ± 2.0 NS | 37.0 ± 0.4 *** (↓) | 412 ± 7.5 *** (↑) |
| Efraloctocog α | 34.5 ± 0.8 ** (↓) | 692 ± 26.0 ** (↑) | 38.7 ± 1.0 ** (↓) | 254 ± 6.5 NS | 39.5 ± 0.9 ** (↓) | 432 ± 4.8 *** (↑) |
| Reference plasma | 37.3 ± 1.1 | 536 ± 66.8 | 41.2 ± 0.9 | 260 ± 20.1 | 42.1 ± 1.1 | 342 ± 9.8 |
| APTT Reagents | FVIII Concentrates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross Eight MC | Confact F | Turoctocog α | Turoctocog α Pegol | Octocog β | Damoctocog α Pegol | Rurioctocog α | Rurioctocog α Pegol | Lonoctocog α | Efraloctocog α | RP | |
| (a) Peak Time (s) | |||||||||||
| APTT-SP | 38.1 ± 1.1 | 39.1 ± 0.8 | 40.3 ± 0.7 | 50.9 ± 1.1 * | 38.4 ± 0.7 * | 69.3 ± 8.1 * | 36.9 ± 0.7 * | 43.5 ± 0.9 | 36.3 ± 0.4 * | 38.7 ± 1.0 | 41.2 ± 0.9 |
| APTT-SS | 30.2 ± 0.6 * | 30.0 ± 0.4 * | 27.0 ± 0.3 * | 30.6 ± 0.4 * | 30.5 ± 0.7 | 32.0 ± 0.2 | 26.4 ± 0.5 * | 30.0 ± 0.3 * | 30.0 ± 0.5 * | 30.9 ± 0.8 * | 39.6 ± 0.9 |
| STA Cepha-screen | 36.4 ± 0.6 | 39.7 ± 0.4 * | 39.0 ± 0.4 * | 38.3 ± 0.3 * | 34.9 ± 0.2 * | 32.6 ± 0.3 * | 35.2 ± 0.4 | 37.8 ± 0.4 * | 35.5 ± 0.4 | 36.9 ± 0.7 | 35.5 ± 0.8 |
| APTT-N | 27.9 ± 0.3 * | 29.6 ± 0.4 * | 27.4 ± 0.1 * | 26.3 ± 0.4 * | 27.7 ± 0.3 * | 27.2 ± 0.5 * | 25.0 ± 0.3 * | 27.8 ± 0.4 * | 29.2 ± 0.2 * | 28.0 ± 0.3 * | 33.9 ± 0.5 |
| APTT PSL | 44.0 ± 0.4 * | 42.9 ± 0.6 * | 44.0 ± 0.3 * | 44.3 ± 0.3 * | 40.7 ± 0.2 * | 43.5 ± 3.4 | 42.3 ± 0.5 * | 41.9 ± 0.2 * | 42.4 ± 0.9 * | 49.5 ± 0.6 * | 46.4 ± 0.6 |
| C.K. Prest | 33.9 ± 0.3 * | 32.2 ± 0.7 * | 34.3 ± 0.4 * | 43.7 ± 0.3 * | 31.4 ± 0.4 * | 36.4 ± 0.3 | 32.5 ± 0.6 * | 34.6 ± 0.4 * | 32.3 ± 0.3 * | 33.1 ± 0.5 * | 36.9 ± 0.6 |
| APTT-SLA | 30.1 ± 0.5 * | 29.8 ± 0.4 * | 30.4 ± 0.3 * | 30.9 ± 0.3 * | 27.7 ± 0.3 * | 25.7 ± 0.3 * | 28.2 ± 0.5 * | 31.4 ± 0.5 | 28.8 ± 0.3 * | 30.6 ± 0.4 * | 31.9 ± 0.2 |
| TC APTT | 35.9 ± 0.4 | 35.0 ± 0.4 * | 38.1 ± 0.3 * | 36.8 ± 0.2 * | 31.3 ± 0.4 * | 28.6 ± 0.4 * | 34.3 ± 0.6 | 37.5 ± 0.4 * | 34.9 ± 0.2 * | 36.9 ± 0.3 * | 35.8 ± 0.3 |
| APTT FS | 36.2 ± 0.5 | 36.0 ± 0.6 | 38.2 ± 0.3 * | 39.8 ± 0.4 * | 33.1 ± 0.3 * | 29.8 ± 0.3 * | 36.3 ± 0.4 | 40.2 ± 0.4 * | 38.9 ± 0.1 * | 38.2 ± 0.5 * | 36.8 ± 0.1 |
