Anatomical Variants of the Renal Veins and Their Relationship with Morphofunctional Alterations of the Kidney: A Systematic Review and Meta-Analysis of Prevalence
Abstract
1. Introduction
2. Methodology
2.1. Protocol and Registration
2.2. Electronic Search
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Collection Process
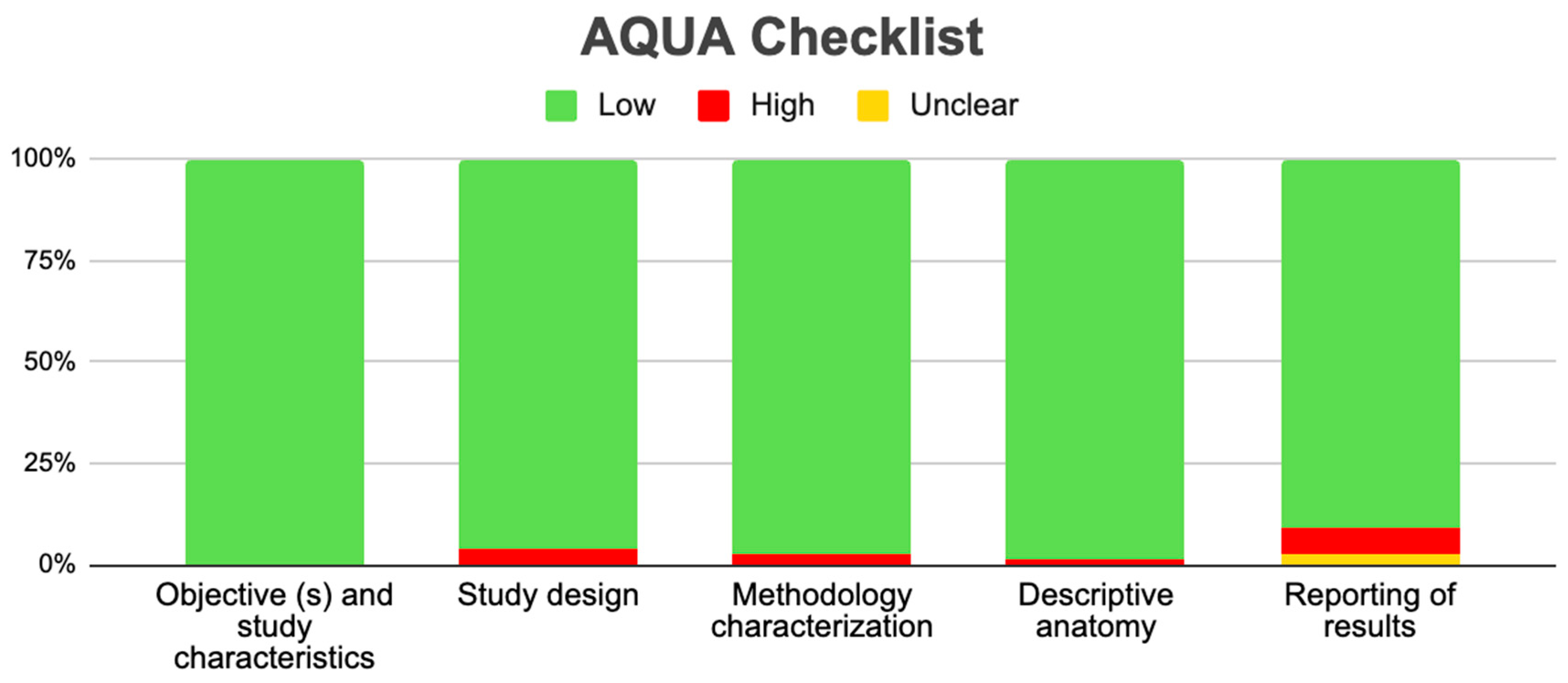
2.6. Assessment of the Methodological Quality of the Included Studies
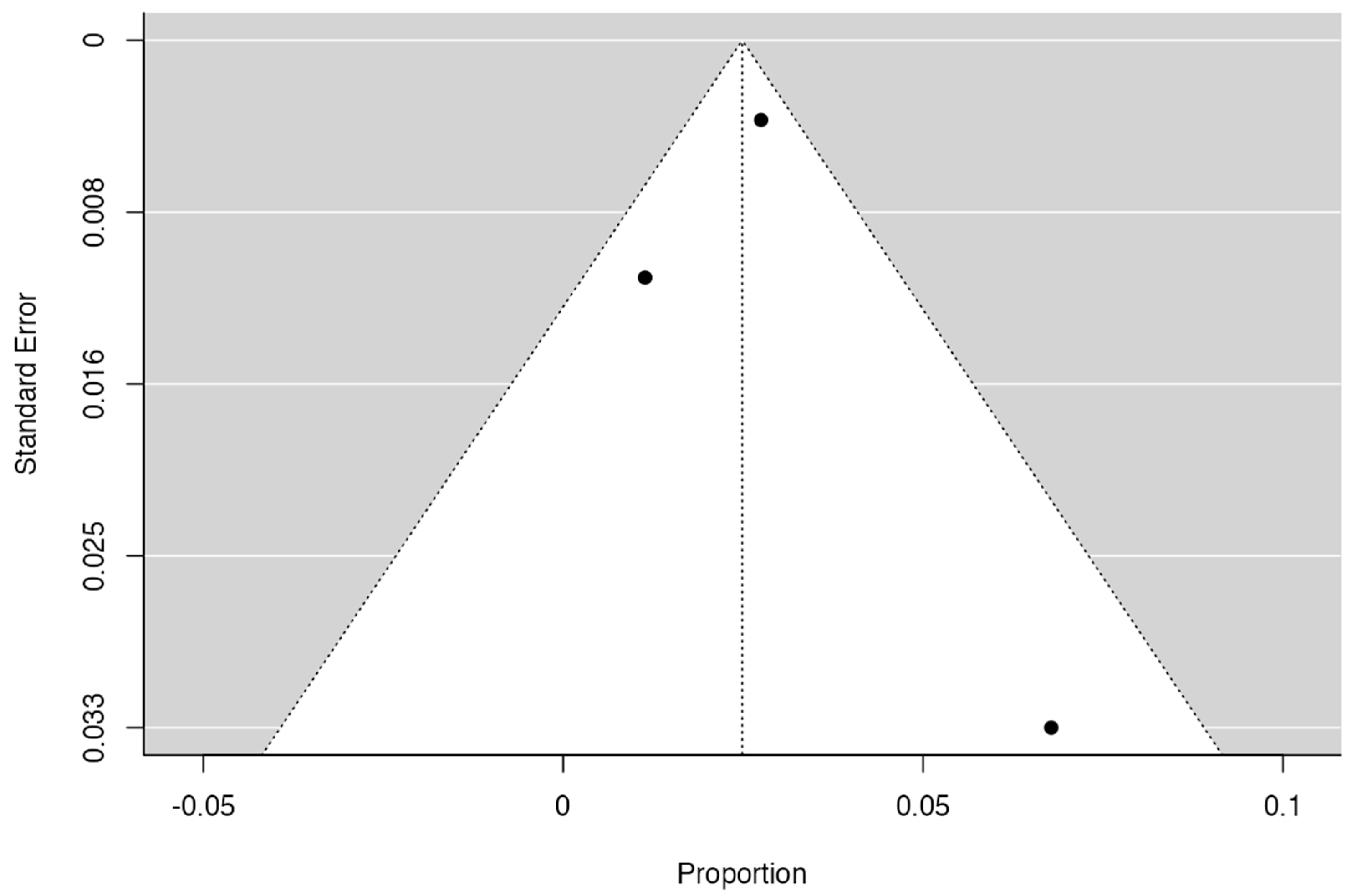
2.7. Publication Bias
2.8. Statistical Methods
3. Results
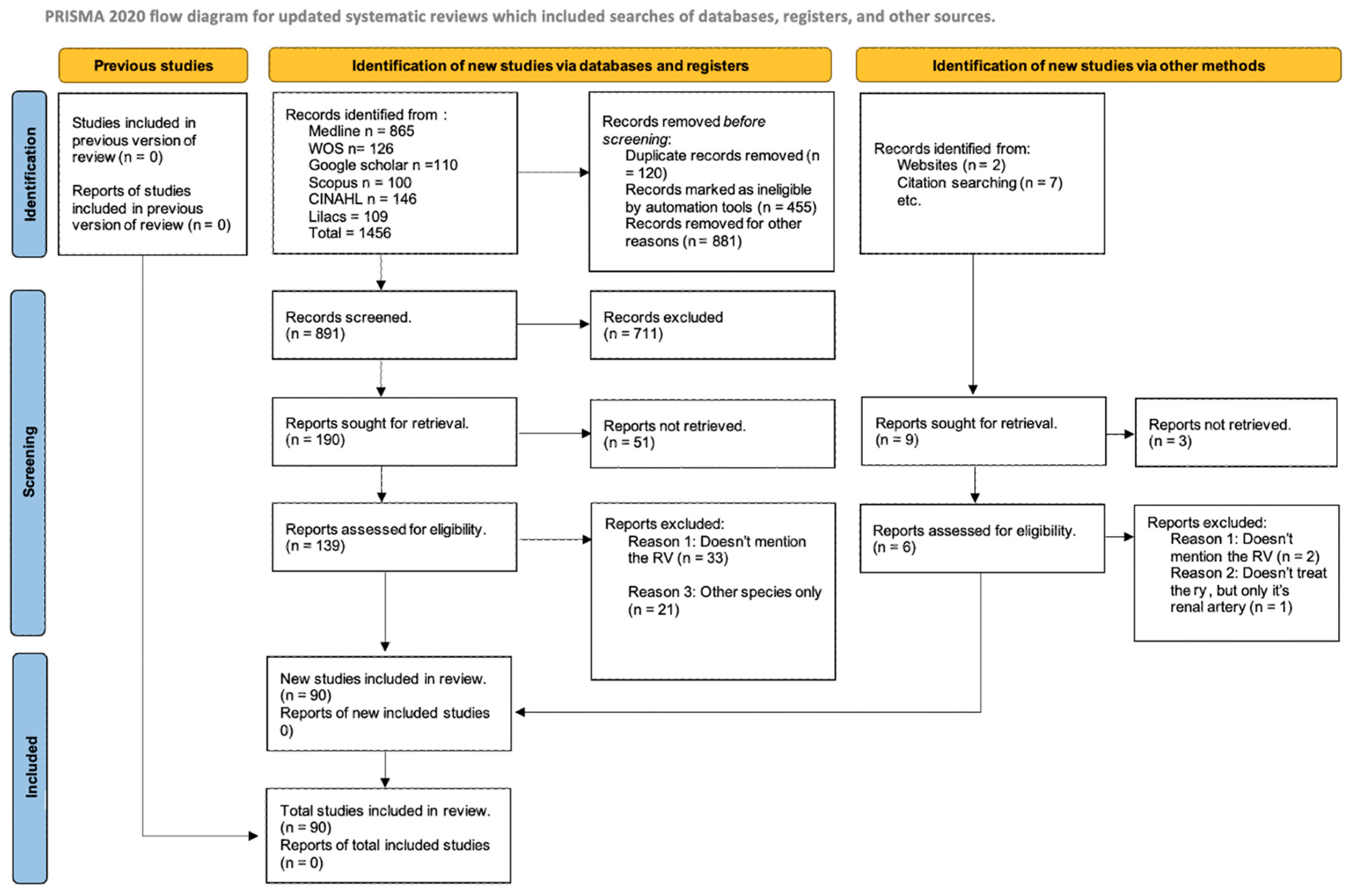
3.1. Included Articles
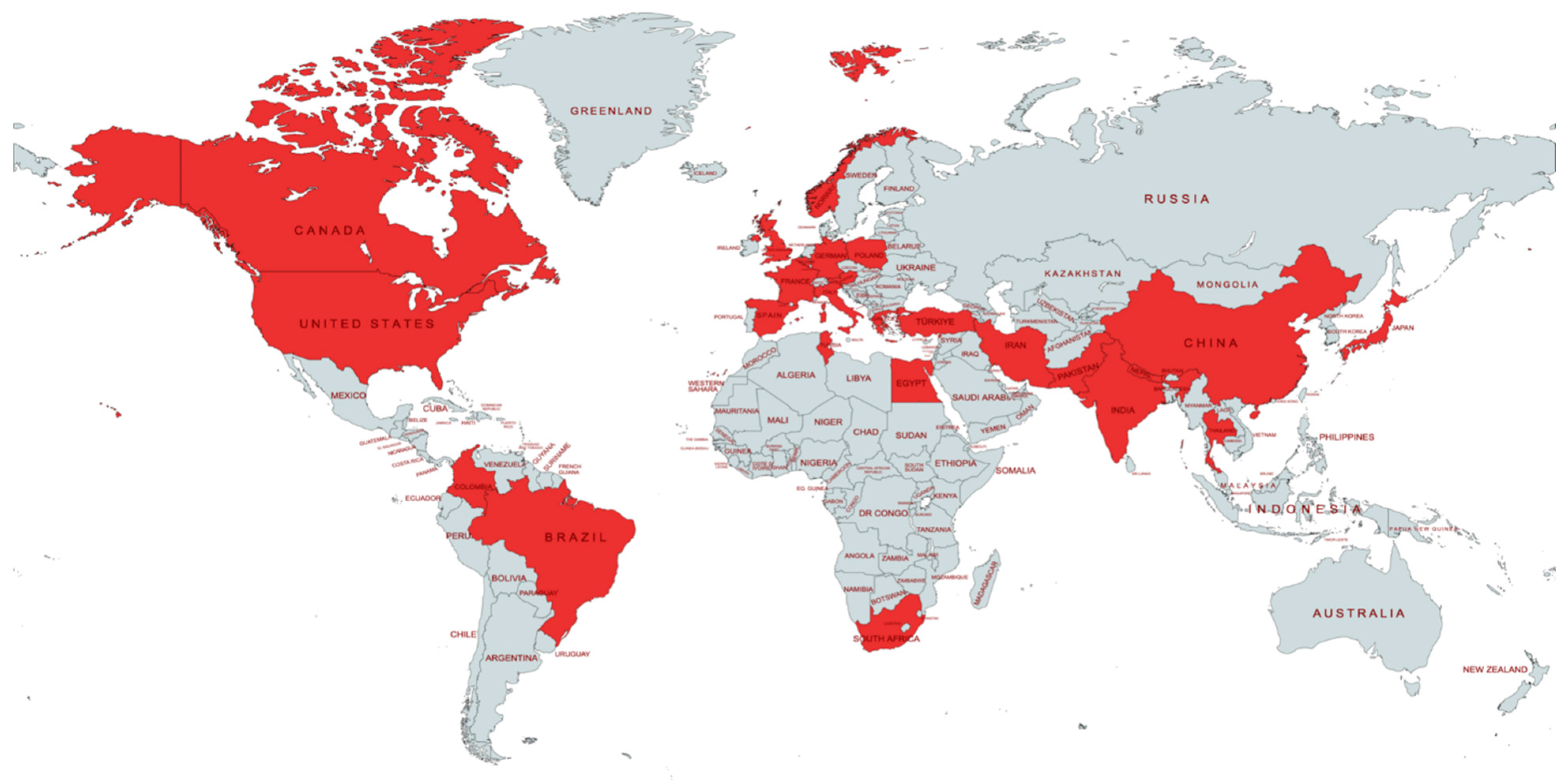
3.2. Characteristics of the Studies and the Study Population
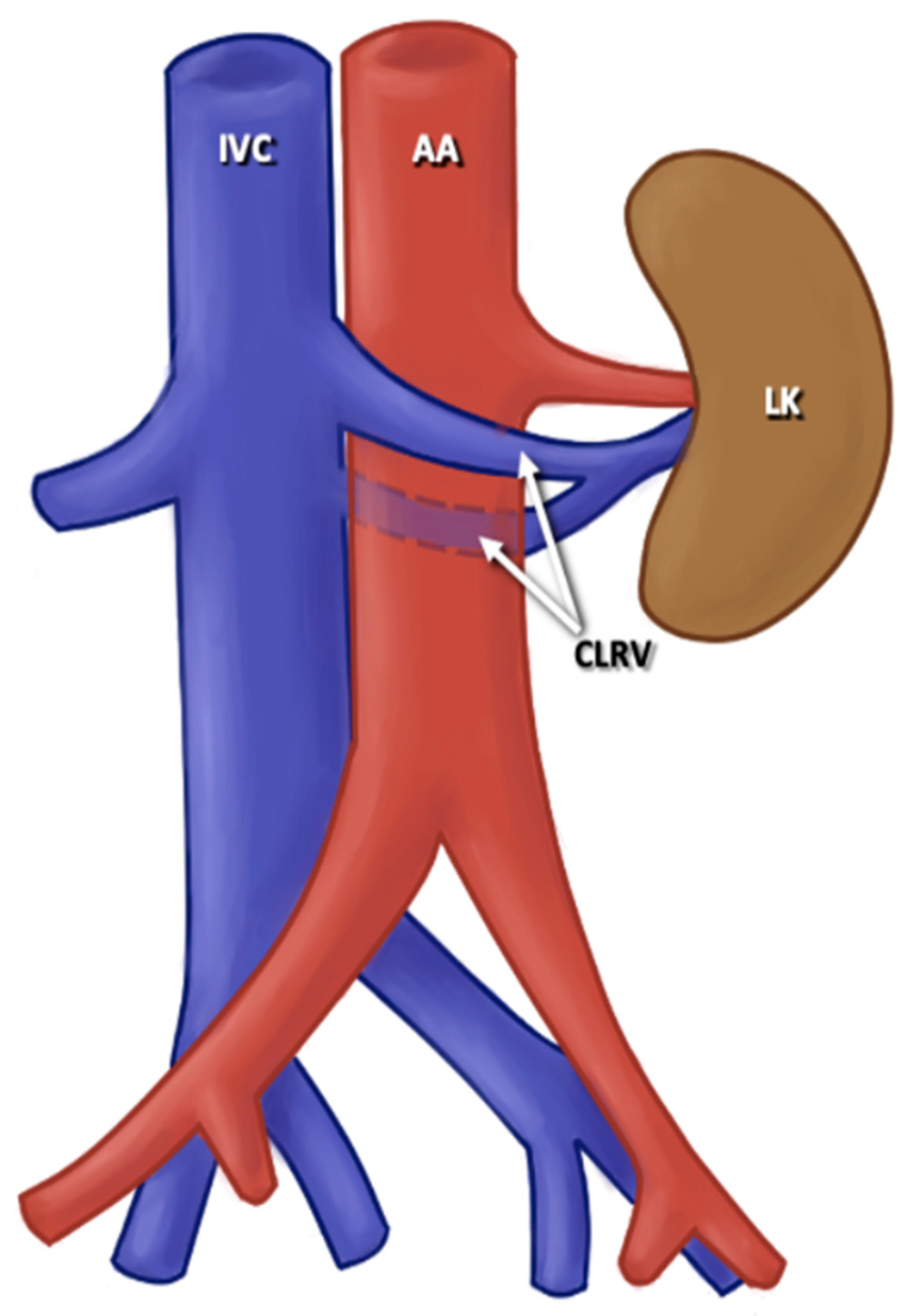
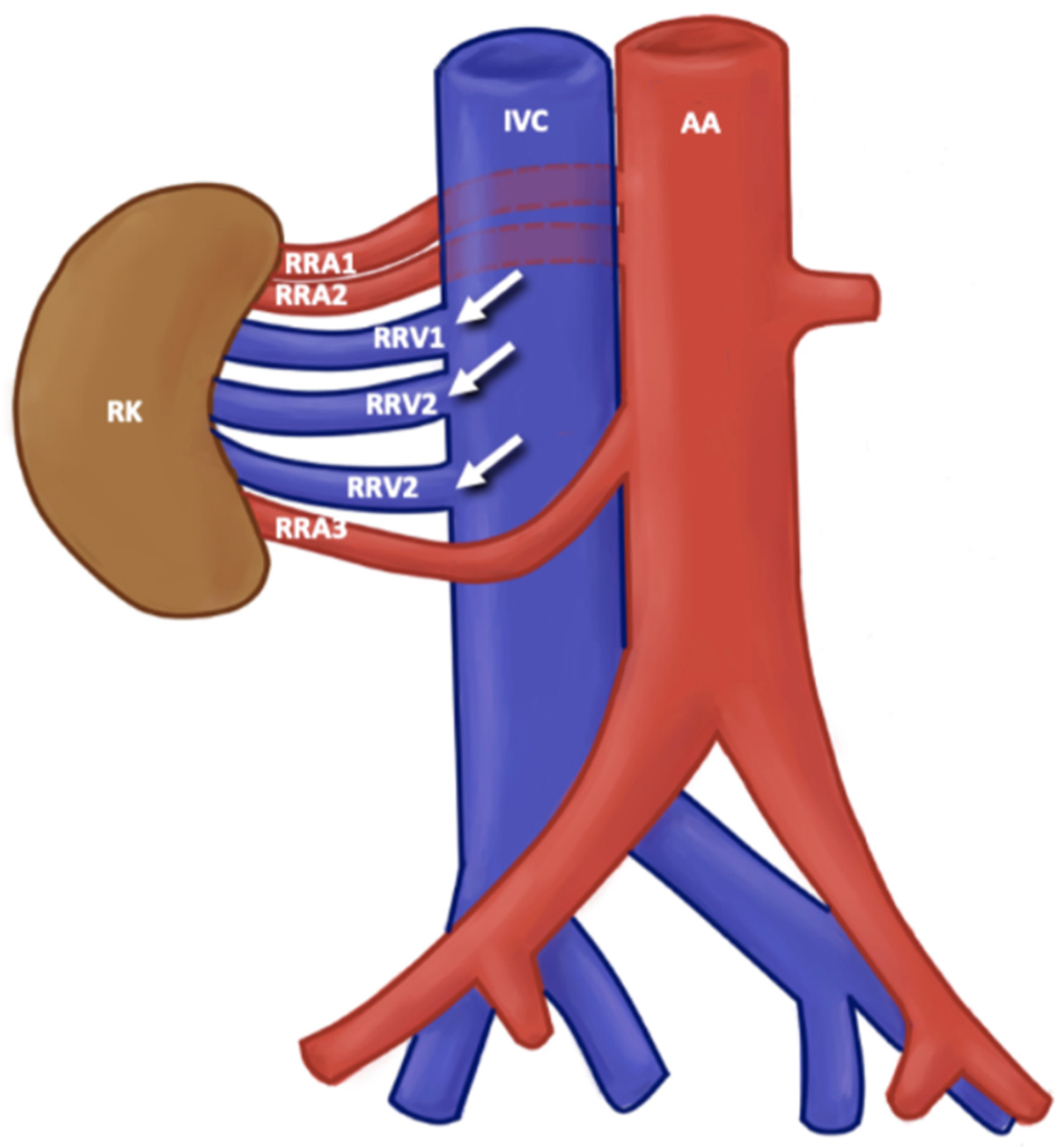
3.3. Description of Variants
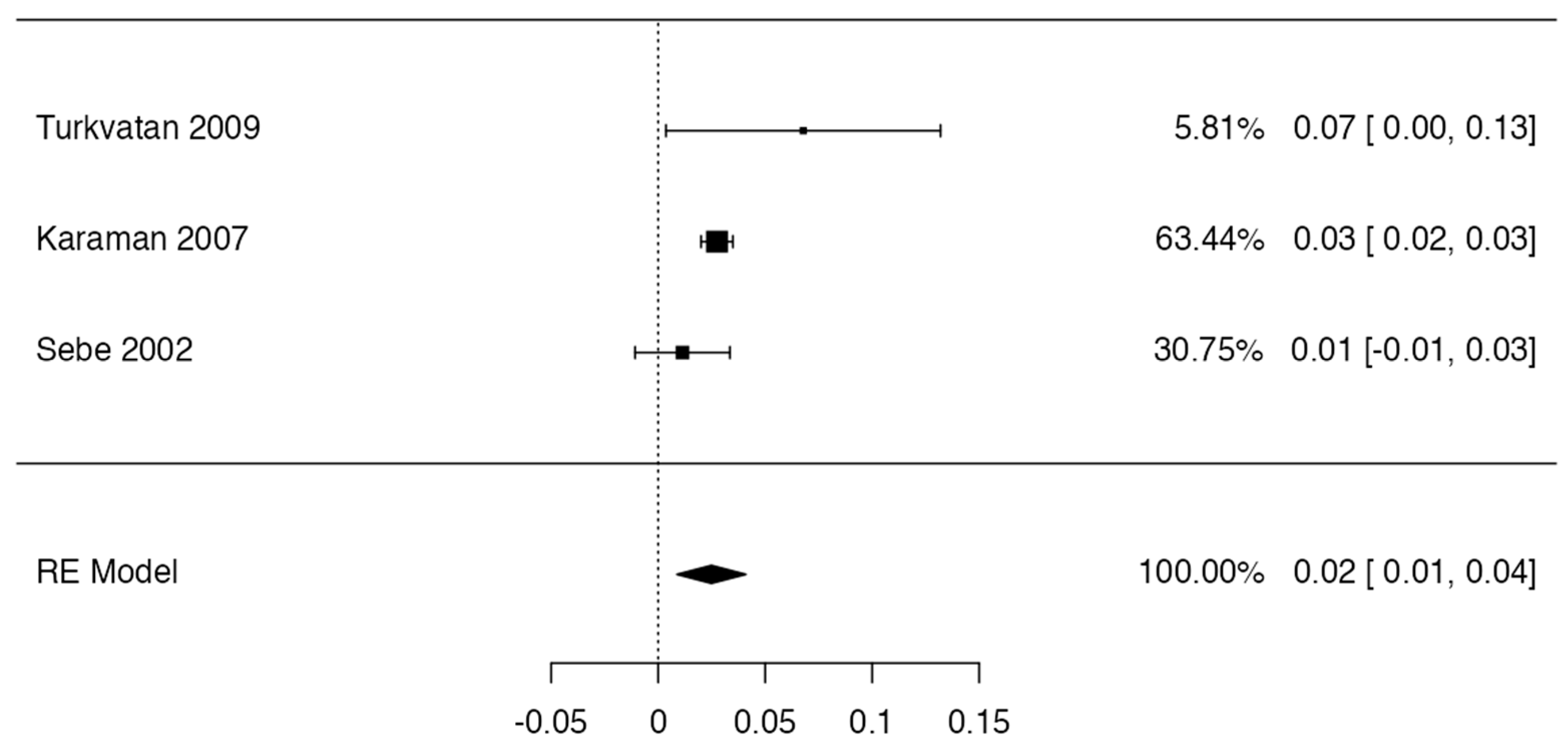
3.4. Prevalence
3.5. Risk of Bias of Included Articles
3.6. Clinical Considerations
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Itoh, M.; Moriyama, H.; Tokunaga, Y.; Miyamoto, K.; Nagata, W.; Satriotomo, I.; Shimada, K.; Takeuchi, Y. Embryological consideration of drainage of the left testicular vein into the ipsilateral renal vein: Analysis of cases of a double inferior vena cava. Int. J. Androl. 2001, 24, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Bowdino, C.S.; Owens, J.; Shaw, P.M. Anatomy, Abdomen and Pelvis, Renal Veins. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hoeltl, W.; Hruby, W.; Aharinejad, S. Renal vein anatomy and its implications for retroperitoneal surgery. J. Urol. 1990, 143, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Iimura, A.; Oguchi, T.; Matsuo, M.; Hayashi, S.; Moriyama, H.; Itoh, M. Anatomical study of the coexistence of the postaortic left brachiocephalic vein with the postaortic left renal vein with a review of the literature. Okajimas Folia Anat. Jpn. 2014, 91, 73–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Famurewa, O.C.; Asaleye, C.M.; Ibitoye, B.O.; Ayoola, O.O.; Aderibigbe, A.S.; Badmus, T.A. Variations of renal vascular anatomy in a nigerian population: A computerized tomography studys. Niger. J. Clin. Pract. 2018, 21, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, R.; Jamil, M.N.; Farooq, U. Anatomic Patterns of Right Renal Vein. J. Ayub Med. Coll. Abbottabad. 2019, 31, 55–59. [Google Scholar] [PubMed]
- Janschek, E.C.; Rothe, A.U.; Hölzenbein, T.J.; Langer, F.; Brugger, P.C.; Pokorny, H.; Domenig, C.M.; Rasoul-Rockenschaub, S.; Mühlbacher, F. Anatomic basis of right renal vein extension for cadaveric kidney transplantation. Urology 2004, 63, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.S.; Mitre, A.I.; Sheepmaker, R.; Nahas, W.C.; Dénes, F.T.; Coelho, R.F.; David Neto, E.; Srougi, M. Evaluation of cadaveric renal vein lengths and their extension loss with three types of ligature and section. J. Endourol. 2009, 23, 995–1000. [Google Scholar] [CrossRef]
- Vu, L.N.; Nghia, N.Q.; Thanh, D.T.; Giang, T.B.; Nga, V.T.; Bui, L.M.; Chu, D.T. Laparoscopic living donor right nephrectomy: Assessment of outcome and association of BMI to length of right renal vein. Actas Urol. Esp. (Engl. Ed.) 2019, 43, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Tomaszewski, K.A.; Walocha, J.A. Methods of Evidence-Based Anatomy: A guide to conducting systematic reviews and meta-analysis of anatomical studies. Ann. Anat. 2016, 205, 16–21. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. (2021) (Version 4.0) [Computer Software]. R Packages Retrieved from MRAN Snapshot 2021-04-01. Available online: https://cran.r-project.org (accessed on 20 March 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799, Correction in Rev. Esp. Cardiol. (Engl. Ed.) 2022, 75, 192. [Google Scholar]
- Pedrão, I.; Valeri, M.T.A.; Gonsalves, D.G.; Afonso, N.M.; Signori, A.G.; Rissi, R. Anomalous drainage of the right gonadal vein. Anat. Sci. Int. 2023, 98, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.A.; Buonfiglio, V.B. Triple Retroaortic Renal Vein with Tumor Thrombus. Urology 2021, 154, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, R.; Amatya, I.; Kayastha, P.; Paudel, S.; Suwal, S.; Ghimire, R.K. Normal Anatomy and Variants of Renal Vasculature with Multidetector Computed Tomography in a Tertiary Care Hospital: A Descriptive Cross-sectional Study. JNMA J. Nepal Med. Assoc. 2020, 58, 911–914. [Google Scholar] [CrossRef]
- Salimy, M.S.; Luiselli, G.A.; Yuen, M.; Healy, R.C.; Shah, S.G.; Giannaris, E.L.; Das, M.; Wink, A.E. A case of solitary kidney with duplex collecting systems and renal vascular variants in an adult male cadaver. Folia Morphol. 2021, 80, 722–725. [Google Scholar] [CrossRef]
- Fontana, F.; Coppola, A.; Ossola, C.; Beneventi, A.; Macchi, E.; Fugazzola, C. Hypoplasia of right renal vein with aberrant drainage into ipsilateral spermatic vein: Case report. Radiol. Case Rep. 2018, 14, 156–159. [Google Scholar] [CrossRef]
- Tatarano, S.; Enokida, H.; Yamada, Y.; Nishimura, H.; Yoshino, H.; Ishihara, T.; Yonemori, M.; Eura, R.; Sakaguchi, T.; Nakagawa, M. Anatomical Variations of the Left Renal Vein During Laparoscopic Donor Nephrectomy. Transplant. Proc. 2019, 51, 1311–1313. [Google Scholar] [CrossRef] [PubMed]
- Dunnwald, M.; Pizzimenti, M.A. A Case Study of Malrotated Kidneys with Asymmetric Multiple Renal Arteries, Variant Venous Drainage, and Unilateral Ureteral Duplication. Case Rep. Vasc. Med. 2019, 2019, 1893137. [Google Scholar] [CrossRef]
- Hassan, S.S.; El-Shaarawy, E.A.; Johnson, J.C.; Youakim, M.F.; Ettarh, R. Incidence of variations in human cadaveric renal vessels. Folia Morphol. 2017, 76, 394–407. [Google Scholar] [CrossRef]
- Nambur, B. A study of renal veins. Int. J. Anat. Res. 2017, 5, 4463–4468. [Google Scholar] [CrossRef][Green Version]
- Ayaz, S.; Ayaz, Ü.Y. Detection of retroaortic left renal vein and circumaortic left renal vein by PET/CT images to avoid misdiagnosis and support possible surgical procedures. Hell. J. Nucl. Med. 2016, 19, 135–139. [Google Scholar]
- Çınar, C.; Türkvatan, A. Prevalence of renal vascular variations: Evaluation with MDCT angiography. Diagn. Interv. Imaging 2016, 97, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.T.C.; Ferreira, H. Anatomical Study of Retro-Aortic Left Renal Vein. J. Chem. Pharm. Res. 2016, 8, 1011–1018. [Google Scholar]
- Kumaresan, M.; Sankaran, P.K.; Gunapriya, R.; Karthikeyan, G.; Priyadarshini, A. Morphometric study of renal vein and its variations using CT. Indian J. Med. Res. Pharm. Sci. 2016, 3, 41–49. [Google Scholar] [CrossRef]
