Evolution of Stemless Reverse Shoulder Arthroplasty: Current Indications, Outcomes, and Future Prospects
Abstract
1. Introduction
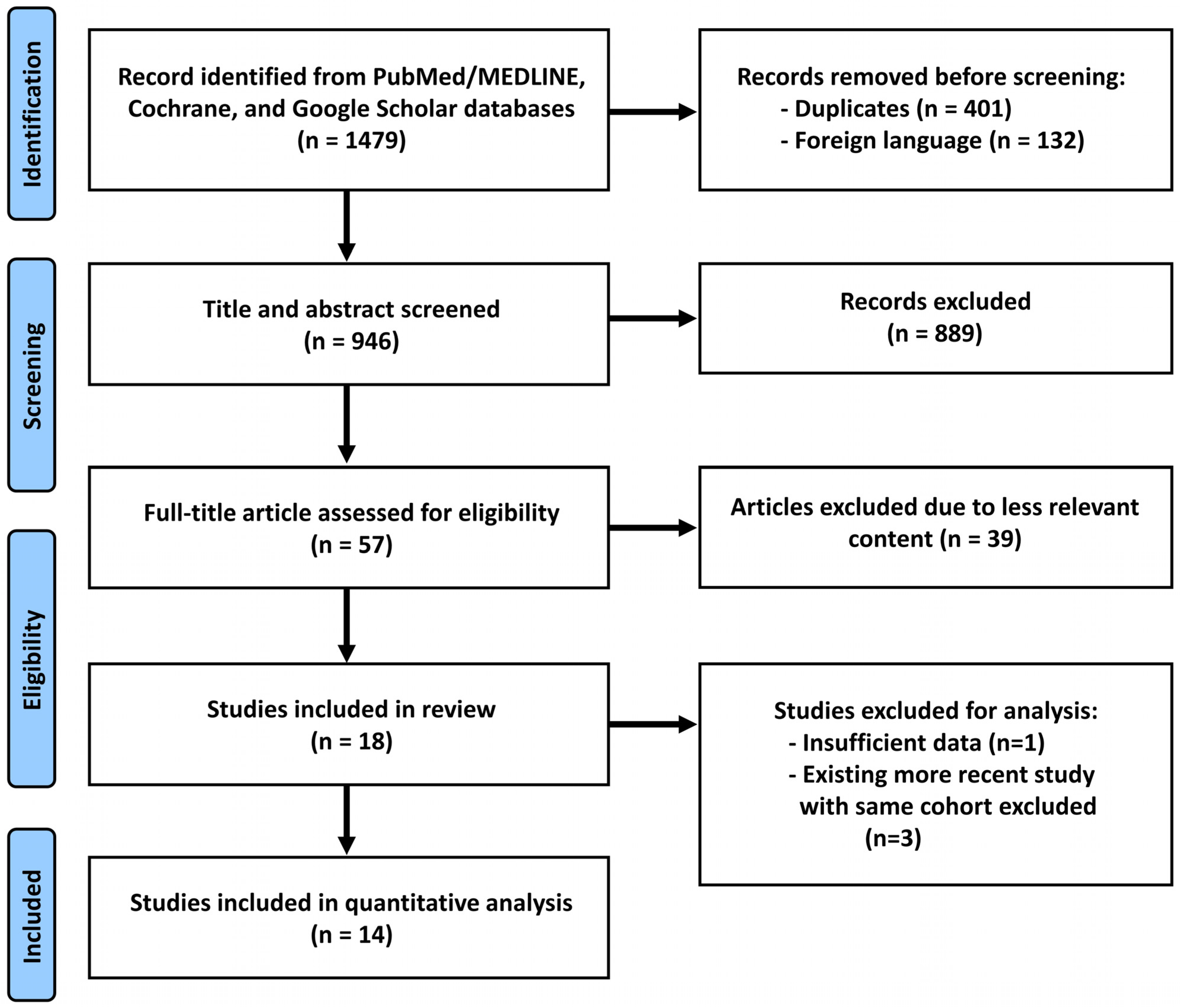
2. Methods
2.1. Literature Search and Data Extraction
2.2. Quality Assessment
2.3. Statistical Analyses
3. Evolution of Stemless rTSA
3.1. Implant Design
3.2. Implant Material
3.3. Biomechanical Properties
4. Indication Considerations
4.1. Young Age
4.2. Obesity
4.3. Osteoporosis
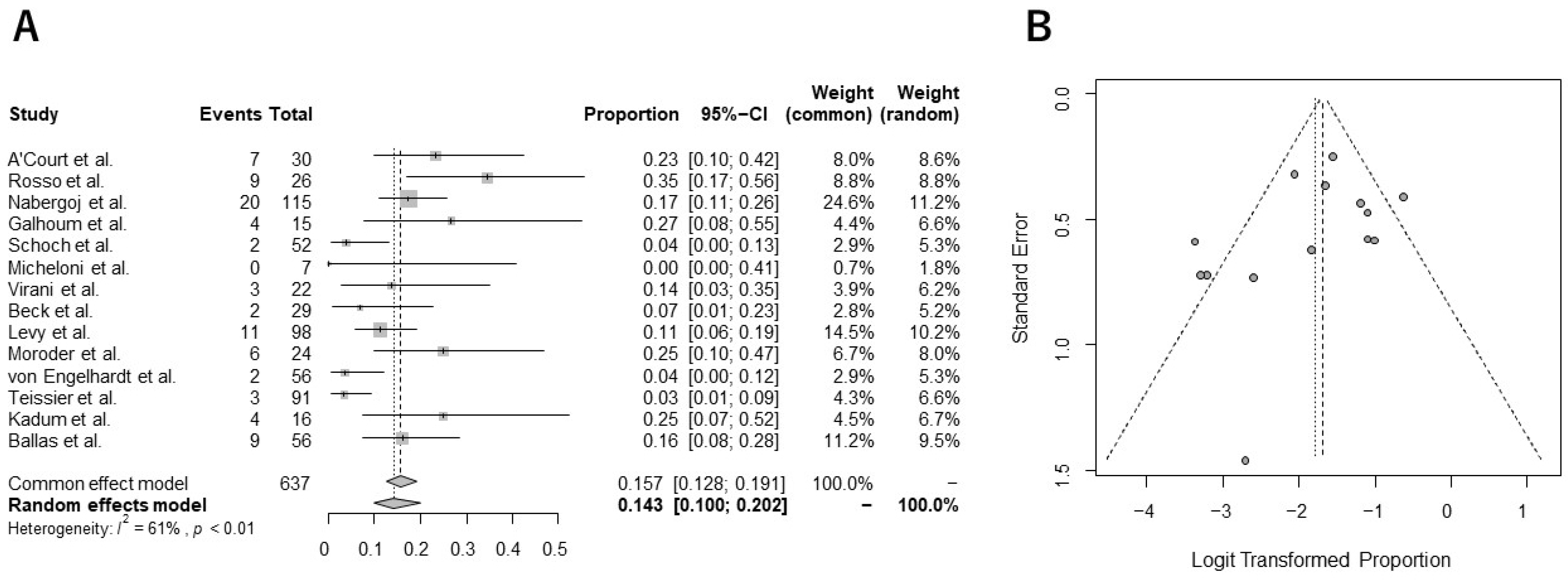
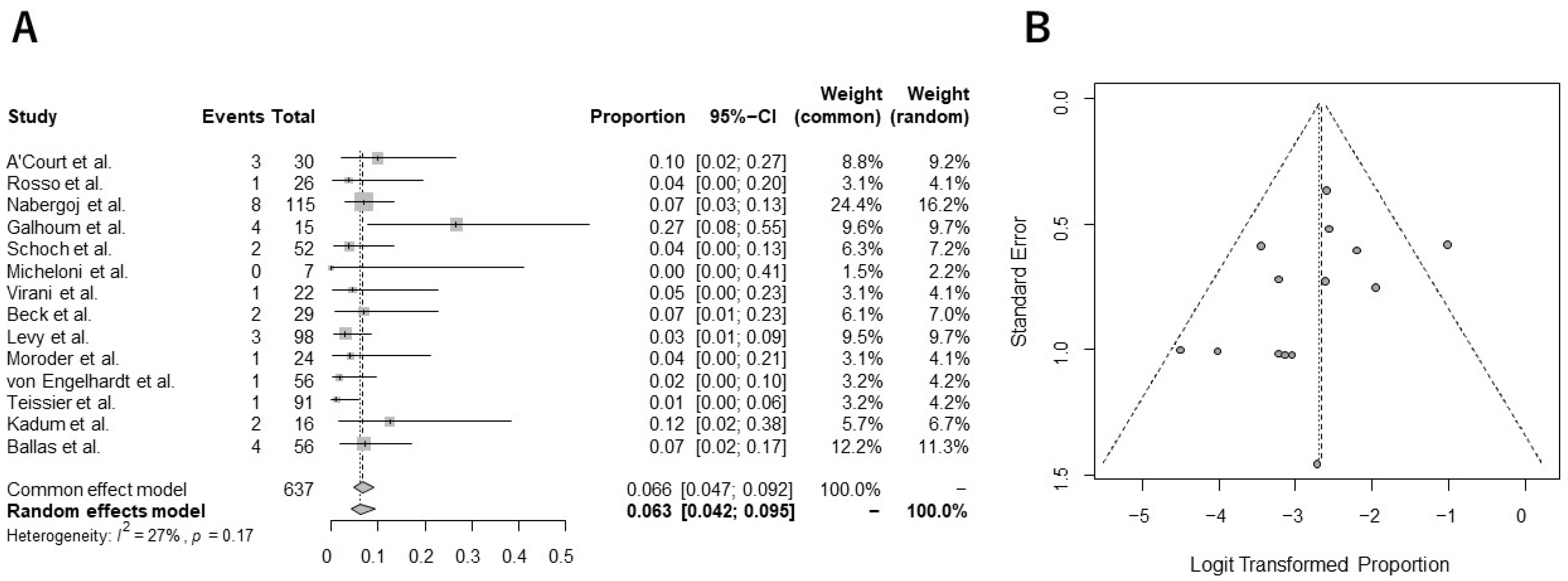
5. Outcomes from Quantitative Analysis
Incidence of Complications and Revision Surgery from Meta-Analysis
6. Comparative Analysis of Stemmed and Stemless rTSA
6.1. Clinical Outcomes
6.2. Complications
7. Conclusions
- The current meta-analysis demonstrated that the pooled overall complication and revision rates were 14.3% and 6.3%, respectively;
- Comparative studies may indicate equivalent functional recovery and incidence of complications between stemmed and stemless prostheses;
- Further long-term studies comparing the survivorship between stemless and stemmed rTSAs are required to determine the gold standard for selecting stemless rTSA.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walch, G.; Mesiha, M.; Boileau, P.; Edwards, T.B.; Levigne, C.; Moineau, G.; Young, A. Three-dimensional assessment of the dimensions of the osteoarthritic glenoid. Bone Jt. J. 2013, 95-B, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Grammont, P.M.; Baulot, E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin. Orthop. Relat. Res. 2011, 469, 2424. [Google Scholar] [CrossRef] [PubMed]
- Baulot, E.; Sirveaux, F.; Boileau, P. Grammont’s idea: The story of Paul Grammont’s functional surgery concept and the development of the reverse principle. Clin. Orthop. Relat. Res. 2011, 469, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Trofa, D.; Rajaee, S.S.; Smith, E.L. Nationwide trends in total shoulder arthroplasty and hemiarthroplasty for osteoarthritis. Am. J. Orthop. 2014, 43, 166–172. [Google Scholar]
- Gao, R.; van der Merwe, M.; Coleman, B.; Boyle, M.J.; Frampton, C.M.; Hirner, M. Outcomes of reverse shoulder arthroplasty in patients under 55 years old: Results from the New Zealand joint registry. Shoulder Elb. 2023, 15, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kim, S.H.; Kim, S.C.; Park, J.H.; Kim, J.S.; Kim, B.T.; Lee, S.M.; Yoo, J.C. Return to Sports Activity After Reverse Total Shoulder Arthroplasty. Orthop. J. Sports Med. 2023, 11, 23259671231208959. [Google Scholar] [CrossRef]
- Su, F.; Kucirek, N.; Goldberg, D.; Feeley, B.T.; Ma, C.B.; Lansdown, D.A. Incidence, risk factors, and complications of acromial stress fractures after reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2024, 33, 65–72. [Google Scholar] [CrossRef]
- Bacle, G.; Nove-Josserand, L.; Garaud, P.; Walch, G. Long-Term Outcomes of Reverse Total Shoulder Arthroplasty: A Follow-up of a Previous Study. J. Bone Jt. Surg. 2017, 99, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Cuff, D.J.; Pupello, D.R.; Santoni, B.G.; Clark, R.E.; Frankle, M.A. Reverse Shoulder Arthroplasty for the Treatment of Rotator Cuff Deficiency: A Concise Follow-up, at a Minimum of 10 Years, of Previous Reports. J. Bone Jt. Surg. 2017, 99, 1895–1899. [Google Scholar] [CrossRef]