| APTT FSL | 30.0 ± 0.7 * | 29.9 ± 0.7 * | 26.3 ± 0.2 * | 28.1 ± 0.3 * | 27.4 ± 0.5 * | 26.8 ± 0.3 * | 24.9 ± 0.2 * | 27.9 ± 0.5 * | 27.3 ± 0.4 * | 28.6 ± 0.3 * | 36.3 ± 0.6 |
| PTT ACT | 37.2 ± 0.5 * | 36.2 ± 0.5 * | 40.9 ± 1.3 * | 42.2 ± 0.6 * | 34.9 ± 0.2 | 29.7 ± 0.4 * | 38.2 ± 0.5 * | 40.7 ± 0.5 * | 39.4 ± 0.3 * | 40.5 ± 0.3 * | 33.1 ± 0.8 |
| STA PTT A | 39.8 ± 0.5 | 37.9 ± 0.4 | 42.0 ± 0.6 | 56.7 ± 0.4 * | 33.9 ± 0.3 * | 74.8 ± 0.5 * | 37.5 ± 0.4 | 44.1 ± 0.3 | 41.3 ± 0.4 | 39.9 ± 0.3 | 41.7 ± 3.3 |
| (b) Peak heights (mabs) | |||||||||||
| APTT-SP | 266 ± 9.0 | 270 ± 8.2 | 251 ± 2.4 | 204 ± 6.2 * | 259 ± 6.3 | 115 ± 4.9 * | 263 ± 3.4 | 237 ± 4.5 | 255 ± 1.9 | 255 ± 5.7 | 260 ± 17.9 |
| APTT-SS | 201 ± 5.0 | 202 ± 5.6 | 192 ± 6.4 | 188 ± 2.9 | 188 ± 1.5 | 193 ± 1.6 | 199 ± 3.0 | 187 ± 3.7 | 200 ± 2.7 | 204 ± 2.6 | 188 ± 22.6 |
| STA Cepha-screen | 251 ± 7.1 | 241 ± 6.1 * | 227 ± 5.7 * | 230 ± 2.4 * | 241 ± 1.5 * | 258 ± 1.6 | 236 ± 5.5 * | 231 ± 4.8 * | 231 ± 1.1 * | 265 ± 4.4 | 271 ± 12.2 |
| APTT-N | 220 ± 5.0 | 217 ± 5.4 | 221 ± 4.0 | 212 ± 4.1 | 220 ± 3.1 | 221 ± 4.9 | 229 ± 4.3 | 217 ± 3.2 | 208 ± 1.1 | 227 ± 6.3 | 221 ± 10.9 |
| APTT PSL | 189 ± 4.0 * | 195 ± 5.0 * | 180 ± 6.8 * | 144 ± 10.7 * | 196 ± 4.2 * | 155 ± 3.2 * | 184 ± 3.7 * | 169 ± 4.4 * | 180 ± 1.9 * | 192 ± 2.1 * | 212 ± 5.2 |
| C.K. Prest | 153 ± 3.5 | 158 ± 3.5 | 142 ± 3.1 * | 128 ± 2.5 * | 168 ± 4.8 | 153 ± 4.0 | 150 ± 3.7 | 138 ± 3.3 | 155 ± 2.5 | 156 ± 4.8 | 169 ± 13.5 |
| APTT-SLA | 283 ± 3.1 * | 285 ± 5.4 * | 274 ± 4.3 * | 271 ± 6.3 * | 295 ± 4.1 * | 320 ± 7.7 | 280 ± 2.7 * | 276 ± 5.0 * | 272 ± 3.6 * | 306 ± 3.1 * | 332 ± 4.8 |
| TC APTT | 251 ± 3.2 * | 260 ± 6.3 | 247 ± 3.3 * | 246 ± 2.4 * | 257 ± 4.8 | 283 ± 3.1 | 248 ± 3.6 * | 261 ± 4.2 | 240 ± 1.3 * | 264 ± 3.4 | 272 ± 4.7 |
| APTT FS | 255 ± 4.7 * | 252 ± 5.0 * | 240 ± 6.3 | 251 ± 1.6 * | 291 ± 6.0 | 307 ± 5.4 | 249 ± 4.4 * | 250 ± 3.5 * | 253 ± 1.6 * | 276 ± 3.4 * | 302 ± 3.9 |
| APTT FSL | 253 ± 4.6 * | 256 ± 5.9 * | 256 ± 4.9 | 256 ± 2.4 * | 268 ± 5.8 | 297 ± 3.1 * | 261 ± 3.1 * | 260 ± 3.5 * | 252 ± 1.9 * | 279 ± 3.9 | 280 ± 6.0 |