- Pandya, V.K.; Patel, A.S.; Sutariya, H.C.; Gandhi, S.P. Evaluation of renal vascular anatomy in live renal donors: Role of multi detector computed tomography. Urol. Ann. 2016, 8, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Staśkiewicz, G.; Jajko, K.; Torres, K.; Czekajska-Chehab, E.; Maciejewski, R.; Drop, A. Supernumerary renal vessels: Analysis of frequency and configuration in 996 computed tomography studies. Folia Morphol. 2016, 75, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Bouzouita, A.; Kerkeni, W.; Bouchiba, N.; Allouche, M.; Mighri, M.M.; Hamdoun, M.; Chebil, M. Anatomical variations of renal venous vascularisation. A study of 71 three-dimensional kidney endocasts. Tunis. Med. 2015, 93, 16–20. [Google Scholar] [PubMed]
- Heidler, S.; Hruby, S.; Schwarz, S.; Sellner-Zwieauer, Y.; Hoeltl, W.; Albrecht, W. Prevalence and incidence of clinical symptoms of the retroaortic left renal vein. Urol. Int. 2015, 94, 173–176. [Google Scholar] [CrossRef]
- Reginelli, A.; Somma, F.; Izzo, A.; Urraro, F.; D’Andrea, A.; Grassi, R.; Cappabianca, S. Renovascular anatomic variants at CT angiography. Int. Angiol. 2015, 34 (Suppl. 1), 36–42. [Google Scholar]
- Resorlu, M.; Sariyildirim, A.; Resorlu, B.; Sancak, E.B.; Uysal, F.; Adam, G.; Akbas, A.; Aylanc, N.; Gulpinar, M.T.; Karatag, O.; et al. Association of congenital left renal vein anomalies and unexplained hematuria: Multidetector computed tomography findings. Urol. Int. 2015, 94, 177–180. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, L.; Yang, Z.; Zhou, H.; Tang, G. Classification of the renal vein variations: A study with multidetector computed tomography. Surg. Radiol. Anat. 2015, 37, 667–675. [Google Scholar] [CrossRef]
- Ballesteros, L.E.; Saldarriaga, V.; Ramirez, L.M. Morphologic evaluation of the renal veins: A study with autopsy material from Colombian subjects. Rom. J. Morphol. Embryol. 2014, 55, 77–81. [Google Scholar] [PubMed]
- Boyaci, N.; Karakas, E.; Dokumacı, D.S.; Yildiz, S.; Cece, H. Evaluation of left renal vein and inferior vena cava variations through routine abdominal multi-slice computed tomography. Folia Morphol. 2014, 73, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.H. Variaciones anatómicas de vasos renales y testiculares bilaterales. Rev. CES Med. 2014, 28, 273–281. [Google Scholar]
- Lavy, M.; Martin, L.; Eouzan, D.; Turco, C.; Heyd, B.; Mantion, G.; Parratte, B.; Tatu, L. An unusual case of Y-shaped right renal vein. Surg. Radiol. Anat. 2015, 37, 101–104. [Google Scholar] [CrossRef]
- Rashid, R.J.; Tarzemani, M.K.; Mohtasham, M.A.; Zomorrodi, A.; Kakaei, F.; Jalili, J.; Habibzadeh, A. Diagnostic accuracy of 64-MDCT angiography in the preoperative evaluation of renal vessels and compared with laparotomy findings in living donor kidney. Ren. Fail. 2014, 36, 327–331. [Google Scholar] [CrossRef][Green Version]
- Șahin, C.; Kaçira, O.K.; Tüney, D. The retroaortic left renal vein abnormalities in cross-sectional imaging. Folia Med. (Plovdiv) 2014, 56, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Dilli, A.; Ayaz, U.Y.; Kaplanoğlu, H.; Saltas, H.; Hekimoglu, B. Evaluation of the left renal vein variations and inferior vena cava variations by means of helical computed tomography. Clin. Imaging 2013, 37, 530–535. [Google Scholar] [CrossRef]
- Eid, N.; Ito, Y.; Otsuki, Y. The left inferior vena cava: A surgically important variant tributary of left renal vein. Surg. Radiol. Anat. 2013, 35, 455–456. [Google Scholar] [CrossRef]
- Poyraz, A.K.; Firdolas, F.; Onur, M.R.; Kocakoc, E. Evaluation of left renal vein entrapment using multidetector computed tomography. Acta Radiol. 2013, 54, 144–148. [Google Scholar] [CrossRef]
- Tao, X.F.; Zhu, J.Q.; Wu, Y.W.; Tang, G.Y.; Shi, Y.Z.; Zhang, L.; Lin, Y.; Wang, Z.Q. Dual-energy computed tomography angiography for evaluating the renal vascular variants. Chin. Med. J. (Engl.) 2013, 126, 650–654. [Google Scholar] [CrossRef]
- Apisarnthanarak, P.; Suvannarerg, V.; Muangsomboon, K.; Taweemonkongsap, T.; Hargrove, N.S. Renal vascular variants in living related renal donors: Evaluation with CT angiography. J. Med. Assoc. Thai. 2012, 95, 941–948. [Google Scholar]
- Atalar, M.H.; Kosar, M.I.; Salk, I.; Isleyen, M. Left renal vein abnormalities detected during routine abdominal computed tomography imaging: Clinico-radiological significance. Folia Morphol. 2012, 71, 168–172. [Google Scholar]
- Bouali, O.; Labarre, D.; Molinier, F.; Lopez, R.; Benouaich, V.; Lauwers, F.; Moscovici, J. Anatomic variations of the renal vessels: Focus on the precaval right renal artery. Surg. Radiol. Anat. 2012, 34, 441–446. [Google Scholar] [CrossRef]
- Dilli, A.; Ayaz, U.Y.; Karabacak, O.R.; Tatar, I.G.; Hekimoglu, B. Study of the left renal variations by means of magnetic resonance imaging. Surg. Radiol. Anat. 2012, 34, 267–270. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, R.; Singal, R. Congenital Variations of Renal Veins: Embryological Background and Clinical Implications. J. Chem. Pharm. Res. 2011, 5, 1140–1143. [Google Scholar]
- Yi, S.Q.; Ueno, Y.; Naito, M.; Ozaki, N.; Itoh, M. The three most common variations of the left renal vein: A review and meta-analysis. Surg. Radiol. Anat. 2012, 34, 799–804. [Google Scholar] [CrossRef]
- Costa, H.C.; Moreira, R.J.; Fukunaga, P.; Fernandes, R.C.; Boni, R.C.; Matos, A.C. Anatomic variations in vascular and collecting systems of kidneys from deceased donors. Transplant. Proc. 2011, 43, 61–63. [Google Scholar] [CrossRef]
- Kulkarni, S.; Emre, S.; Arvelakis, A.; Asch, W.; Bia, M.; Formica, R.; Israel, G. Multidetector CT angiography in living donor renal transplantation: Accuracy and discrepancies in right venous anatomy. Clin. Transplant. 2011, 25, 77–82. [Google Scholar] [CrossRef]
- Li, G.; Dong, J.; Lu, J.S.; Zu, Q.; Yang, S.X.; Li, H.Z.; Ma, X.; Zhang, X. Anatomical variation of the posterior lumbar tributaries of the left renal vein in retroperitoneoscopic left living donor nephrectomy. Int. J. Urol. 2011, 18, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Favaro, W.J.; Santos, T.D.; Cagnon, V.H. Venous communication between the right and left kidneys: A rare anatomic variation. Int. J. Morphol. 2009, 27, 117–120. [Google Scholar] [CrossRef][Green Version]
- Turkvatan, A.; Akinci, S.; Yildiz, S.; Olçer, T.; Cumhur, T. Multidetector computed tomography for preoperative evaluation of vascular anatomy in living renal donors. Surg. Radiol. Anat. 2009, 31, 227–235. [Google Scholar] [CrossRef]
- Kaneko, N.; Kobayashi, Y.; Okada, Y. Anatomic variations of the renal vessels pertinent to transperitoneal vascular control in the management of trauma. Surgery 2008, 143, 616–622. [Google Scholar] [CrossRef]
- Mir, N.S.; Ul Hassan, A.; Rangrez, R.; Hamid, S.; Sabia Tabish, S.A.; Iqbal Suhaila Masarat Rasool, Z. Bilateral Duplication of Renal Vessels: Anatomical, Medical and Surgical perspective. Int. J. Health Sci. (Qassim) 2008, 2, 179–185. [Google Scholar]