- Favard, L.; Levigne, C.; Nerot, C.; Gerber, C.; De Wilde, L.; Mole, D. Reverse prostheses in arthropathies with cuff tear: Are survivorship and function maintained over time? Clin. Orthop. Relat. Res. 2011, 469, 2469–2475. [Google Scholar] [CrossRef]
- Mazaleyrat, M.; Favard, L.; Boileau, P.; Berhouet, J. Humeral osteolysis after reverse shoulder arthroplasty using cemented or cementless stems comparative retrospective study with a mean follow-up of 9 years. Orthop. Traumatol. Surg. Res. 2021, 107, 102916. [Google Scholar] [CrossRef] [PubMed]
- Ek, E.T.; Neukom, L.; Catanzaro, S.; Gerber, C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: Results after five to fifteen years. J. Shoulder Elb. Surg. 2013, 22, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Guery, J.; Favard, L.; Sirveaux, F.; Oudet, D.; Mole, D.; Walch, G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J. Bone Jt. Surg. 2006, 88, 1742–1747. [Google Scholar] [CrossRef]
- Cuff, D.; Clark, R.; Pupello, D.; Frankle, M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: A concise follow-up, at a minimum of five years, of a previous report. J. Bone Jt. Surg. 2012, 94, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Chelli, M.; Boileau, P.; Domos, P.; Clavert, P.; Berhouet, J.; Collin, P.; Walch, G.; Favard, L. Survivorship of Reverse Shoulder Arthroplasty According to Indication, Age and Gender. J. Clin. Med. 2022, 11, 2677. [Google Scholar] [CrossRef] [PubMed]
- Porcellini, G.; Combi, A.; Merolla, G.; Bordini, B.; Stea, S.; Zanoli, G.; Paladini, P. The experience of the RIPO, a shoulder prosthesis registry with 6-year follow-up. Musculoskelet. Surg. 2018, 102, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Berth, A.; Pap, G. Stemless shoulder prosthesis versus conventional anatomic shoulder prosthesis in patients with osteoarthritis: A comparison of the functional outcome after a minimum of two years follow-up. J. Orthop. Traumatol. 2013, 14, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Magosch, P.; Lichtenberg, S.; Habermeyer, P. Survival of stemless humeral head replacement in anatomic shoulder arthroplasty: A prospective study. J. Shoulder Elb. Surg. 2021, 30, e343–e355. [Google Scholar] [CrossRef]
- Maier, M.W.; Lauer, S.; Klotz, M.C.; Bulhoff, M.; Spranz, D.; Zeifang, F. Are there differences between stemless and conventional stemmed shoulder prostheses in the treatment of glenohumeral osteoarthritis? BMC Musculoskelet. Disord. 2015, 16, 275. [Google Scholar] [CrossRef]
- Simon, M.J.K.; Coghlan, J.A.; Hughes, J.; Wright, W.; Dallalana, R.J.; Bell, S.N. Mid-term outcomes of a stemless ceramic head anatomic total shoulder replacement. BMC Musculoskelet. Disord. 2022, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Spranz, D.M.; Bruttel, H.; Wolf, S.I.; Zeifang, F.; Maier, M.W. Functional midterm follow-up comparison of stemless total shoulder prostheses versus conventional stemmed anatomic shoulder prostheses using a 3D-motion-analysis. BMC Musculoskelet. Disord. 2017, 18, 478. [Google Scholar] [CrossRef] [PubMed]