| PTT ACT | 299 ± 6.3 * | 298 ± 5.9 * | 271 ± 3.8 * | 295 ± 2.7 * | 312 ± 4.3 | 352 ± 4.2 | 294 ± 6.6 * | 279 ± 4.3 * | 281 ± 1.9 * | 319 ± 2.4 | 338 ± 11.3 |
| STA PTT A | 218 ± 6.2 | 228 ± 7.8 | 202 ± 4.7 * | 153 ± 1.9 * | 279 ± 5.6 | 131 ± 4.0 * | 207 ± 4.1 * | 180 ± 3.7 * | 219 ± 1.6 * | 242 ± 33.3 | 249 ± 12.5 |
| Method | Cross Eight MC | Confact F | Turoctocog α | Turoctocog α Pegol | Octocog β | Damoctocog α Pegol | Rurioctocog α | Rurioctocog α Pegol | Lonoctocog α | Efraloctocog α |
|---|---|---|---|---|---|---|---|---|---|---|
| Chromogenic assay (IU/mL) | 1.00 | 1.01 | 1.01 | 1.00 | 1.04 | 1.03 | 1.00 | 1.03 | 1.04 | 1.00 |
| APTT (IU/mL) | 1.04 | 1.05 | 0.80 | 0.48 | 0.96 | 0.12 | 1.01 | 0.81 | 1.03 | 1.02 |
| sTT (IU/mL) | 1.60 | 1.78 | 1.34 | 1.40 | 1.25 | 1.20 | 1.51 | 1.31 | 1.44 | 1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, H.; Shiraki, K.; Matsumoto, T.; Shimpo, H.; Sakano, Y.; Nishii, H.; Tamaki, S.; Suzuki, K.; Tawara, I.; Yamashita, Y.; et al. Evaluating Factor VIII Concentrates Using Clot Waveform Analysis. J. Clin. Med. 2024, 13, 3857. https://doi.org/10.3390/jcm13133857
Wada H, Shiraki K, Matsumoto T, Shimpo H, Sakano Y, Nishii H, Tamaki S, Suzuki K, Tawara I, Yamashita Y, et al. Evaluating Factor VIII Concentrates Using Clot Waveform Analysis. Journal of Clinical Medicine. 2024; 13(13):3857. https://doi.org/10.3390/jcm13133857
Chicago/Turabian StyleWada, Hideo, Katsuya Shiraki, Takeshi Matsumoto, Hideto Shimpo, Yumi Sakano, Hiroko Nishii, Shigehisa Tamaki, Kei Suzuki, Isao Tawara, Yoshiki Yamashita, and et al. 2024. "Evaluating Factor VIII Concentrates Using Clot Waveform Analysis" Journal of Clinical Medicine 13, no. 13: 3857. https://doi.org/10.3390/jcm13133857
APA StyleWada, H., Shiraki, K., Matsumoto, T., Shimpo, H., Sakano, Y., Nishii, H., Tamaki, S., Suzuki, K., Tawara, I., Yamashita, Y., & Shimaoka, M. (2024). Evaluating Factor VIII Concentrates Using Clot Waveform Analysis. Journal of Clinical Medicine, 13(13), 3857. https://doi.org/10.3390/jcm13133857