- Natsis, K.; Tsitouridis, I.; Totlis, T.; Levva, S.; Tsikaras, P.; Skandalakis, P. Proposal for classification of the circumaortic renal collar’s morphology. Am. Surg. 2008, 74, 1190–1194. [Google Scholar] [CrossRef]
- Tombul, S.T.; Aki, F.T.; Gunay, M.; Inci, K.; Hazirolan, T.; Karcaaltincaba, M.; Erkan, I.; Bakkaloglu, A.; Yasavul, U.; Bakkaloglu, M. Preoperative evaluation of hilar vessel anatomy with 3-D computerized tomography in living kidney donors. Transplant. Proc. 2008, 40, 47–49. [Google Scholar] [CrossRef]
- Yagci, B.; Tavasli, B.; Karabulut, N.; Kiroglu, Y. Clinical significance and renal haemodynamics of incidentally detected retroaortic left renal vein: Assessment with venous Doppler sonography. Br. J. Radiol. 2008, 81, 187–191. [Google Scholar] [CrossRef]
- Holt, P.J.; Adshead, J.M.; Filiadis, I.; Christmas, T.J. Retroperitoneal anomalies in men with testicular germ cell tumours. BJU Int. 2007, 99, 344–346. [Google Scholar] [CrossRef]
- Karaman, B.; Koplay, M.; Ozturk, E.; Basekim, C.C.; Ogul, H.; Mutlu, H.; Kizilkaya, E.; Kantarci, M. Retroaortic left renal vein: Multidetector computed tomography angiography findings and its clinical importance. Acta Radiol. 2007, 48, 355–360. [Google Scholar] [CrossRef]
- Karazincir, S.; Balci, A.; Görür, S.; Sumbas, H.; Kiper, A.N. Incidence of the retroaortic left renal vein in patients with varicocele. J. Ultrasound Med. 2007, 26, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Koc, Z.; Ulusan, S.; Oguzkurt, L.; Tokmak, N. Venous variants and anomalies on routine abdominal multi-detector row CT. Eur. J. Radiol. 2007, 61, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.S.; Pojchamarnwiputh, S.; Muangsomboon, K.; Schulam, P.G.; Gritsch, H.A.; Lu, D.S. Surgically relevant normal and variant renal parenchymal and vascular anatomy in preoperative 16-MDCT evaluation of potential laparoscopic renal donors. AJR Am. J. Roentgenol. 2007, 188, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, S.; Kalra, M.K.; Waldrop, S.M.; Mittal, P.K.; Small, W.C. Multidetector row CT angiography of living related renal donors: Is there a need for venous phase imaging? Eur. J. Radiol. 2006, 59, 442–452. [Google Scholar] [CrossRef]
- Arslan, H.; Etlik, O.; Ceylan, K.; Temizoz, O.; Harman, M.; Kavan, M. Incidence of retro-aortic left renal vein and its relationship with varicocele. Eur. Radiol. 2005, 15, 1717–1720. [Google Scholar] [CrossRef]
- Kawamoto, S.; Lawler, L.P.; Fishman, E.K. Evaluation of the renal venous system on late arterial and venous phase images with MDCT angiography in potential living laparoscopic renal donors. AJR Am. J. Roentgenol. 2005, 184, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Klemm, P.; Fröber, R.; Köhler, C.; Schneider, A. Vascular anomalies in the paraaortic region diagnosed by laparoscopy in patients with gynaecologic malignancies. Gynecol. Oncol. 2005, 96, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Steinberg, A.P.; Ramani, A.P.; Abreu, S.C.; Desai, M.M.; Kaouk, J.; Goldfarb, D.A.; Gill, I.S. Laparoscopic live donor nephrectomy in the presence of circumaortic or retroaortic left renal vein. J. Urol. 2004, 171, 44–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yeh, B.M.; Coakley, F.V.; Meng, M.V.; Breiman, R.S.; Stoller, M.L. Precaval right renal arteries: Prevalence and morphologic associations at spiral CT. Radiology 2004, 230, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Yeşildağ, A.; Adanir, E.; Köroğlu, M.; Baykal, B.; Oyar, O.; Gülsoy, U.K. Rutin abdomen BT’de sol renal ven anomalilerinin görülme sikliği [Incidence of left renal vein anomalies in routine abdominal CT scans]. Tani. Girisim. Radyol. 2004, 10, 140–143. (In Turkish) [Google Scholar] [PubMed]
- Senecail, B.; Bobeuf, J.; Forlodou, P.; Nonent, M. Two rare anomalies of the left renal vein. Surg. Radiol. Anat. 2003, 25, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Duques, P.; Rodrigues, J.R.; Silva Neto, F.B.; SNeto, E.M.; Tolêdo, E.S. Estudo anatômico da veia renal izquierda de cadáveres humanos brasileiros. Medicina 2002, 35, 184–191. [Google Scholar] [CrossRef]
- Sèbe, P.; Peyromaure, M.; Raynaud, A.; Delmas, V. Anatomical variations in the drainage of the principal adrenal veins: The results of 88 venograms. Surg. Radiol. Anat. 2002, 24, 222–225. [Google Scholar] [CrossRef]
- Aljabri, B.; MacDonald, P.S.; Satin, R.; Stein, L.S.; Obrand, D.I.; Steinmetz, O.K. Incidence of major venous and renal anomalies relevant to aortoiliac surgery as demonstrated by computed tomography. Ann. Vasc. Surg. 2001, 15, 615–618. [Google Scholar] [CrossRef]
- Shindo, S.; Kubota, K.; Kojima, A.; Iyori, K.; Ishimoto, T.; Kobayashi, M.; Kamiya, K.; Tada, Y. Anomalies of inferior vena cava and left renal vein: Risks in aortic surgery. Ann. Vasc. Surg. 2000, 14, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Kawai, K.; Kodama, K. An anatomical study of the retroaortic left renal vein. Okajimas Folia Anat. Jpn. 2000, 77, 47–52. [Google Scholar] [CrossRef]
- Satyapal, K.S.; Kalideen, J.M.; Haffejee, A.A.; Singh, B.; Robbs, J.V. Left renal vein variations. Surg. Radiol. Anat. 1999, 21, 77–81. [Google Scholar] [CrossRef]
- Pozniak, M.A.; Balison, D.J.; Lee, F.T., Jr.; Tambeaux, R.H.; Uehling, D.T.; Moon, T.D. CT angiography of potential renal transplant donors. Radiographics 1998, 18, 565–587. [Google Scholar] [CrossRef]
- Trigaux, J.P.; Vandroogenbroek, S.; De Wispelaere, J.F.; Lacrosse, M.; Jamart, J. Congenital anomalies of the inferior vena cava and left renal vein: Evaluation with spiral CT. J. Vasc. Interv. Radiol. 1998, 9, 339–345. [Google Scholar] [CrossRef]
- Baptista-Silva, J.C.; Veríssimo, M.J.; Castro, M.J.; Câmara, A.L.; Pestana, J.O. Anatomical study of the renal veins observed during 342 living-donor nephrectomies. Sao Paulo Med. J. 1997, 115, 1456–1459. [Google Scholar] [CrossRef]
- Hicks, M.E.; Malden, E.S.; Vesely, T.M.; Picus, D.; Darcy, M.D. Prospective anatomic study of the inferior vena cava and renal veins: Comparison of selective renal venography with cavography and relevance in filter placement. J. Vasc. Interv. Radiol. 1995, 6, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.A.; Waltman, A.C.; Rivitz, S.M.; Geller, S.G. Anatomical observations on the renal veins and inferior vena cava at magnetic resonance angiography. Cardiovasc. Interv. Radiol. 1995, 18, 153–157. [Google Scholar] [CrossRef]
- Satyapal, K.S. Classification of the drainage patterns of the renal veins. J. Anat. 1995, 186 Pt 2, 329–333. [Google Scholar] [PubMed]
- Benedetti-Panici, P.; Maneschi, F.; Scambia, G.; Greggi, S.; Mancuso, S. Anatomic abnormalities of the retroperitoneum encountered during aortic and pelvic lymphadenectomy. Am. J. Obstet. Gynecol. 1994, 170 Pt 1, 111–116. [Google Scholar] [CrossRef]