- Uschok, S.; Magosch, P.; Moe, M.; Lichtenberg, S.; Habermeyer, P. Is the stemless humeral head replacement clinically and radiographically a secure equivalent to standard stem humeral head replacement in the long-term follow-up? A prospective randomized trial. J. Shoulder Elb. Surg. 2017, 26, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Moroder, P.; Ernstbrunner, L.; Zweiger, C.; Schatz, M.; Seitlinger, G.; Skursky, R.; Becker, J.; Resch, H.; Krifter, R.M. Short to mid-term results of stemless reverse shoulder arthroplasty in a selected patient population compared to a matched control group with stem. Int. Orthop. 2016, 40, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.Y.; Kord, D.; Horner, N.S.; Leroux, T.; Alolabi, B.; Khan, M. Stemless anatomic total shoulder arthroplasty: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2020, 29, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.I.P.; Hoffmann, J.; Sierevelt, I.N.; van den Bekerom, M.P.J.; Alta, T.D.W.; van Noort, A. Results of stemless shoulder arthroplasty: A systematic review and meta-analysis. EFORT Open Rev. 2021, 6, 35–49. [Google Scholar] [CrossRef]
- Sears, B.W.; Creighton, R.A.; Denard, P.J.; Griffin, J.W.; Lichtenberg, S.; Lederman, E.S.; Werner, B.C. Stemless components lead to improved radiographic restoration of humeral head anatomy compared with short-stemmed components in total shoulder arthroplasty. J. Shoulder Elb. Surg. 2023, 32, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.E.; Spangenberg, G.W.; Langohr, G.D.G.; Athwal, G.S.; Johnson, J.A. Stemless reverse humeral component neck-shaft angle has an influence on initial fixation. J. Shoulder Elb. Surg. 2024, 33, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.T.; Jost, B.; Hertel, R.; Zipeto, C.; Van Rooij, F.; Zumstein, M.A. Patient-specific instrumentation reduces deviations between planned and postosteotomy humeral retrotorsion and height in shoulder arthroplasty. J. Shoulder Elb. Surg. 2022, 31, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, D.A.; Yin, C.X.; Hamid, H.S.; Wiater, B.P.; Martusiewicz, A.; Wiater, J.M. Stemless reverse total shoulder arthroplasty: A systematic review. J. Shoulder Elb. Surg. 2022, 31, 1083–1095. [Google Scholar] [CrossRef]
- Kadum, B.; Mafi, N.; Norberg, S.; Sayed-Noor, A.S. Results of the Total Evolutive Shoulder System (TESS): A single-centre study of 56 consecutive patients. Arch. Orthop. Trauma Surg. 2011, 131, 1623–1629. [Google Scholar] [CrossRef]
- Atoun, E.; Van Tongel, A.; Hous, N.; Narvani, A.; Relwani, J.; Abraham, R.; Levy, O. Reverse shoulder arthroplasty with a short metaphyseal humeral stem. Int. Orthop. 2014, 38, 1213–1218. [Google Scholar] [CrossRef]
- Leonidou, A.; Virani, S.; Buckle, C.; Yeoh, C.; Relwani, J. Reverse shoulder arthroplasty with a cementless short metaphyseal humeral prosthesis without a stem: Survivorship, early to mid-term clinical and radiological outcomes in a prospective study from an independent centre. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 89–96. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Phillips, A.N. Meta-analysis: Principles and procedures. BMJ 1997, 315, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Churchill, R.S.; Athwal, G.S. Stemless shoulder arthroplasty-current results and designs. Curr. Rev. Musculoskelet. Med. 2016, 9, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Churchill, R.S.; Chuinard, C.; Wiater, J.M.; Friedman, R.; Freehill, M.; Jacobson, S.; Spencer, E., Jr.; Holloway, G.B.; Wittstein, J.; Lassiter, T.; et al. Clinical and Radiographic Outcomes of the Simpliciti Canal-Sparing Shoulder Arthroplasty System: A Prospective Two-Year Multicenter Study. J. Bone Jt. Surg. 2016, 98, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Harmer, L.; Throckmorton, T.; Sperling, J.W. Total shoulder arthroplasty: Are the humeral components getting shorter? Curr. Rev. Musculoskelet. Med. 2016, 9, 17–22. [Google Scholar] [CrossRef]
