Increased Risk for Infections and Allergic Disease in Hereditary Hemorrhagic Telangiectasia
Abstract
1. Introduction
2. Materials and Methods
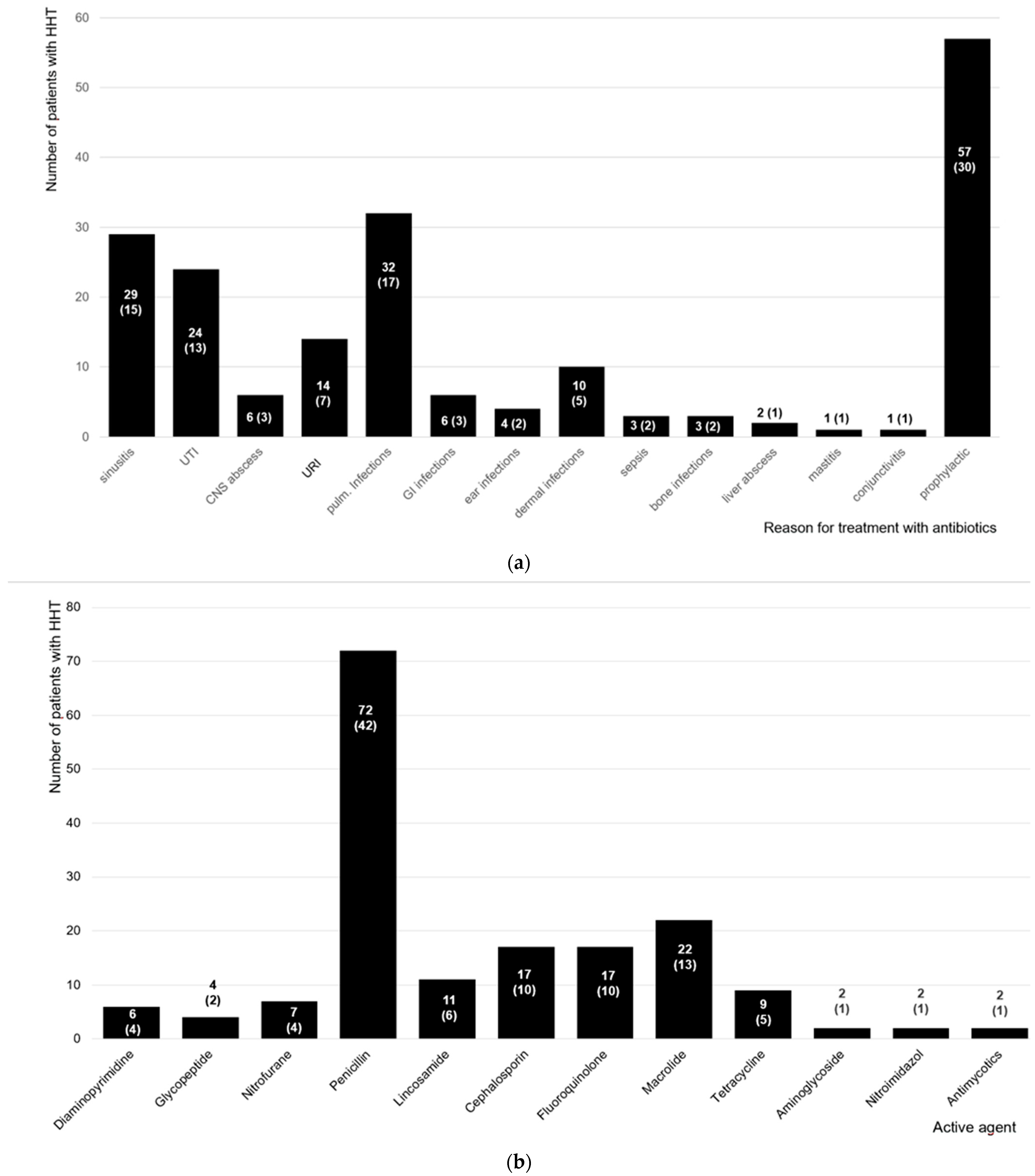
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kjeldsen, A.D.; Vase, P.; Green, A. Hereditary haemorrhagic telangiectasia: A population-based study of prevalence and mortality in Danish patients. J. Intern. Med. 1999, 245, 31–39. [Google Scholar] [CrossRef]
- Guttmacher, A.E.; Marchuk, D.A.; White, R.I., Jr. Hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 1995, 333, 918–924. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Guttmacher, A.E.; Buscarini, E.; Faughnan, M.E.; Hyland, R.H.; Westermann, C.J.; Kjeldsen, A.D.; Plauchu, H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am. J. Med. Genet. 2000, 91, 66–67. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Hughes, J.M.; Tuddenham, E.G.; Temperley, I.; Perembelon, Y.F.; Scott, J.; Seidman, C.E.; Seidman, J.G. A gene for hereditary haemorrhagic telangiectasia maps to chromosome 9q3. Nat. Genet. 1994, 6, 205–209. [Google Scholar] [CrossRef]
- McAllister, K.A.; Grogg, K.M.; Johnson, D.W.; Gallione, C.J.; Baldwin, M.A.; Jackson, C.E.; Helmbold, E.A.; Markel, D.S.; McKinnon, W.C.; Murrell, J.; et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994, 8, 345–351. [Google Scholar] [CrossRef]
- Gallione, C.J.; Repetto, G.M.; Legius, E.; Rustgi, A.K.; Schelley, S.L.; Tejpar, S.; Mitchell, G.; Drouin, E.; Westermann, C.J.; Marchuk, D.A. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 2004, 363, 852–859. [Google Scholar] [CrossRef]
- Amati, L.; Passeri, M.E.; Resta, F.; Triggiani, V.; Jirillo, E.; Sabba, C. Ablation of T-helper 1 cell derived cytokines and of monocyte-derived tumor necrosis factor-alpha in hereditary hemorrhagic telangiectasia: Immunological consequences and clinical considerations. Curr. Pharm. Des. 2006, 12, 1201–1208. [Google Scholar] [CrossRef]
- Cirulli, A.; Loria, M.P.; Dambra, P.; Di Serio, F.; Ventura, M.T.; Amati, L.; Jirillo, E.; Sabba, C. Patients with Hereditary Hemorrhagic Telangectasia (HHT) exhibit a deficit of polymorphonuclear cell and monocyte oxidative burst and phagocytosis: A possible correlation with altered adaptive immune responsiveness in HHT. Curr. Pharm. Des. 2006, 12, 1209–1215. [Google Scholar] [CrossRef]
- Lenato, G.M.; Suppressa, P.; Giordano, P.; Guanti, G.; Guastamacchia, E.; Triggiani, V.; Amati, L.; Resta, F.; Covelli, V.; Jirillo, E.; et al. Hereditary haemorrhagic telangiectasia: A rare disease as a model for the study of human atherosclerosis. Curr. Pharm. Des. 2007, 13, 3656–3664. [Google Scholar] [CrossRef]