- Martinez-Almagro, A.; Almenar Garcia, V.; Martinez Sanjuan, V.; Hernandez Gil de Tejada, T.; Lorente Montalvo, P. Retroaortic left renal vein: A report of six cases. Surg. Radiol. Anat. 1992, 14, 361–366. [Google Scholar] [CrossRef]
- Monkhouse, W.S.; Khalique, A. The adrenal and renal veins of man and their connections with azygos and lumbar veins. J. Anat. 1986, 146, 105–115. [Google Scholar] [PubMed]
- Mayo, J.; Gray, R.; St Louis, E.; Grosman, H.; McLoughlin, M.; Wise, D. Anomalies of the inferior vena cava. AJR Am. J. Roentgenol. 1983, 140, 339–345. [Google Scholar] [CrossRef]
- Reed, M.D.; Friedman, A.C.; Nealey, P. Anomalies of the left renal vein: Analysis of 433 CT scans. J. Comput. Assist. Tomogr. 1982, 6, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.J.; Gewertz, B.L.; Lu, C.T.; Zarins, C.K. New criteria for placement of a prophylactic vena cava filter. Surg. Gynecol. Obstet. 1986, 163, 405–409. [Google Scholar] [PubMed]
- Beckmann, C.F.; Abrams, H.L. Renal venography: Anatomy, technique, applications, analysis of 132 venograms, and a review of the literature. Cardiovasc. Interv. Radiol. 1980, 3, 45–70. [Google Scholar] [CrossRef]
- Kramer, B. The incidence of the renal venous collar in South African blacks. S. Afr. Med. J. 1980, 57, 875–876. [Google Scholar]
- Lien, H.H.; Kolbenstvedt, A. Phlebographic appearances of the left renal and left testicular veins. Acta Radiol. Diagn. 1977, 18, 321–332. [Google Scholar] [CrossRef]
- Goswami, A.P. Anatomical variation of the renal veins with varicosity presenting as pseudotumor of the kidney. J. Urol. 1976, 116, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Royster, T.S.; Lacey, L.; Marks, R.A. Abdominal aortic surgery and the left renal vein. Am. J. Surg. 1974, 127, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.J., Jr.; Lundberg, G.D. Retroaortic left renal vein, a relatively frequent anomaly. Am. J. Clin. Pathol. 1968, 50, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Samuel, E.; Millar, D.R. Variations in the renal vascular pedicle. (An anatomical and radiological study with particular reference to renal transplantation). Br. J. Urol. 1961, 33, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.H.; Esenther, G. Variations in the pattern of renal vessels and their relation to the type of posterior vena cava in man. Am. J. Anat. 1959, 104, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Royster, T.S. Thrombophlebitis after vena caval clipping. Am. J. Surg. 1967, 113, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Mazengenya, P. Multiple variations of the renal and testicular vessels: Possible embryological basis and clinical importance. Surg. Radiol. Anat. 2016, 38, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Raheem, O.A.; O’Brien, M.; Glacken, P.; Mohan, P.; Hickey, D.P. A review of the anatomical variations of the posterior tributaries of the left renal vein. Ir. J. Med. Sci. 2008, 177, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, J.; Kopecky, K.K.; Tann, M.; Persohn, S.A.; Leapman, S.B.; Filo, R.S.; Shalhav, A.L. Evaluation of prospective living renal donors for laparoscopic nephrectomy with multisection CT: The marriage of minimally invasive imaging with minimally invasive surgery. Radiographics 2001, 21, S223–S236. [Google Scholar] [CrossRef]
- Hostiuc, S.; Rusu, M.C.; Negoi, I.; Dorobanțu, B.; Grigoriu, M. Anatomical variants of renal veins: A meta-analysis of prevalence. Sci. Rep. 2019, 9, 10802. [Google Scholar] [CrossRef]
- Steinmuller, D.; Novick, A.; Braun, W.; Vidt, D.; Nakamoto, S. Renal transplantation of patients on chronic peritoneal dialysis. Am. J. Kidney Dis. 1984, 3, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Stratta, R.J.; D’Alessandro, A.M.; Belzer, F.O. Renal vein reconstruction with interposition allografts in cadaveric renal transplantation. Transpl. Int. 1988, 1, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, S.P. Acute renal failure in the newborn. Semin. Perinatol. 2004, 28, 112–123. [Google Scholar] [CrossRef]
- Lorenz, J.M.; Regalado, S.; Navuluri, R.; Zangan, S.; Vanha, T.; Funaki, B. Transhepatic guidance of translumbar hemodialysis catheter placement in the setting of chronic infrarenal IVC occlusion. Cardiovasc. Interv. Radiol. 2010, 33, 635–638. [Google Scholar] [CrossRef]

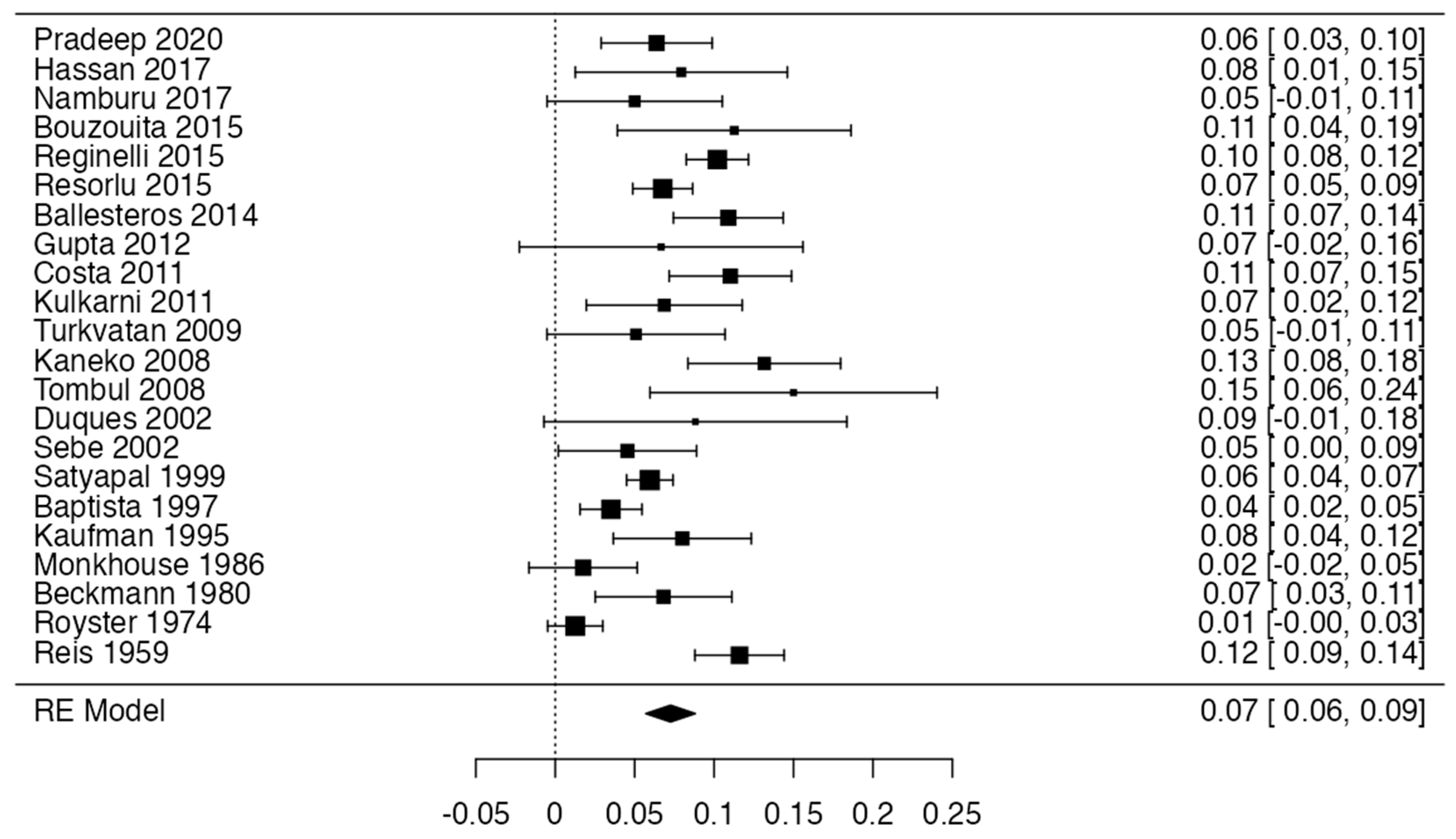


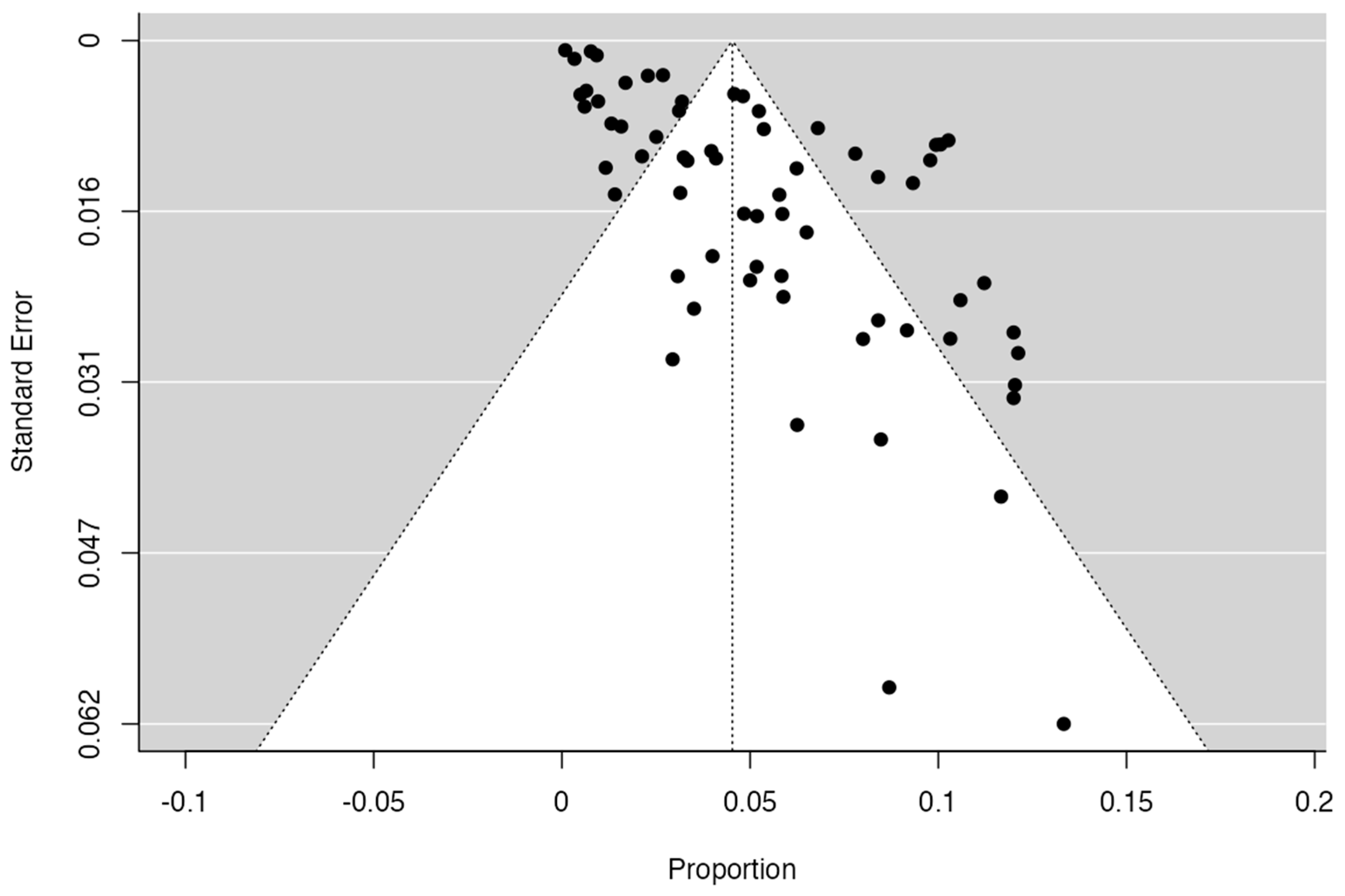
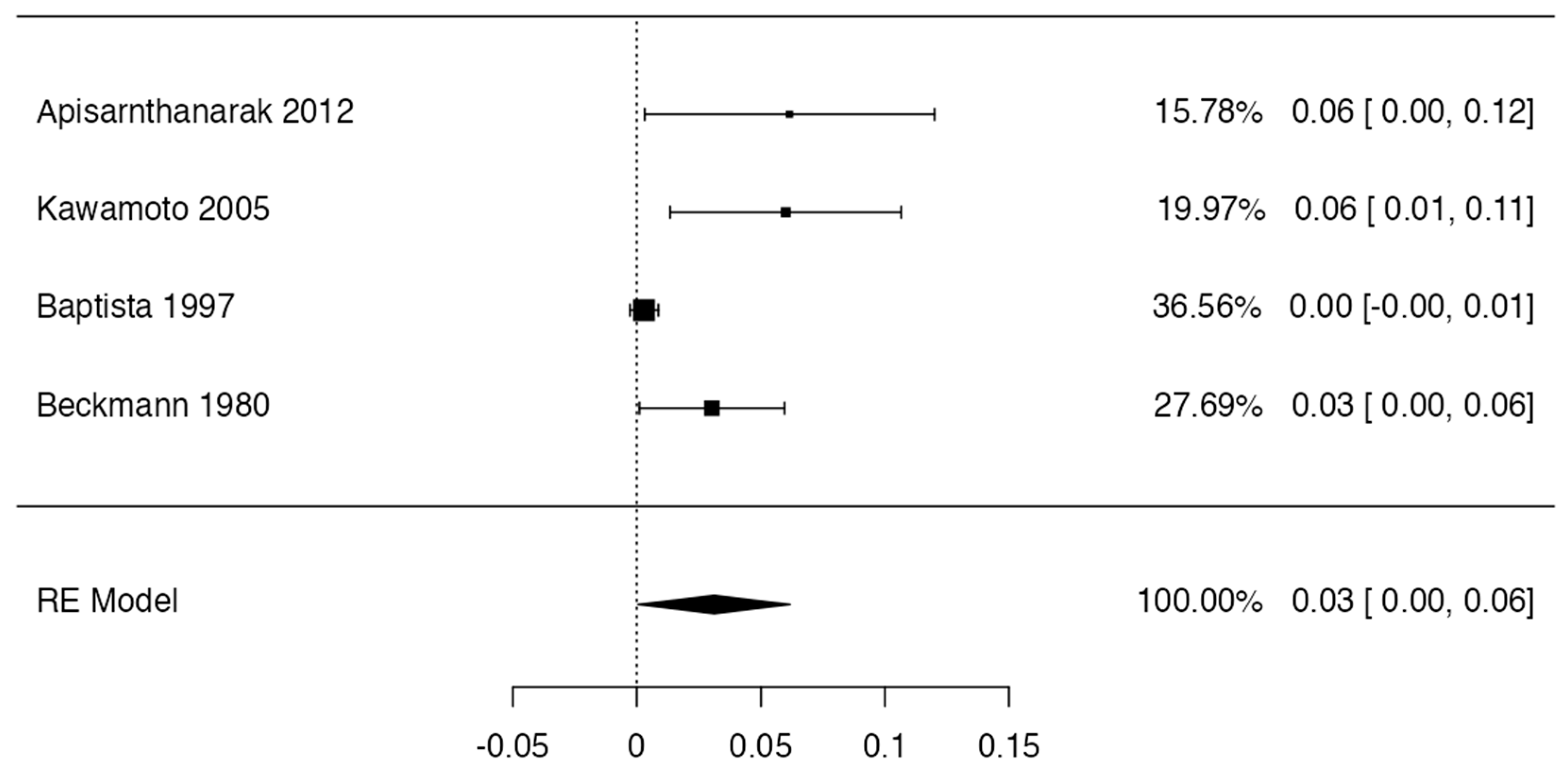
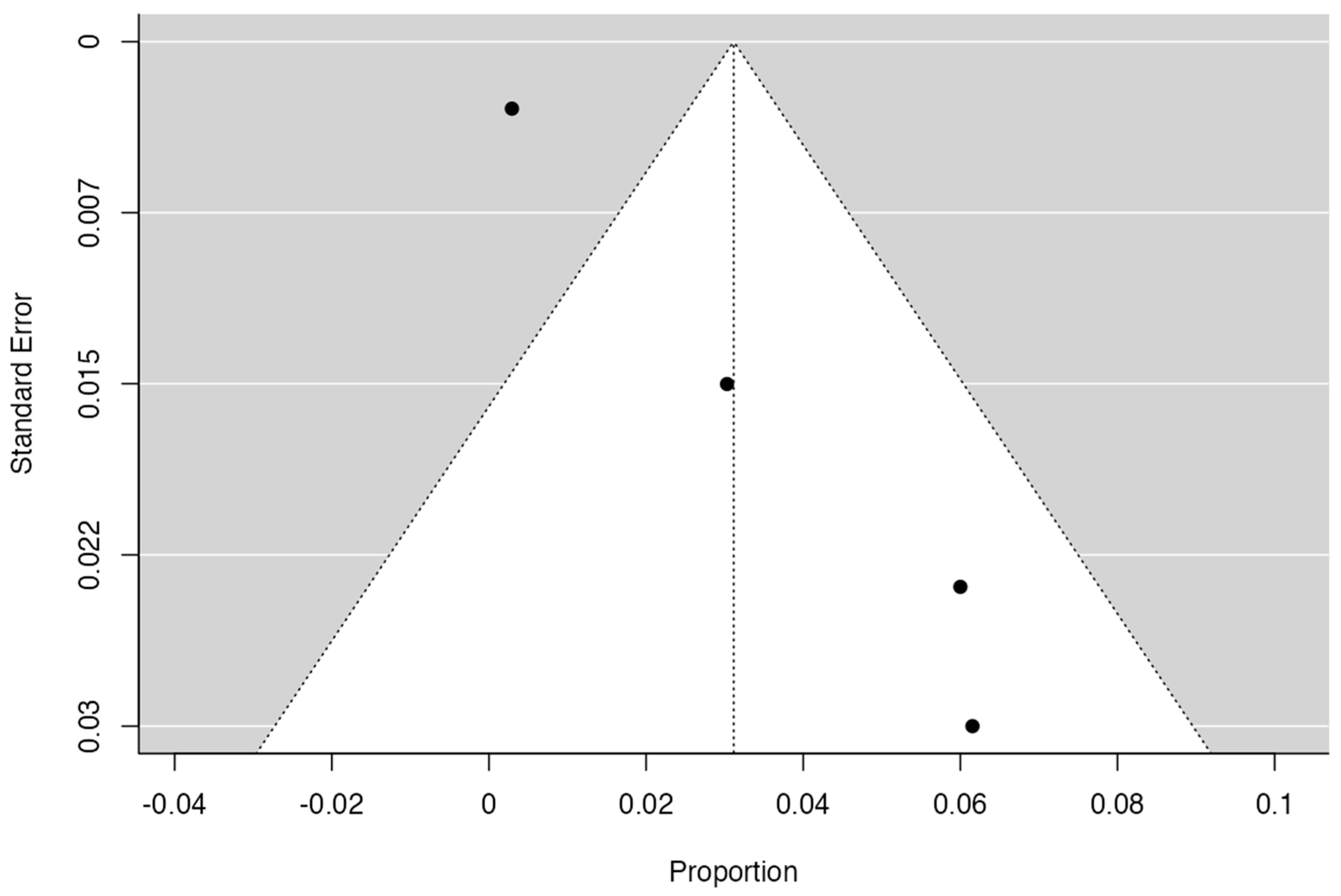
| Author and Year | Number of Patients | Incidence and Characteristics | Statistical Value | Geographic Region | Sex | Laterality |
|---|---|---|---|---|---|---|
| Pedrao, 2023 [13] | Observational N = 1 | Double RRV. | Not mentioned. | Brazil | 1 male | Right |
| Silva, 2021 [14] | Observational N = 1 | Multiple retroaortic LRV. | Not mentioned. | Brazil | 1 male | Left |
| Pradeep, 2020 [15] | Observational N = 188 | Double RRV (12 cases—6.3%). Retroaortic LRV (4 cases—2.1%). | Not mentioned. | Nepal | Not specified | Bilateral |
| Salimy et al., 2020 [16] | Observational N = 1 | Double RRV. Right kidney with double collector system. | Not mentioned. | USA | 1 male | Right |
| Fontana, 2018 [17] | Observational N = 1 | Duplication of circumaortic RV. | Not mentioned. | Italy | 1 male | Left |
| Tatarano et al., 2019 [18] | Observational N = 120 | Circumaortic LRV (6 cases). Retroaortic LRV (1 case). | There was no significant incidence between donors with or without abnormalities on their LRV. | Japan | Not specified | Left |
| Dunnwald, 2019 [19] | Observational N = 1 | Triple RRV (1 case). | Not mentioned. | USA | 1 male | Bilateral |
| Shaheen, 2018 [6] | Observational N = 50 | Double RRV (28 cases—56%), triple (13 cases—26%), quadruple (5 cases—10%). | Not mentioned. | Pakistan | 50 males | Bilateral |
| Hassan, 2017 [20] | Observational N = 63 | Double RV (2 cases—3%), triple (2 cases—3%), quadruple (1 case—2%). | No significant differences between cadavers with or without variations in renal vasculature and age of death (p = 0.67) or gender (p = 0.71). | Egypt | 32 males 31 females | Bilateral |
| Nambur, 2017 [21] | Observational N = 60 | Circumaortic LRV (2 cases—3.3%). Retroaortic LRV (5 cases—8.3%). Double RRV (3 cases—5%). Right gonadal vein draining into RRV (1 case—0.3%). | Not mentioned. | India | Not specified | Bilateral |
| Ayaz, 2016 [22] | Observational N = 222 | Circumaortic LRV (7 cases—3.15%). Retroaortic LRV (6 cases—2.7%). | Not mentioned. | Turkey | 116 males 106 females | Left |
| Çınar, 2016 [23] | Observational N = 504 | Circumaortic LRV (26 cases—5.2%). Retroaortic LRV (21 cases—4.2%). RRV: double (97 cases—19.2%), triple (11 cases—2.2%), quadruple (1 case—0.2%). | No associations were found between sex and the presence of RA or RV variations (p = 0.630 and 0.650, respectively). | Turkey | 317 males 187 females | Bilateral |