- Teissier, J.; Teissier, P. Stemless shoulder arthroplasty. Orthop. Traumatol. Surg. Res. 2023, 109, 103460. [Google Scholar] [CrossRef]
- Ballas, R.; Béguin, L. Results of a stemless reverse shoulder prosthesis at more than 58 months mean without loosening. J. Shoulder Elb. Surg. 2013, 22, e1–e6. [Google Scholar] [CrossRef]
- Beck, S.; Patsalis, T.; Busch, A.; Dittrich, F.; Dudda, M.; Jager, M.; Wegner, A. Long-term results of the reverse Total Evolutive Shoulder System (TESS). Arch. Orthop. Trauma Surg. 2019, 139, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Kadum, B.; Mukka, S.; Englund, E.; Sayed-Noor, A.; Sjoden, G. Clinical and radiological outcome of the Total Evolutive Shoulder System (TESS(R)) reverse shoulder arthroplasty: A prospective comparative non-randomised study. Int. Orthop. 2014, 38, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Teissier, P.; Teissier, J.; Kouyoumdjian, P.; Asencio, G. The TESS reverse shoulder arthroplasty without a stem in the treatment of cuff-deficient shoulder conditions: Clinical and radiographic results. J. Shoulder Elb. Surg. 2015, 24, 45–51. [Google Scholar] [CrossRef] [PubMed]
- von Engelhardt, L.V.; Manzke, M.; Filler, T.J.; Jerosch, J. Short-term results of the reverse Total Evolutive Shoulder System (TESS) in cuff tear arthropathy and revision arthroplasty cases. Arch. Orthop. Trauma Surg. 2015, 135, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Narvani, A.; Hous, N.; Abraham, R.; Relwani, J.; Pradhan, R.; Bruguera, J.; Sforza, G.; Atoun, E. Reverse shoulder arthroplasty with a cementless short metaphyseal humeral implant without a stem: Clinical and radiologic outcomes in prospective 2- to 7-year follow-up study. J. Shoulder Elb. Surg. 2016, 25, 1362–1370. [Google Scholar] [CrossRef]
- Virani, S.; Holmes, N.; Al-Janabi, M.; Watts, C.; Brooks, C.; Relwani, J. Intermediate to long term results of stemless metaphyseal reverse shoulder arthroplasty: A five to nine year follow-up. J. Clin. Orthop. Trauma 2021, 23, 101611. [Google Scholar] [CrossRef]
- Nabergoj, M.; Ladermann, A.; Authom, T.; Beaudouin, E.; Azar, M.; Wahab, H.; Leger, O.; Haight, H.; Harris, H.; Collin, P. Stemless reverse shoulder arthroplasty: Clinical and radiologic outcomes with minimum 2 years’ follow-up. J. Shoulder Elb. Surg. 2023, 32, e464–e474. [Google Scholar] [CrossRef] [PubMed]
- Galhoum, M.S.; Elsheikh, A.A.; Wood, A.; Yin, Q.; Frostick, S.P. Anatomic and Reverse Stemless Shoulder Arthroplasty: Functional and Radiological Evaluation. J. Shoulder Elb. Arthroplast. 2022, 6, 24715492221118765. [Google Scholar] [CrossRef]
- Schoch, C.; Plath, J.E.; Ambros, L.; Geyer, M.; Dittrich, M. Clinical and radiological outcomes of a stemless reverse shoulder implant: A two-year follow-up in 56 patients. JSES Int. 2021, 5, 1042–1048. [Google Scholar] [CrossRef]
- Rosso, C.; Kränzle, J.; Delaney, R.; Grezda, K. Radiologic, clinical, and patient-reported outcomes in stemless reverse shoulder arthroplasty at a mean of 46 months. J. Shoulder Elb. Surg. 2024, 33, 1324–1330. [Google Scholar] [CrossRef]