- Ojeda-Fernandez, L.; Recio-Poveda, L.; Aristorena, M.; Lastres, P.; Blanco, F.J.; Sanz-Rodriguez, F.; Gallardo-Vara, E.; de las Casas-Engel, M.; Corbi, A.; Arthur, H.M.; et al. Mice Lacking Endoglin in Macrophages Show an Impaired Immune Response. PLoS Genet. 2016, 12, e1005935. [Google Scholar] [CrossRef]
- Moussouttas, M.; Fayad, P.; Rosenblatt, M.; Hashimoto, M.; Pollak, J.; Henderson, K.; Ma, T.Y.; White, R.I. Pulmonary arteriovenous malformations: Cerebral ischemia and neurologic manifestations. Neurology 2000, 55, 959–964. [Google Scholar] [CrossRef]
- Dong, S.L.; Reynolds, S.F.; Steiner, I.P. Brain abscess in patients with hereditary hemorrhagic telangiectasia: Case report and literature review. J. Emerg. Med. 2001, 20, 247–251. [Google Scholar] [CrossRef]
- Suddock, J.T.; Crookston, K.P. Transfusion Reactions. In StatPearls; Ineligible Companies: Treasure Island, FL, USA, 2024. [Google Scholar]
- Guilhem, A.; Malcus, C.; Clarivet, B.; Plauchu, H.; Dupuis-Girod, S. Immunological abnormalities associated with hereditary haemorrhagic telangiectasia. J. Intern. Med. 2013, 274, 351–362. [Google Scholar] [CrossRef]
- Droege, F.; Lueb, C.; Thangavelu, K.; Stuck, B.A.; Lang, S.; Geisthoff, U. Nasal self-packing for epistaxis in Hereditary Hemorrhagic Telangiectasia increases quality of life. Rhinology 2019, 57, 231–239. [Google Scholar] [CrossRef]
- Hosman, A.E.; Devlin, H.L.; Silva, B.M.; Shovlin, C.L. Specific cancer rates may differ in patients with hereditary haemorrhagic telangiectasia compared to controls. Orphanet J. Rare Dis. 2013, 8, 195. [Google Scholar] [CrossRef]
- Hoag, J.B.; Terry, P.; Mitchell, S.; Reh, D.; Merlo, C.A. An epistaxis severity score for hereditary hemorrhagic telangiectasia. Laryngoscope 2010, 120, 838–843. [Google Scholar] [CrossRef]
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. In Vitamin and Mineral Nutrition Information System; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Droege, F.; Pylaeva, E.; Siakaeva, E.; Bordbari, S.; Spyra, I.; Thangavelu, K.; Lueb, C.; Domnich, M.; Lang, S.; Geisthoff, U.; et al. Impaired Release of Neutrophil Extracellular Traps and Anemia-Associated T Cell Deficiency in Hereditary Hemorrhagic Telangiectasia. J. Clin. Med. 2020, 9, 767. [Google Scholar] [CrossRef]
- Aagaard, K.S.; Kjeldsen, A.D.; Torring, P.M.; Green, A. Comorbidity among HHT patients and their controls in a 20 years follow-up period. Orphanet J. Rare Dis. 2018, 13, 223. [Google Scholar] [CrossRef]
- Musso, M.; Capone, A.; Chinello, P.; Di Bella, S.; Galati, V.; Noto, P.; Taglietti, F.; Topino, S.; Petrosillo, N. Extra-cerebral severe infections associated with haemorrhagic hereditary telangiectasia (Rendu-Osler-Weber Disease): Five cases and a review of the literature. Infez. Med. 2014, 22, 50–56. [Google Scholar]