| Duran, 2016 [24] | Observational N = 23 | Retroaortic LRV (2 cases—8.7%). | Not mentioned. | Colombia | 12 males 1 females | Left |
| Kumaresan, 2016 [25] | Observational N = 100 | Retroaortic LRV (4 cases—4%). Multiple RV (19 cases—19%) | Not mentioned. | India | Not specified | Bilateral |
| Pandya, 2016 [26] | Observational N = 200 | Circumaortic LRV (8 cases—4%). Retroaortic LRV (5 cases—2.5%). Double LRV (2 cases—1%) Double RRV (61 cases—30.5%), triple (5 cases—2.5%). | Not mentioned. | India | Not specified | Bilateral |
| Staśkiewicz, 2016 [27] | Observational N = 996 | Circumaortic or retroaortic courses of the LRV in 99 cases (10%). | No significant difference was observed in the type of RV course between men and women (c2 = 1.22, p = 0.543). | Poland | 481 males 515 females | Bilateral |
| Bouzouita et al., 2015 [28] | Observational N = 71 | Retroaortic LRV (1 case—1.4%). Double LRV (3 cases—4.2%). Double RRV (5 cases—7%). | Not mentioned. | Tunisia | Not specified | Bilateral |
| Heidler, 2015 [29] | Observational N = 7929 | Retroaortic LRV (61 cases—0.77%). | Not mentioned. | Austria | 4781 males 3148 females | Left |
| Reginelli, 2015 [30] | Observational N = 921 | Multiple RV (94 cases—10.2%). Retroaortic RV (219 cases—23.8%). | Not mentioned. | Italy | 418 males 503 females | Bilateral |
| Resorlu, 2015 [31] | Observational N = 680 | Retroaortic LRV (36 cases—5.4%). Circumaortic LRV (17 cases—2.5%). Multiple RV (46 cases—6.8%). | Hematuria was detected in 23.5% of patients with circumaortic LRV anomaly and 10.1% of patients without anomaly (p = 0.074). Hematuria was found in 21.7% of patients with multiple RV and 9.6% in those without the anomaly (p = 0.009). | Turkey | 391 males 289 females | Left |
| Zhu, 2015 [32] | Observational N = 1452 | Circumaortic LRV (31 cases—2.1%). Retroaortic LRV (30 cases—2.1%). | No statistically significant correlation found between left/right RV variations and sex (p > 0.05). | China | Not specified | Bilateral |
| Ballesteros, 2014 [33] | Observational N = 312 | Circumaortic LRV (1 case—0.32%). Retroaortic LRV (2 cases—0.64%). Double LRV (1 case—0.32%). Double RRV (28 cases—8.9%), triple (5 cases—1.6%). | No significant difference between the presence of additional veins in men and women (p = 0.452) and extrahilar origin between RRV and LRV (p = 0.768). | Colombia | 129 males 27 females | Bilateral |
| Boyaci, 2014 [34] | Observational N = 746 | Circumaortic LRV (18 cases—2.4%). Retroaortic LRV (55 cases—7.4%). | No significant difference between the presence of variations [LRV (p = 0.801), RLRV (p = 0.551), CLRV (p = 0.823)] and sex. | Turkey | 395 males 351 females | Left |
| Ferreira, 2014 [35] | Observational N = 1 | Retroaortic LRV. Multiple RV. | 29 to 65% of pyeloureteral obstructions were related to anomalies in the path of the vessels crossing the renal pelvis. | Colombia | 1 males | Bilateral |
| Lavy et al., 2015 [36] | Observational N = 1 | Multiple RV. | Not mentioned. | France | 1 males | Bilateral |
| Rashid, 2014 [37] | Observational N = 100 | Circumaortic LRV (3 cases). Retroaortic LRV (5 cases). Double RRV (16 cases—16%), triple (1 case—1%). | Not mentioned. | Iran | 91 males 9 females | Bilateral |
| Șahin, 2014 [38] | Observational N = 2189 | Circumaortic LRV (6 cases—0.3%). Retroaortic LRV (44 cases—2%). | Not mentioned. | Turkey | Not specified | Left |
| Dilli, 2013 [39] | Observational N = 1204 | Circumaortic LRV (25 cases—2.1%). Retroaortic LRV (38 cases—3.2%). | Significant correlation between retroaortic LRV and gender (p = 0.036). | Turkey | 642 males 562 females | Left |
| Eid et al., 2013 [40] | Observational N = 1 | LRV origin: IVC. End: RRV. | Not mentioned. | Japan | 1 male | Left |
| Poyraz, 2013 [41] | Observational N = 1000 | Circumaortic LRV (3 cases—0.3%). Retroaortic LRV (65 cases—6.5%). | Diameters of the RRV and LRV were not significantly different (p = 0.1). Diameter of the anterior LRV was significantly greater than contralateral RV in its widest portion (p = 0.04). | Turkey | 537 males 463 females | Left |
| Tao, 2013 [42] | Observational N = 378 | Circumaortic LRV (8 cases—2.1%). Retroaortic LRV (7 cases—1.85%). | Not mentioned. | China | 197 males 181 females | Bilateral |
| Apisarnthanarak, 2012 [43] | Observational N = 65 | Circumaortic LRV (1 case—1.5%). Retroaortic LRV (1 case—1.5%). Double LRV (1 case—1.5%). Double RRV (19 cases—29.2%), triple (4 cases—6.2%). Right gonadal vein draining into the RRV (4 cases—6.2%). | Not mentioned. | Thailand | 25 males 40 females | Bilateral |
| Atalar, 2012 [44] | Observational N = 739 | Circumaortic LRV (6 cases—0.8%). Retroaortic LRV (17 cases—2.3%). | Not mentioned. | Turkey | 425 males 314 females | Left |
| Bouali et al., 2012 [45] | Observational N = 120 | Circumaortic LRV (6 cases—5%). Retroaortic LRV (5 cases—4.17%). Double RRV (22 cases—18.3%), triple (2 cases—1.7%). | Not mentioned. | France | 79 males 41 females | Bilateral |
| Dilli, 2012 [46] | Observational N = 2644 | Circumaortic LRV (27 cases—1.02%). Retroaortic LRV (44 cases—1.66%). | No statistically significant gender difference was found between LRV variations (p = 0.83). | Turkey | 1204 males 1440 females | Left |
| Gupta, 2011 [47] | Observational N = 30 | Circumaortic LRV (2 cases—6.6%). Retroaortic LRV (2 cases—6.6%). Double LRV (1 case—3.3%). Double RRV (1 case—3.3%). | Not mentioned. | India | Not specified | Bilateral |
| Yi et al., 2012 [48] | Observational N = 3 | Circumaortic LRV (1 case). Retroaortic LRV (1 case). | Not mentioned. | Japan | 1 male 2 females | Left |
| Costa et al., 2011 [49] | Observational N = 254 | Circumaortic LRV (1 case). Retroaortic LRV (3 cases). Double LRV (4 cases—1.5%). Double RRV (24 cases—9.8%). | Dominance of venous variations on the right side, 12 times greater than on the left. | Brazil | Not specified | Bilateral |
| Kulkarni, 2011 [50] | Observational N = 102 | Circumaortic RV (5 cases—5%). Retroaortic RV (1 case—1%). Multiple RV (7 cases—7%). | Not mentioned. | USA | Not specified | Not specified |
| Li et al., 2011 [51] | Observational N = 61 | Anastomosis between the LRV and hemiazygos vein (51 cases—83.6%). | Significant differences when comparing operation time. Type 4 took longer (p < 0.05), type 5 shorter time (p < 0.05). | China | 32 males 29 females | Left |
| Favaro et al., 2009 [52] | Observational N = 1 | Venous communication between LRV and RRV. Kidneys without any relation to the IVC or common iliac veins. | Not mentioned. | Brazil | 1 male | Bilateral |
| Turkvatan, 2009 [53] | Observational N = 59 | Circumaortic LRV (2 cases—3.3%). Retroaortic LRV (3 cases—5%). Multiple RV (3 cases—5%). | Greater sensitivity and specificity of MDCT for renal venous anomalies. | Turkey | 32 males 27 females | Bilateral |