- A’Court, J.J.; Chatindiara, I.; Fisher, R.; Poon, P.C. Stemless reverse arthroplasty: Does the stemless compare to a conventional stemmed implant? Clinical and radiographic evaluation 2 years minimum follow up. J. Shoulder Elb. Surg. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, M.T.; Hannon, C.P. The Evolution, Current Indications and Outcomes of Cementless Total Knee Arthroplasty. J. Clin. Med. 2022, 11, 6608. [Google Scholar] [CrossRef] [PubMed]
- Furlong, R.J.; Osborn, J.F. Fixation of hip prostheses by hydroxyapatite ceramic coatings. J. Bone Jt. Surg. Br. 1991, 73, 741–745. [Google Scholar] [CrossRef]
- Toksvig-Larsen, S.; Jorn, L.P.; Ryd, L.; Lindstrand, A. Hydroxyapatite-enhanced tibial prosthetic fixation. Clin. Orthop. Relat. Res. 2000, 370, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Dumbleton, J.; Manley, M.T. Hydroxyapatite-coated prostheses in total hip and knee arthroplasty. J. Bone Jt. Surg. 2004, 86, 2526–2540. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Bjorkman, A.; Besjakov, J.; Onsten, I. Cemented tibial component fixation performs better than cementless fixation: A randomized radiostereometric study comparing porous-coated, hydroxyapatite-coated and cemented tibial components over 5 years. Acta Orthop. 2005, 76, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Winther, N.S.; Jensen, C.L.; Jensen, C.M.; Lind, T.; Schroder, H.M.; Flivik, G.; Petersen, M.M. Comparison of a novel porous titanium construct (Regenerex(R)) to a well proven porous coated tibial surface in cementless total knee arthroplasty—A prospective randomized RSA study with two-year follow-up. Knee 2016, 23, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Ryan, W.K.; Vander Voort, W.D.; Saad, M.A.; Wu, E.; Garcia-Nolen, T.C.; Bayne, C.O.; Szabo, R.M. The effect of shoulder prosthesis stem length on failure due to torsional loading. A biomechanical study in composite humeri. JSES Int. 2023, 7, 819–826. [Google Scholar] [CrossRef]
- Colasanti, C.A.; Lin, C.C.; Simovitch, R.W.; Virk, M.S.; Zuckerman, J.D. International consensus statement on the management of glenohumeral arthritis in patients ≤ 50 years old. J. Shoulder Elb. Surg. 2023, 32, e329–e342. [Google Scholar] [CrossRef]
- McBride, A.P.; Ross, M.; Duke, P.; Hoy, G.; Page, R.; Dyer, C.; Taylor, F. Shoulder joint arthroplasty in young patients: Analysis of 8742 patients from the Australian Orthopaedic Association National Joint Replacement Registry. Shoulder Elb. 2023, 15, 41–52. [Google Scholar] [CrossRef]
- Hatta, T.; Werthel, J.D.; Wagner, E.R.; Itoi, E.; Steinmann, S.P.; Cofield, R.H.; Sperling, J.W. Effect of smoking on complications following primary shoulder arthroplasty. J. Shoulder Elb. Surg. 2017, 26, 1–6. [Google Scholar] [CrossRef]
- Gruson, K.I.; Lo, Y.; Rothchild, E.; Shah, P.; Tabeayo, E.; Qawasmi, F. Does Morbid Obesity (BMI >/=40 kg/m2) Impact Operative Time, Blood Loss, Length of Stay, or Complications Following Anatomic Total Shoulder Arthroplasty? Arch. Bone Jt. Surg. 2023, 11, 389–397. [Google Scholar] [CrossRef]
- Theodoulou, A.; Krishnan, J.; Aromataris, E. Risk of poor outcomes in patients who are obese following total shoulder arthroplasty and reverse total shoulder arthroplasty: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2019, 28, e359–e376. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Pasqualini, I.; Barros, H.; Menendez, M.E.; Horinek, J.L.; Ardebol, J.; Denard, P.J. Lesser Tuberosity Osteotomy Healing in Stemmed and Stemless Anatomic Shoulder Arthroplasty Is Higher with a Tensionable Construct and Affected by Body Mass Index and Tobacco Use. J. Clin. Med. 2023, 12, 834. [Google Scholar] [CrossRef]