- Duval, X.; Djendli, S.; Le Moing, V.; Longuet, P.; Barry, B.; Leport, C.; Vilde, J.L. Recurrent Staphylococcus aureus extracerebral infections complicating hereditary hemorrhagic telangiectasia (Osler-Rendu-Weber disease). Am. J. Med. 2001, 110, 671–672. [Google Scholar] [CrossRef]
- Hull, H.F.; Mann, J.M.; Sands, C.J.; Gregg, S.H.; Kaufman, P.W. Toxic shock syndrome related to nasal packing. Arch. Otolaryngol. 1983, 109, 624–626. [Google Scholar] [CrossRef]
- Herzon, F.S. Bacteremia and local infections with nasal packing. Arch. Otolaryngol. 1971, 94, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Boumis, E.; Capone, A.; Galati, V.; Venditti, C.; Petrosillo, N. Probiotics and infective endocarditis in patients with hereditary hemorrhagic telangiectasia: A clinical case and a review of the literature. BMC Infect. Dis. 2018, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Barham, H.P.; Zhang, A.S.; Christensen, J.M.; Sacks, R.; Harvey, R.J. Acute radiology rarely confirms sinus disease in suspected recurrent acute rhinosinusitis. Int. Forum. Allergy Rhinol. 2017, 7, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Danai, P.; Martin, G.S. Epidemiology of sepsis: Recent advances. Curr. Infect. Dis. Rep. 2005, 7, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Tiemersma, E.W.; Monnet, D.L.; Bruinsma, N.; Skov, R.; Monen, J.C.; Grundmann, H. Staphylococcus aureus bacteremia, Europe. Emerg. Infect. Dis. 2005, 11, 1798–1799. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S. Bidirectional regulation of macrophage function by TGF-beta. Microbes Infect. 1999, 1, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K. Nutrition and the immune system from birth to old age. Eur. J. Clin. Nutr. 2002, 56 (Suppl. S3), S73–S76. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, C.; Wu, Q.; An, P.; Huang, L.; Wang, J.; Chen, C.; Chen, X.; Zhang, F.; Ma, L.; et al. Iron-dependent histone 3 lysine 9 demethylation controls B cell proliferation and humoral immune responses. Nat. Commun. 2019, 10, 2935. [Google Scholar] [CrossRef]
- Elmadfa, I.; Meyer, A.L. The Role of the Status of Selected Micronutrients in Shaping the Immune Function. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1100–1115. [Google Scholar] [CrossRef]
- Cherayil, B.J. Iron and immunity: Immunological consequences of iron deficiency and overload. Arch. Immunol. Ther. Exp. 2010, 58, 407–415. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Buscarini, E.; Sabba, C.; Mager, H.J.; Kjeldsen, A.D.; Pagella, F.; Sure, U.; Ugolini, S.; Torring, P.M.; Suppressa, P.; et al. The European Rare Disease Network for HHT Frameworks for management of hereditary haemorrhagic telangiectasia in general and speciality care. Eur. J. Med. Genet. 2022, 65, 104370. [Google Scholar] [CrossRef]