| Kaneko et al., 2008 [54] | Observational N = 190 | Multiple RV (25 cases—13%). | Not mentioned. | Japan | Not specified | Bilateral |
| Mir et al., 2008 [55] | Observational N = 1 | Double RV bilaterally. | Not mentioned. | India | Not specified | Bilateral |
| Natsis, 2008 [56] | Observational N = 319 | Circumaortic LRV (8 cases—2.5%). | Not mentioned. | Greece | 173 males 146 females | Left |
| Tombul, 2008 [57] | Observational N = 60 | Multiple RV (9 cases—15%) | Sensitivity of MDCT angiography for veins was 93%. | Turkey | Not specified | Bilateral |
| Yagci, 2008 [58] | Observational N = 783 | Circumaortic LRV (15 cases—2%). Retroaortic LRV (23 cases—3%). Double retroaortic vein (4 cases—0.5%). | No statistically significant difference in ages. | Turkey | Not specified | Left |
| Holt, 2007 [59] | Observational N = 278 | Retroaortic LRV (9 cases—3.2%). | Not mentioned. | UK | 278 males | Left |
| Karaman, 2007 [60] | Observational N = 1856 | Circumaortic LRV (17 cases—8.9%). Retroaortic LRV (68 cases—3.6%). | Not mentioned. | Turkey | Not specified | Left |
| Karazincir, 2007 [61] | Observational N = 277 | Retroaortic LRV in patients (13 cases—9.3%) and controls (3 cases—2.2%). | Significantly higher incidence of varicocele in patients compared to controls (p = 0.018). | Turkey | Not specified | Left |
| Koc, 2007 [62] | Observational N = 1120 | Circumaortic RV (62 cases—5.5%). Retroaortic RV (53 cases—4.7%). Multiple RV (210 cases—18.8%). | Not mentioned. | Turkey | 588 males 532 females | Bilateral |
| Raman, 2007 [63] | Observational N = 126 | Circumaortic LRV (10 cases—8%). Retroaortic LRV (3 cases—2%). Double LRV (10 cases—8%). Double RRV (28 cases—22%), triple (2 cases—2%). | Not mentioned. | USA | 57 males 69 females | Bilateral |
| Namasivayam, 2006 [64] | Observational N = 48 | Circumaortic LRV (1 case—2%). Retroaortic LRV (2 cases—4%). Double RRV (13 cases—27%), triple (1 case—2%). | Venous phase images showed significantly greater opacification of the left renal, gonadal, adrenal, and lumbar veins (p < 0.05). | USA | 20 males 28 females | Bilateral |
| Arslan, 2005 [65] | Observational N = 1125 | Retroaortic LRV (19 cases—1.68%). | Not mentioned. | Turkey | 573 males 552 females | Left |
| Kawamoto, 2005 [66] | Observational N = 100 | Circumaortic LRV (3 cases—3%). Retroaortic LRV (2 cases—2%). Small posterior branch that runs behind the aorta and drains into the IVC (6 cases—6%). | Not mentioned. | USA | Not specified | Left |
| Klemm, 2005 [67] | Observational N = 86 | Retroaortic LRV (1 case). | Not mentioned. | Germany | 86 females | Left |
| Janschek, 2004 [7] | Observational N = 119 | Circumaortic LRV (7 cases—6%). Retroaortic LRV (3 cases—2.5%). Double LRV (7 cases—5.9%), triple (1 case—0.8%). Double RRV (21 cases—18%), triple (6 cases—5%). | Not mentioned. | Austria | 58 males 61 females | Bilateral |
| Lin, 2004 [68] | Observational N = 170 | Circumaortic LRV (16 cases—9.4%). Retroaortic LRV (2 cases—1.2%). | Groups 1 and 2 were similar in operation time (p = 0.90), blood loss (p = 0.45), warm ischemia time (p = 0.14), and hospital stay (p = 0.45). | USA | Not specified | Left |
| Yeh, 2004 [69] | Observational N = 186 | Precaval RRV (9 cases—4.8%). | Not mentioned. | USA | Not specified | Right |
| Yesidag, 2004 [70] | Observational N = 1003 | Circumaortic LRV (23 cases—3.2%). Retroaortic LRV (9 cases—0.9%). | Not mentioned. | Turkey | Not specified | Left |
| Senecail et al., 2003 [71] | Observational N = 2 | Circumaortic LRV (1 case). Retroaortic LRV (1 case). | Not mentioned. | France | 1 male 1 female | Left |
| Duques, 2002 [72] | Observational N = 34 | Circumaortic LRV (1 case—2.9%). Double LRV (3 cases—8.9%). | Not mentioned. | Brazil | 24 males 10 females | Left |
| Sebe et al., 2002 [73] | Observational N = 88 | Left adrenal vein that drains into a double RV (4 cases—4.5%). | Not mentioned. | France | Not specified | Bilateral |
| Aljabri, 2001 [74] | Observational N = 1788 | Circumaortic LRV (29 cases—1.62%). Retroaortic LRV (57 cases—3.18%). | Not mentioned. | Canada | 929 males 859 females | Left |
| Shindo, 2000 [75] | Observational N = 166 | Circumaortic LRV (1 case). | Not mentioned. | Japan | 3 males 1 females | Left |
| Yoshinag, 2000 [76] | Observational N = 203 | Retroaortic LRV (1 case). | Not mentioned. | Japan | Not specified | Left |
| Satyapal, 1999 [77] | Observational N = 1008 | Circumaortic LRV (301 cases—30%). Retroaortic LRV (71 cases—7.1%). Additional RV (60 cases—6%). | Not mentioned. | South Africa | Not specified | Left |
| Pozniak, 1998 [78] | Observational N = 205 | Circumaortic LRV (17 cases—8.3%). Retroaortic LRV (6 cases—2.9%). | Not mentioned. | USA | 90 males 115 females | Bilateral |
| Trigaux, 1998 [79] | Observational N = 1014 | Circumaortic LRV, (64 cases—6.3%). Retroaortic LRV (38 cases—3.7%). | The distance between the entrance to the IVC in case of a circumaortic variation and the distance in the case of retroaortic RV were not statistically different (p = 0.6). | Belgium | 572 males 442 females | Left |
| Baptista-Silva et al., 1997 [80] | Observational N = 342 | Circumaortic LRV (6 cases—1.75%). Retroaortic LRV (8 cases—2.3%). Double RRV (9 cases—2.63%), triple (3 cases—0.87%). Right gonadal vein draining into the RRV (1 case—0.3%). | Not mentioned. | Brazil | 134 males 208 females | Bilateral |
| Hicks, 1995 [81] | Observational N = 108 | Circumaortic LRV (11 cases—10%). Retroaortic LRV (2 cases—1.85%). Double LRV (5 cases—4.6%), triple (1 case—0.92%). Double RRV (18 cases—16.6%), triple (4 cases—3.7%). | No statistically significant difference between the 108 patients included and the 78 excluded regarding the indication for the procedure or demographic information such as sex, age, height, or weight. Renal venography was more sensitive in detecting both significant (p < 0.001) and insignificant (p < 0.001) abnormalities. | USA | 51 males 57 females | Bilateral |
| Kaufman, 1995 [82] | Observational N = 150 | Circumaortic LRV (8 cases—5%). Retroaortic LRV (10 cases—7%). Multiple RRV (12 cases—8%). | Not mentioned. | USA | Not specified | Bilateral |
| Satyapal, 1995 [83] | Observational N = 153 | Double RRV (40 cases—26%), triple (5 cases—3.2%). Double LRV (4 cases—2.6%). | Not mentioned. | South Africa | 131 males 22 females | Bilateral |
| Benedetti-Panici, 1994 [84] | Observational N = 309 | Circumaortic RV (3 cases—0.97%). | Not mentioned. | Italy | 309 females | Bilateral |
| Martinez-Almagro, 1992 [85] | Observational N = 116 | Retroaortic LRV (6 cases—5%). | Not mentioned. | Spain | 94 males 22 females | Left |
| Hoeltl, 1990 [3] | Observational N = 4520 | Circumaortic LRV (4 cases—0.08%). Retroaortic LRV (29 cases—0.6%). | Not mentioned. | Austria | Not specified | Left |
| Observational N = 354 | Circumaortic LRV (2 cases—0.5%). Retroaortic LRV (4 cases—1.2%). | |||||