- Zdravkovic, V.; Kaufmann, R.; Neels, A.; Dommann, A.; Hofmann, J.; Jost, B. Bone mineral density, mechanical properties, and trabecular orientation of cancellous bone within humeral heads affected by advanced shoulder arthropathy. J. Orthop. Res. 2020, 38, 1914–1919. [Google Scholar] [CrossRef]
- Leafblad, N.; Asghar, E.; Tashjian, R.Z. Innovations in Shoulder Arthroplasty. J. Clin. Med. 2022, 11, 2799. [Google Scholar] [CrossRef] [PubMed]
- Hatta, T.; Shinagawa, K.; Kawakami, J.; Kanazawa, K.; Hayakawa, T.; Yamamoto, N.; Yamakado, K. A survey and biomechanical analysis of the feasibility of the thumb test for determining the cancellous bone quality for stemless shoulder prosthesis. J. Orthop. Surg. 2023, 31, 10225536231218869. [Google Scholar] [CrossRef]
- Athwal, G.S. Spare the Canal: Stemless Shoulder Arthroplasty Is Finally Here: Commentary on an article by R. Sean Churchill, MD; et al.: “Clinical and Radiographic Outcomes of the Simpliciti Canal-Sparing Shoulder Arthroplasty System. A Prospective Two-Year Multicenter Study”. J. Bone Jt. Surg. 2016, 98, e28. [Google Scholar] [CrossRef]
- Favre, P.; Henderson, A.D. Prediction of stemless humeral implant micromotion during upper limb activities. Clin. Biomech 2016, 36, 46–51. [Google Scholar] [CrossRef]
- Favre, P.; Seebeck, J.; Thistlethwaite, P.A.; Obrist, M.; Steffens, J.G.; Hopkins, A.R.; Hulme, P.A. In vitro initial stability of a stemless humeral implant. Clin. Biomech 2016, 32, 113–117. [Google Scholar] [CrossRef]
- Micheloni, G.M.; Salmaso, G.; Berti, M.; Bortolato, S.; Zecchinato, G.; Momoli, A.; Giaretta, S. Cementless metaphyseal reverse shoulder arthroplasty: Our preliminary experience. Acta Biomed. 2019, 90, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.A.; Erickson, B.J.; Costouros, J.; Long, N.; Klassen, J.; Araghi, A.; Brown, J.; Setter, K.; Port, J.; Tyndall, W.; et al. Eclipse stemless shoulder prosthesis vs. Univers II shoulder prosthesis: A multicenter, prospective randomized controlled trial. J. Shoulder Elb. Surg. 2020, 29, 2200–2212. [Google Scholar] [CrossRef] [PubMed]
- Wiater, J.M.; Levy, J.C.; Wright, S.A.; Brockmeier, S.F.; Duquin, T.R.; Wright, J.O.; Codd, T.P. Prospective, Blinded, Randomized Controlled Trial of Stemless Versus Stemmed Humeral Components in Anatomic Total Shoulder Arthroplasty: Results at Short-Term Follow-up. J. Bone Jt. Surg. 2020, 102, 1974–1984. [Google Scholar] [CrossRef]
- Mariotti, U.; Motta, P.; Stucchi, A.; Ponti di Sant’Angelo, F. Stemmed versus stemless total shoulder arthroplasty: A preliminary report and short-term results. Musculoskelet. Surg. 2014, 98, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Looney, A.M.; Day, J.; Johnson, J.L.; Johnston, P.S. Outcomes Between Stemmed and Stemless Total Shoulder Arthroplasty: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2022, 6, e22.00077. [Google Scholar] [CrossRef]
- Parada, S.A.; Flurin, P.H.; Wright, T.W.; Zuckerman, J.D.; Elwell, J.A.; Roche, C.P.; Friedman, R.J. Comparison of complication types and rates associated with anatomic and reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2021, 30, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Rajabzadeh-Oghaz, H.; Kumar, V.; Berry, D.B.; Singh, A.; Schoch, B.S.; Aibinder, W.R.; Gobbato, B.; Polakovic, S.; Elwell, J.; Roche, C.P. Impact of Deltoid Computer Tomography Image Data on the Accuracy of Machine Learning Predictions of Clinical Outcomes after Anatomic and Reverse Total Shoulder Arthroplasty. J. Clin. Med. 2024, 13, 1273. [Google Scholar] [CrossRef] [PubMed]