- Swanson, D.L.; Dahl, M.V. Embolic abscesses in hereditary hemorrhagic telangiectasia. J. Am. Acad. Dermatol. 1991, 24, 580–583. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Giraud, S.; Decullier, E.; Lesca, G.; Cottin, V.; Faure, F.; Merrot, O.; Saurin, J.C.; Cordier, J.F.; Plauchu, H. Hemorrhagic hereditary telangiectasia (Rendu-Osler disease) and infectious diseases: An underestimated association. Clin. Infect. Dis. 2007, 44, 841–845. [Google Scholar] [CrossRef]
- Kallenbach, M.; Dittberner, A.; Boeger, D.; Buentzel, J.; Kaftan, H.; Hoffmann, K.; Jecker, P.; Mueller, A.; Radtke, G.; Guntinas-Lichius, O. Hospitalization for epistaxis: A population-based healthcare research study in Thuringia, Germany. Eur. Arch. Otorhinolaryngol. 2020, 277, 1659–1666. [Google Scholar] [CrossRef]
- De Angelis, G.; Murthy, A.; Beyersmann, J.; Harbarth, S. Estimating the impact of healthcare-associated infections on length of stay and costs. Clin. Microbiol. Infect. 2010, 16, 1729–1735. [Google Scholar] [CrossRef]
- Roberts, R.R.; Scott, R.D., 2nd; Hota, B.; Kampe, L.M.; Abbasi, F.; Schabowski, S.; Ahmad, I.; Ciavarella, G.G.; Cordell, R.; Solomon, S.L.; et al. Costs attributable to healthcare-acquired infection in hospitalized adults and a comparison of economic methods. Med. Care 2010, 48, 1026–1035. [Google Scholar] [CrossRef]
- Tiemersma, E.W.; Bronzwaer, S.L.; Lyytikainen, O.; Degener, J.E.; Schrijnemakers, P.; Bruinsma, N.; Monen, J.; Witte, W.; Grundman, H.; European Antimicrobial Resistance Surveillance System Participants. Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002. Emerg. Infect. Dis. 2004, 10, 1627–1634. [Google Scholar] [CrossRef]
- Faughnan, M.E.; Mager, J.J.; Hetts, S.W.; Palda, V.A.; Ratjen, F. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann. Intern. Med. 2021, 174, 1035–1036. [Google Scholar] [CrossRef]
- Brinjikji, W.; Iyer, V.N.; Wood, C.P.; Lanzino, G. Prevalence and characteristics of brain arteriovenous malformations in hereditary hemorrhagic telangiectasia: A systematic review and meta-analysis. J. Neurosurg. 2017, 127, 302–310. [Google Scholar] [CrossRef]
- Vase, P.; Grove, O. Gastrointestinal lesions in hereditary hemorrhagic telangiectasia. Gastroenterology 1986, 91, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Sabba, C.; Pompili, M. Review article: The hepatic manifestations of hereditary haemorrhagic telangiectasia. Aliment Pharmacol. Ther. 2008, 28, 523–533. [Google Scholar] [CrossRef] [PubMed]


| All Patients (n = 430) | Female (n = 295) | Male (n = 135) | |||
|---|---|---|---|---|---|
| Family history | yes | 402 (94) | 281 (95) | 121 (90) | |
| no | 17 (4) | 7 (2) | 10 (7) | ||
| n.k. | 11 (3) | 7 (2) | 4 (3) | ||
| mv | 0 | 0 | 0 | ||
| Epistaxis | yes | 411 (96) | 279 (95) | 132 (96) | |
| no | 18 (4) | 16 (5) | 2 (2) | ||
| mv | 1 (0.2) | 0 | 1 (2) | ||
| TAE | yes | 412 (96) | 279 (95) | 133 (99) | |