| Observational N = 215 | Circumaortic LRV (2 cases—0.9%). Retroaortic LRV (6 cases—2.8%). | |||||
| Monkhouse, 1986 [86] | Observational N = 57 | Circumaortic LRV (2 cases—3.5%). Double RRV (1 case—1.7%). RRV drains into IVC lower than LRV (22 cases—38.5%). RRV drains into IVC upper than LRV (4 cases—7%). | Not mentioned. | UK | 25 embalmed (9 males and 16 females); 32 fresh postmortem (8 males and 24 females) | Bilateral |
| Mayo, 1983 [87] | Observational N = 1140 | Circumaortic LRV (1 case—0.08%). | Not mentioned. | Canada | Not specified | Left |
| Reed, 1982 [88] | Observational N = 433 | Circumaortic LRV (19 cases—4.4%). Retroaortic LRV (8 cases—1.8%). | Not mentioned. | USA | Not specified | Left |
| Alexander, 1981 [89] | Observational N = 1200 | Circumaortic LRV (3 cases—0.25%). Retroaortic LRV (1 case—0.08%). | Not mentioned. | USA | Not specified | Left |
| Beckmann, 1980 [90] | Observational N = 132 | Circumaortic venous ring (8 cases—6.06%). Retroaortic LRV (1 case—0.75%). Double RRV (13 cases—9.84%), triple (3 cases—2.27%). Right gonadal vein draining into the RRV (4 cases—3%). | Not mentioned. | USA | Not specified | Bilateral |
| Kramer, 1980 [91] | Observational N = 193 | Circumaortic RV (10 cases—5%). | Not mentioned. | South Africa | 140 males 53 females | Left |
| Lien, 1977 [92] | Observational N = 100 | Circumaortic LRV (10 cases—10%). Retroaortic LRV (2 cases—2%). | Not mentioned. | Norway | 100 males | Left |
| Goswami, 1976 [93] | Observational N = 1 | Double LRV. | Not mentioned. | USA | 1 female | Left |
| Royster, 1974 [94] | Observational N = 159 | Circumaortic LRV (1 case—0.6%). Retroaortic LRV (3 cases—1.8%), Double LRV (1 case—0.6%). | Not mentioned. | USA | Not specified | Left |
| Royster, 1974 [94] | Observational N = 228 | Circumaortic LRV (1 case—0.43%). Retroaortic LRV (2 cases—0.8%). | Not mentioned. | USA | Not specified | Left |
| Davis, 1968 [95] | Observational N = 270 | Circumaortic LRV (4 cases—1.5%). Retroaortic LRV (5 cases—1.8%). | Not mentioned. | USA | 9 males | Left |
| Ross, 1961 [96] | Observational N = 34 | Double RRV (7 cases—20.5%). Double LRV (1 case—3%). | Not mentioned. | Scotland | 16 males 18 females | Bilateral |
| Reis, 1959 [97] | Observational N = 500 | Circumaortic RV (30 cases—6%). Retroaortic LRV (12 cases—2.4%). Double LRV (4 cases—0.8%) Double RRV (51 cases—10.2%), triple (3 cases—0.6%). | Not mentioned. | USA | 437 males 63 females | Bilateral |
| Author and Year | Total N | Prevalence | Multiple RV | RV Course (Circumaortic or Retroaortic) | RV Ramifications | Unusual Origin of RV |
|---|---|---|---|---|---|---|
| Pedrao, 2023 [13] | 1 | Multiple RV: 1 | 1 | Not mentioned | Not mentioned | Not mentioned |
| Silva 2021 [14] | 1 | Multiple RV: 1 RV course: 1 | 1 | 1 | Not mentioned | Not mentioned |
| Pradeep, 2020 [15] | 188 | Multiple RV: 12 RV course: 4 | 12 | 4 | Not mentioned | Not mentioned |
| Salimy et al., 2020 [16] | 1 | Multiple RV: 1 | 1 | Not mentioned | Not mentioned | Not mentioned |
| Fontana, 2018 [17] | 1 | Multiple RV: 1 RV course: 1 | 1 | 1 | Not mentioned | Not mentioned |
| Tatarano et al., 2019 [18] | 120 | RV course: 7 | Not mentioned | 7 | Not mentioned | Not mentioned |
| Dunnwald, 2019 [19] | 1 | Multiple RV: 1 | 1 | Not mentioned | Not mentioned | Not mentioned |
| Shaheen, 2018 [6] | 50 | Multiple RV: 46 | 46 | Not mentioned | Not mentioned | Not mentioned |
| Hassan, 2017 [20] | 63 | Multiple RV: 5 | 5 | Not mentioned | Not mentioned | Not mentioned |
| Nambur, 2017 [21] | 60 | Multiple RV: 3 RV course: 7 | 3 | 7 | Not mentioned | Not mentioned |
| Ayaz, 2016 [22] | 222 | RV course: 13 | Not mentioned | 13 | Not mentioned | Not mentioned |
| Çınar, 2016 [23] | 504 | Multiple RV: 109 RV course: 47 | 109 | 47 | Not mentioned | Not mentioned |
| Duran, 2016 [24] | 23 | RV course: 2 | Not mentioned | 2 | Not mentioned | Not mentioned |
| Kumaresan, 2016 [25] | 100 | Multiple RV: 19 RV course: 4 | 19 | 4 | Not mentioned | Not mentioned |
| Pandya, 2016 [26] | 200 | Multiple RV: 66 RV course: 13 | 66 | 13 | Not mentioned | Not mentioned |
| Staśkiewicz, 2016 [27] | 996 | RV course: 99 | Not mentioned | 99 | Not mentioned | Not mentioned |
| Bouzouita et al., 2015 [28] | 71 | Multiple RV: 8 RV course: 1 | 8 | 1 | Not mentioned | Not mentioned |
| Heidler, 2015 [29] | 7929 | RV course: 61 | Not mentioned | 61 | Not mentioned | Not mentioned |
| Mazengenya, 2015 [99] | 1 | Multiple RV: 1 | 1 | Not mentioned | Not mentioned | Not mentioned |
| Reginelli, 2015 [30] | 921 | Multiple RV: 94 RV course: 219 | 94 | 219 | Not mentioned | Not mentioned |
| Resorlu, 2015 [31] | 680 | Multiple RV: 46 RV course: 53 | 46 | 53 | Not mentioned | Not mentioned |
| Ballesteros, 2014 [33] | 312 | Multiple RV: 34 RV course: 3 | 34 | 3 | Not mentioned | Not mentioned |
| Boyaci, 2014 [34] | 746 | RV course: 73 | Not mentioned | 73 | Not mentioned | Not mentioned |
| Ferreira, 2014 [35] | 1 | Multiple RV: 1 RV course: 1 | 1 | 1 | Not mentioned | Not mentioned |
| Lavy et al., 2015 [36] | 1 | Multiple RV: 1 | 1 | Not mentioned | Not mentioned | Not mentioned |
| Rashid, 2014 [37] | 100 | Multiple RV: 17 RV course: 8 | 17 | 8 | Not mentioned | Not mentioned |
| Șahin, 2014 [38] | 2189 | RV course: 50 | Not mentioned | 50 | Not mentioned | Not mentioned |
| Dilli, 2013 [39] | 1204 | RV course: 63 | Not mentioned | 63 | Not mentioned | Not mentioned |
| Poyraz, 2013 [41] | 1000 | RV course: 68 | Not mentioned | 68 | Not mentioned | Not mentioned |
| Tao, 2013 [42] | 378 | RV course: 15 | Not mentioned | 15 | Not mentioned | Not mentioned |
| Apisarnthanarak, 2012 [43] | 65 | Multiple RV: 24 RV course: 2 RV ramifications: 4 | 24 | 2 | 4 | Not mentioned |
| Atalar, 2012 [44] | 739 | RV course: 23 | Not mentioned | 23 | Not mentioned | Not mentioned |
| Bouali et al., 2012 [45] | 120 | Multiple RV: 24. RV course: 11 | 24 | 11 | Not mentioned | Not mentioned |
| Dilli, 2012 [46] | 2644 | RV course: 71 | Not mentioned | 71 | Not mentioned | Not mentioned |
| Gupta, 2011 [47] | 30 | Multiple RV: 2 RV course: 4 | 2 | 4 | Not mentioned | Not mentioned |
| Yi et al., 2012 [48] | 3 | RV course: 2 | Not mentioned | 2 | Not mentioned | Not mentioned |
| Costa et al., 2011 [49] | 254 | Multiple RV: 28 RV course: 4 | 28 | 4 | Not mentioned | Not mentioned |
| Kulkarni, 2011 [50] | 102 | Multiple RV: 7 RV course: 6 | 7 | 6 | Not mentioned | Not mentioned |
| Li et al., 2011 [51] | 61 | RV ramification: 51 | Not mentioned | Not mentioned | 51 | Not mentioned |
| Turkvatan, 2009 [53] | 59 | Multiple RV: 3 RV course: 5 | 3 | 5 | Not mentioned | 4 |
| Kaneko et al., 2008 [54] | 190 | Multiple RV: 25 | 25 | Not mentioned | Not mentioned | Not mentioned |