- Habermeyer, P.; Lichtenberg, S.; Tauber, M.; Magosch, P. Midterm results of stemless shoulder arthroplasty: A prospective study. J. Shoulder Elb. Surg. 2015, 24, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Fram, B.; Elder, A.; Namdari, S. Periprosthetic Humeral Fractures in Shoulder Arthroplasty. JBJS Rev. 2019, 7, e6. [Google Scholar] [CrossRef]
- Dukan, R.; Juvenspan, M.; Scheibel, M.; Moroder, P.; Teissier, P.; Werthel, J.D. Non-operative management of humeral periprosthetic fracture after stemless shoulder arthroplasty. Int. Orthop. 2024, 48, 253–259. [Google Scholar] [CrossRef]
| Study | Year | Mean Age Year (SD) | Sex (Male %) | Final Number of rTSA in Analysis | Implant | Mean Follow-Up Months (SD) |
|---|---|---|---|---|---|---|
| A’Court et al. [51] | 2024 | 64.3 (11.4) | 40 | 30 | SMR | 37.5 (14.0) |
| Rosso et al. [50] | 2024 | 70.1 | 54 | 26 | SMR | 46.8 |
| Nabergoj et al. [47] | 2023 | 68.7 | 47 | 115 | EasyTech | 24 |
| Galhoum et al. [48] | 2022 | 70 (7) | NS | 15 | Nano | 27 (6) |
| Schoch et al. [49] | 2021 | 61.2 | 62 | 52 | SMR | 29.3 |
| Micheloni et al. [71] | 2019 | 73.1 (8.0) | 29 | 7 | Verso | 6.4 (1.3) |
| Virani et al. [46] | 2021 | 76 | NS | 22 | Verso | 78 |
| Beck et al. [41] | 2019 | 72.4 (6.7) | 19 | 29 | TESS | 101.6 |
| Levy et al. [45] | 2016 | 74.4 | 20 | 98 | Verso | 50 |
| Moroder et al. [23] | 2016 | 75.6 (4.6) | 29 | 24 | TESS | 35.2 (14.6) |
| von Engelhardt et al. [44] | 2015 | 73.2 (7.8) | NS | 56 | TESS | 17.5 (10.2) |
| Teissier et al. [43] | 2015 | 73 | 70 | 91 | TESS | 41 |
| Kadum et al. [42] | 2014 | 69 | 63 | 16 | TESS | 35 |
| Ballas et al. [40] | 2013 | 74 | 29 | 56 | TESS | 56 |
| Study | Year | Preop. CS (Mean, SD) | Postop. CS (Mean, SD) | Incidence of Complications (%, n) | Incidence of Revision (%, n) |
|---|---|---|---|---|---|
| A’Court et al. [51] | 2024 | NR | NR | 23.3 (7) | 9.0 (3) |
| Rosso et al. [50] | 2024 | 44.1 (18.7) | 83.1 (10.1) | 34.6 (9) | 3.8 (1) |
| Nabergoj et al. [47] | 2023 | 32.5 (10.3) | 61.8 (15.6) | 17.4 (20) | 7.0 (8) |
| Galhoum et al. [48] | 2022 | 30 (18) | 60 (18) | 26.7 (4) | 26.7 (4) |
| Schoch et al. [49] | 2021 | 34.9 (9.8) | 72.4 (8.7) | 3.8 (2) | 3.8 (2) |
| Micheloni et al. [71] | 2019 | 21.6 | 56.9 | 0 (0) | 0 (0) |
| Virani et al. [46] | 2021 | 18 | 72 | 13.6 (3) | 4.5 (1) |
| Beck et al. [41] | 2019 | 13 | 60.5 | 6.9 (2) | 6.9 (2) |
| Levy et al. [45] | 2016 | 14 | 59 | 12.2 (12) | 3.1 (3) |
| Moroder et al. [23] | 2016 | NR | 65.4 (12.9) | 25 (6) | 4.2 (1) |
| von Engelhardt et al. [44] | 2015 | NS | NS | 3.6 (2) | 1.8 (1) |
| Teissier et al. [43] | 2015 | 40 (24) | 68 (12) | 3.3 (3) | 1.1 (1) |
| Kadum et al. [42] | 2014 | NR | NR | 25 (4) | 12.5 (2) |
| Ballas et al. [40] | 2013 | 29 (8) | 62 (12) | 14.3 (8) | 7.1 (4) |
| Shoulders | Incidence (%) | Incidence of All Complications (%) | |
|---|---|---|---|
| Instability and/or dislocation | 16 | 2.5 | 19.5 |
| Humeral implant displacement/malpositioning/migration | 11 | 1.7 | 13.4 |
| Superficial infection | 1 | 0.2 | 1.2 |
| Deep infection | 4 * | 0.6 | 4.9 |
| Hematoma | 4 | 0.6 | 4.9 |