| no | 12 (3) | 10 (3) | 2 (2) | ||
| n.k. | 6 (1) | 6 (2) | 0 | ||
| mv | 0 | 0 | 0 | ||
| Visceral lesions | GI | yes | 148 (36) | 97 (34) | 51 (40) |
| no | 121 (30) | 80 (28) | 41 (32) | ||
| n.k. | 140 (34) | 105 (37) | 35 (28) | ||
| mv | 21 | 13 | 8 | ||
| PAVMs | yes | 185 (44) | 131 (45) | 54 (42) | |
| no | 153 (37) | 101 (35) | 52 (41) | ||
| n.k. | 80 (19) | 58 (20) | 22 (17) | ||
| mv | 12 | 5 | 7 | ||
| HVM | yes | 96 (24) | 72 (26) | 24 (19) | |
| no | 153 (38) | 94 (34) | 59 (47) | ||
| n.k. | 157 (39) | 114 (41) | 43 (34) | ||
| mv | 24 | 15 | 9 | ||
| CVM | yes | 55 (13) | 38 (13) | 17 (13) | |
| no | 247 (60) | 168 (59) | 79 (62) | ||
| n.k. | 108 (26) | 77 (27) | 31 (24) | ||
| mv | 20 | 12 | 8 | ||
| All Patients (n = 175) | Female (n = 124) | Male (n = 51) | |||
| Genetics | HHT Type 1 | 65 (37) | 50 (40) | 15 (29) | |
| HHT Type 2 | 106 (61) | 72 (58) | 34 (67) | ||
| SMAD 4 | 3 (2) | 1 (1) | 2 (4) | ||
| HHT Type 5 | 1 (0.2) | 1 (1) | 0 | ||
| Patients with HHT n (%) | Non-Affected Partner/Friend n (%) | Odds | 95% Confidence Interval | p-Value 1 | |
|---|---|---|---|---|---|
| Infections | |||||
| Sinusitis | 152 (40) | 60 (16) | 2.53 | 1.89–3.55 | 0.001 |
| UTI | 119 (32) | 56 (15) | 2.13 | 1.54–3.03 | 0.001 |
| CNS abscess | 18 (5) | 5 (1) | 3.61 | 1.29–12.40 | 0.011 |
| IUA | 94 (25) | 91 (24) | 1.03 | 0.76–1.41 | 0.883 |
| Pulm. infections | 66 (18) | 32 (9) | 2.06 | 1.35–3.33 | 0.001 |
| GI infections | 75 (20) | 51 (14) | 1.47 | 1.02–2.17 | 0.040 |
| Dermal infections | 38 (10) | 23 (6) | 1.65 | 0.97–3.03 | 0.072 |
| Sepsis | 19 (5) | 15 (4) | 1.26 | 0.60–2.86 | 0.607 |
| Bone infections | 26 (7) | 28 (8) | 0.93 | 0.51–1.67 | 0.892 |
| Liver abscess | 30 (8) | 2 (1) | 15 | 3.81–129.54 | 0.001 |
| Wound infections | 41 (11) | 32 (9) | 1.28 | 0.79–2.12 | 0.349 |
| Allergies | |||||
| Any allergy | 112 (43) | 43 (22) | 2.61 | 1.85–3.91 | 0.001 |
| Hay fever/asthma | 100 (46) | 47 (29) | 2.13 | 1.51–3.15 | 0.001 |
| Drug/food allergies | 82 (77) | 31 (56) | 2.65 | 1.77–4.33 | 0.001 |
| Contact allergies | 41 (79) | 18 (62) | 2.28 | 1.32–4.58 | 0.004 |
| Anemia | ESS | Iron Supp. | Blood Transfusion | Nasal Surgery | Nasal Packing | PAVMs | |
|---|---|---|---|---|---|---|---|
| Sinusitis | 0.27 0.09–0.79 | 1.43 0.64–3.20 | 0.42 0.15–1.15 | 1.24 0.58–2.64 | 1.28 0.69–2.39 | 4.95 2.85–8.59 | 1.41 0.72–2.75 |
| UTI | 0.85 0.36–2.01 | 0.54 0.22–1.37 | 0.84 0.36–1.98 | 0.75 0.33–1.71 | 0.69 0.35–1.38 | 0.31 0.162–0.6 | 1.12 0.54–2.32 |
| CNS abscess | - | - | 0.65 0.06–7.32 | 1.25 0.09–17.65 | 1.73 0.22–13.67 | 3.33 0.38–29.39 | 1.88 0.13–26.32 |
| Other abscesses | - | 2.33 0.74–7.35 | 9.00 0.44–183.97 | - | - | 3.14 0.17–57.08 | - |