| Periprosthetic fracture (humerus) | 12 | 1.9 | 14.6 |
| Periprosthetic fracture (glenoid) | 2 | 0.3 | 2.4 |
| Periprosthetic fracture (unspecified) | 2 | 0.3 | 2.4 |
| Acromion fracture | 6 | 0.9 | 7.3 |
| Scapular spine fracture | 1 | 0.2 | 1.2 |
| Clavicle fracture | 1 | 0.2 | 1.2 |
| Glenosphere disassembly from baseplate | 8 | 1.3 | 9.8 |
| Dysesthesia in the hand | 3 | 0.5 | 3.7 |
| Postoperative stiffness | 3 | 0.5 | 3.7 |
| Subscapularis rupture | 2 | 0.3 | 2.4 |
| Symptomatic mesacromion | 1 | 0.2 | 1.2 |
| Chronic scapulothoracic conflict | 1 | 0.2 | 1.2 |
| Glenoid ossification | 1 | 0.2 | 1.2 |
| Glenoid and humeral loosening | 1 | 0.2 | 1.2 |
| Asymmetrical polyethylene | 1 | 0.2 | 1.2 |
| Incorrectly positioned humeral base plate | 1 | 0.2 | 1.2 |
| Overall complications | 82 | 12.9 | 100.0 |
| Study | Year | Mean Age Year (SD) | Sex (Male %) | Mean BMI kg/m2 (SD) | Final Number of rTSA in Analysis | Implant | Mean Follow-Up Months (SD) | Main Findings |
|---|---|---|---|---|---|---|---|---|
| A’Court et al. [51] | 2024 | 76.5 (6.3) vs. 64.3 (11.4) | 53 vs. 40 | 29.2 (5.7) vs. 28.5 (4.5) | 30 vs. 30 | SMR (Lima Corporate) | 31.3 (8.7) vs. 37.5 (14.0) |
|
| Moroder et al. [23] | 2016 | 74.3 (4.6) vs. 75.6 (4.6) | 29 vs. 29 | NR | 24 vs. 24 | Delta XTEND (Depuy) TESS (Zimmer Biomet) | 34.2 (10.5) vs. 35.2 (14.6) |
|
| Kadum et al. [42] | 2014 | 72 vs. 69 | 27 vs. 63 | NR | 15 vs. 16 | TESS (Zimmer Biomet) | 35 vs. 35 |
|
| Study | Year | Mean Age Year (SD) | Sex (Male %) | Mean BMI kg/m2 (SD) | Final Number of aTSA in Analysis | Implant | Follow-Up | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Romeo et al. [72] | 2020 | 66.0 (median) vs. 66.0 (median) | 73 vs. 69 | 31.8 (median) vs. 30.3 (median) | 68 vs. 143 | Univers II (Arthrex) Eclipse (Arthrex) | 2 years |
|
| Wiater et al. [73] | 2020 | 62.1 (9.6) vs. 63.1 (9.0) | 65 vs. 67 | 30.1 (5.3) vs. 30.6 (5.8) | 123 vs. 116 | Comprehensive (Zimmer Biomet) Nano (Zimmer Biomet) | 2 years |
|
| Uschok et al. [22] | 2017 | 69 vs. 65 | 35 vs. 50 | NR | 18 vs. 15 (2 years) 15 vs. 14 (5 years) | Univers II (Arthrex) Eclipse (Arthrex) | 2 and 5 years |
|
| Mariotti et al. [74] | 2014 | NS | NR | NR | 10 vs. 9 | Aequalis (Stryker Tornier) | 2 years |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatta, T.; Mashiko, R.; Kawakami, J.; Matsuzawa, G.; Ogata, Y.; Hatta, W. Evolution of Stemless Reverse Shoulder Arthroplasty: Current Indications, Outcomes, and Future Prospects. J. Clin. Med. 2024, 13, 3813. https://doi.org/10.3390/jcm13133813
Hatta T, Mashiko R, Kawakami J, Matsuzawa G, Ogata Y, Hatta W. Evolution of Stemless Reverse Shoulder Arthroplasty: Current Indications, Outcomes, and Future Prospects. Journal of Clinical Medicine. 2024; 13(13):3813. https://doi.org/10.3390/jcm13133813
Chicago/Turabian StyleHatta, Taku, Ryosuke Mashiko, Jun Kawakami, Gaku Matsuzawa, Yohei Ogata, and Waku Hatta. 2024. "Evolution of Stemless Reverse Shoulder Arthroplasty: Current Indications, Outcomes, and Future Prospects" Journal of Clinical Medicine 13, no. 13: 3813. https://doi.org/10.3390/jcm13133813
APA StyleHatta, T., Mashiko, R., Kawakami, J., Matsuzawa, G., Ogata, Y., & Hatta, W. (2024). Evolution of Stemless Reverse Shoulder Arthroplasty: Current Indications, Outcomes, and Future Prospects. Journal of Clinical Medicine, 13(13), 3813. https://doi.org/10.3390/jcm13133813