| IUA | 1.16 0.55–2.46 | 0.80 0.37–1.75 | 0.88 0.39–1.97 | 1.16 0.55–2.49 | 1.00 0.54–1.87 | 0.85 0.45–1.62 | 0.95 0.50–1.83 |
| Pulm. infections | 0.89 0.28–2.85 | 0.87 0.29–2.62 | 0.8 0.23–2.78 | 1.49 0.54–4.08 | 1.28 0.51–3.25 | 1.19 0.44–3.22 | 8.84 3.69–21.20 |
| GI infections | 0.82 0.32–2.10 | 0.78 0.27–2.24 | 0.37 0.13–1.08 | 2.98 0.99–8.99 | 1.43 0.66–3.11 | 1.31 0.57–3.02 | 1.01 0.41–2.45 |
| Dermal infections | 2.78 0.59–13.04 | 2.00 0.47–8.49 | - | 1.46 0.36–5.91 | 0.83 0.26–2.68 | 5.30 1.40–20.12 | 1.46 0.45–4.66 |
| Sepsis | 0.46 0.04–4.98 | - | 9.00 0.92–88.16 | 2.5 0.37–16.89 | 0.4 0.07–2.45 | 2.13 0.31–14.73 | 0.4 0.08–2.12 |
| Bone infections | 0.40 0.03–4,74 | 1.50 0.21–10.81 | 0.66 0.13–3.28 | 1.27 0.33–4.98 | 1.14 0.35–3.78 | 0.69 0.20–2.46 | 0.66 0.19–2.31 |
| Wound infections | 1.36 0.31–5.97 | 0.53 0.11–2.43 | 0.96 0.20–4.62 | 1.77 0.61–5.17 | 1.58 0.58–4.29 | 4.53 1.29–16.25 | 2.73 0.94–7.89 |
| Allergies | 1.34 0.77–2.35 | 0.68 0.38–1.21 | 1.22 0.70–2.11 | 1.14 0.69–1.87 | 1.05 0.68–1.64 | 0.92 0.57–1.48 | 1.31 0.82–2.07 |
| Hay fever/asthma | 0.618 0.37–1.04 | 1.03 0.61–1.74 | 1.03 0.61–1.75 | 0.94 0.59–1.50 | 1.39 0.92–2.10 | 1.01 0.65–1.58 | 1.23 0.80–1.91 |
| Drug/food allergies | 0.98 0.55–1.71 | 0.72 0.41–1.26 | 1.48 0.78–2.79 | 0.88 0.53–1.48 | 1.11 0.70–1.76 | 1.17 0.70–1.93 | 1.61 0.98–2.66 |
| Contact allergies | 1.10 0.52–2.30 | 0.63 0.29–1.37 | 1.05 0.49–2.26 | 0.96 0.47–1.94 | 0.81 0.45–1.46 | 1.80 0.87–3.71 | 1.33 0.69–2.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Droege, F.; König, J.; Lang, K.S.; Jablonska, J.; Pylaeva, E.; Huckenbeck, C.; Wrobeln, A.; Duerig, I.; Thangavelu, K.; Lang, S.; et al. Increased Risk for Infections and Allergic Disease in Hereditary Hemorrhagic Telangiectasia. J. Clin. Med. 2024, 13, 3752. https://doi.org/10.3390/jcm13133752
Droege F, König J, Lang KS, Jablonska J, Pylaeva E, Huckenbeck C, Wrobeln A, Duerig I, Thangavelu K, Lang S, et al. Increased Risk for Infections and Allergic Disease in Hereditary Hemorrhagic Telangiectasia. Journal of Clinical Medicine. 2024; 13(13):3752. https://doi.org/10.3390/jcm13133752
Chicago/Turabian StyleDroege, Freya, Jochem König, Karl S. Lang, Jadwiga Jablonska, Ekaterina Pylaeva, Carolin Huckenbeck, Anna Wrobeln, Inga Duerig, Kruthika Thangavelu, Stephan Lang, and et al. 2024. "Increased Risk for Infections and Allergic Disease in Hereditary Hemorrhagic Telangiectasia" Journal of Clinical Medicine 13, no. 13: 3752. https://doi.org/10.3390/jcm13133752
APA StyleDroege, F., König, J., Lang, K. S., Jablonska, J., Pylaeva, E., Huckenbeck, C., Wrobeln, A., Duerig, I., Thangavelu, K., Lang, S., & Geisthoff, U. (2024). Increased Risk for Infections and Allergic Disease in Hereditary Hemorrhagic Telangiectasia. Journal of Clinical Medicine, 13(13), 3752. https://doi.org/10.3390/jcm13133752