| Mir et al., 2008 [55] | 1 | Multiple RV: 1 | 1 | Not mentioned | Not mentioned | Not mentioned |
| Natsis, 2008 [56] | 319 | RV course: 8 | Not mentioned | 8 | Not mentioned | Not mentioned |
| Tombul, 2008 [57] | 60 | Multiple RV: 9 | 9 | Not mentioned | Not mentioned | Not mentioned |
| Yagci, 2008 [58] | 783 | RV course: 42 | Not mentioned | 42 | Not mentioned | Not mentioned |
| Holt, 2007 [59] | 278 | RV course: 9 | Not mentioned | 9 | Not mentioned | Not mentioned |
| Karaman, 2007 [60] | 1856 | RV course: 85 | Not mentioned | 85 | Not mentioned | 89 |
| Karazincir, 2007 [61] | 277 | RV course: 16 | Not mentioned | 16 | Not mentioned | Not mentioned |
| Koc, 2007 [62] | 1120 | Multiple RV: 210 RV course: 115 | 210 | 115 | Not mentioned | Not mentioned |
| Raman, 2007 [63] | 126 | Multiple RV: 40 RV course: 13 | 40 | 13 | Not mentioned | Not mentioned |
| Namasivayam, 2006 [64] | 48 | Multiple RV: 14 RV course: 3 | 14 | 3 | Not mentioned | Not mentioned |
| Arslan, 2005 [65] | 1125 | RV course: 19 | Not mentioned | 19 | Not mentioned | Not mentioned |
| Kawamoto, 2005 [66] | 100 | RV course: 5 RV ramifications: 6 | Not mentioned | 5 | 6 | Not mentioned |
| Klemm, 2005 [67] | 86 | RV course: 1 | Not mentioned | 1 | Not mentioned | Not mentioned |
| Janschek, 2004 [7] | 119 | Multiple RV: 35 RV course: 10 | 35 | 10 | Not mentioned | Not mentioned |
| Lin, 2004 [68] | 170 | RV course: 18 | Not mentioned | 16 | Not mentioned | Not mentioned |
| Yeh, 2004 [69] | 186 | RV course: 9 | Not mentioned | 9 | Not mentioned | Not mentioned |
| Yesidag, 2004 [70] | 1003 | RV course: 32 | Not mentioned | 32 | Not mentioned | Not mentioned |
| Senecail et al., 2003 [71] | 2 | RV course: 2 | Not mentioned | 2 | Not mentioned | Not mentioned |
| Duques, 2002 [72] | 34 | Multiple renal vein: 3 RV course: 1 | 3 | 1 | Not mentioned | Not mentioned |
| Sebe et al., 2002 [73] | 88 | Multiple RV: 4 RV origin: 4 | 4 | Not mentioned | Not mentioned | 4 |
| Aljabri, 2001 [74] | 1788 | RV course: 86 | Not mentioned | 86 | Not mentioned | Not mentioned |
| Shindo, 2000 [75] | 166 | RV course: 1 | Not mentioned | 1 | Not mentioned | Not mentioned |
| Yoshinag, 2000 [76] | 203 | RV course: 1 | Not mentioned | 1 | Not mentioned | Not mentioned |
| Satyapal, 1999 [77] | 1008 | Multiple RV: 60 RV course: 372 | 60 | 372 | Not mentioned | Not mentioned |
| Pozniak, 1998 [78] | 205 | RV course: 23 | Not mentioned | 23 | Not mentioned | Not mentioned |
| Trigaux, 1998 [79] | 1014 | RV course: 102 | Not mentioned | 102 | Not mentioned | Not mentioned |
| Baptista-Silva et al., 1997 [80] | 342 | Multiple RV: 12 RV course: 14 RV ramifications: 1 | 12 | 14 | 1 | Not mentioned |
| Hicks, 1995 [81] | 108 | Multiple RV: 28 RV course: 13 | 28 | 13 | Not mentioned | Not mentioned |
| Kaufman, 1995 [82] | 150 | Multiple RV: 12 RV course: 18 | 12 | 18 | Not mentioned | Not mentioned |
| Satyapal, 1995 [83] | 153 | Multiple RV: 49 | 49 | Not mentioned | Not mentioned | Not mentioned |
| Benedetti-Panici, 1994 [84] | 309 | RV course: 3 | Not mentioned | 3 | Not mentioned | Not mentioned |
| Martinez-Almagro, 1992 [85] | 116 | RV course: 6 | Not mentioned | 6 | Not mentioned | Not mentioned |
| Hoeltl, 1990 [3] | 5089 | RV course: 47 | Not mentioned | 47 | Not mentioned | Not mentioned |
| Monkhouse, 1986 [86] | 57 | Multiple RV: 1 RV course: 2 RV origin: 26 | 1 | 2 | Not mentioned | 26 |
| Mayo, 1983 [87] | 1140 | RV course: 1 | Not mentioned | 1 | Not mentioned | Not mentioned |
| Reed, 1982 [88] | 433 | RV course: 27 | Not mentioned | 27 | Not mentioned | Not mentioned |
| Alexander, 1981 [89] | 1200 | RV course: 4 | Not mentioned | 4 | Not mentioned | Not mentioned |
| Beckmann, 1980 [90] | 132 | Multiple RV: 9 RV course: 16 RV ramifications: 4 | 9 | 16 | 4 | Not mentioned |
| Kramer, 1980 [91] | 193 | RV course: 10 | Not mentioned | 10 | Not mentioned | Not mentioned |
| Lien, 1977 [92] | 100 | RV course: 12 | Not mentioned | 12 | Not mentioned | Not mentioned |
| Goswami, 1976 [93] | 1 | Multiple RV: 1 | 1 | Not mentioned | Not mentioned | Not mentioned |
| Royster, 1974 [94] | 159 | Multiple RV: 2 RV course: 5 | 2 | 5 | Not mentioned | Not mentioned |
| Royster, 1974 [94] | 228 | RV course: 3 | Not mentioned | 3 | Not mentioned | Not mentioned |
| Davis, 1968 [95] | 270 | RV course: 9 | Not mentioned | 9 | Not mentioned | Not mentioned |
| Ross, 1961 [96] | 34 | Multiple RV: 8 | 8 | Not mentioned | Not mentioned | Not mentioned |
| Reis, 1959 [97] | 500 | Multiple RV: 58 RV course: 42 | 58 | 42 | Not mentioned | Notmentioned |
| Author | JBI Q1 | JBI Q2 | JBI Q3 | JBI Q4 | JBI Q5 | JBI Q6 | JBI Q7 | JBI Q8 | Bias Risk |
|---|---|---|---|---|---|---|---|---|---|
| Dunnwald et al., 2019 [19] | Low | ||||||||
| Eid et al., 2013 [40] | Low | ||||||||
| Favaro et al., 2009 [52] | Low | ||||||||
| Ferreira et al., 2014 [35] | Low | ||||||||
| Yi et al., 2012 [48] | Low | ||||||||
| Goswami et al., 1976 [93] | Low | ||||||||
| Mazengenya et al., 2015 [99] | Low | ||||||||
| Mir et al., 2008 [55] | Low | ||||||||
| Pedrao, 2023 [13] | Low | ||||||||
| Salimy et al., 2020 [16] | Low | ||||||||
| Senecail et al., 2003 [71] | Low | ||||||||
| Silva, 2021 [14] | Low |
| (1) Were patient’s demographic characteristics clearly described? | Yes | No | Unclear | Not applicable |
| (2) Was the patient’s history clearly described and presented as a timeline? | Yes | No | Unclear | Not applicable |
| (3) Was the current clinical condition of the patient on presentation clearly described? | Yes | No | Unclear | Not applicable |
| (4) Were diagnostic tests or assessment methods results clearly described? | Yes | No | Unclear | Not applicable |
| (5) Was the intervention(s) or treatment procedure(s) clearly described? | Yes | No | Unclear | Not applicable |
| (6) Was the postintervention clinical condition clearly described? | Yes | No | Unclear | Not applicable |
| (7) Were adverse events (harms) or unanticipated events identified and described? | Yes | No | Unclear | Not applicable |
| (8) Does the case report provide takeaway lessons? | Yes | No | Unclear | Not applicable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela Fuenzalida, J.J.; Vera-Tapia, K.; Urzúa-Márquez, C.; Yáñez-Castillo, J.; Trujillo-Riveros, M.; Koscina, Z.; Orellana-Donoso, M.; Nova-Baeza, P.; Suazo-Santibañez, A.; Sanchis-Gimeno, J.; et al. Anatomical Variants of the Renal Veins and Their Relationship with Morphofunctional Alterations of the Kidney: A Systematic Review and Meta-Analysis of Prevalence. J. Clin. Med. 2024, 13, 3689. https://doi.org/10.3390/jcm13133689
Valenzuela Fuenzalida JJ, Vera-Tapia K, Urzúa-Márquez C, Yáñez-Castillo J, Trujillo-Riveros M, Koscina Z, Orellana-Donoso M, Nova-Baeza P, Suazo-Santibañez A, Sanchis-Gimeno J, et al. Anatomical Variants of the Renal Veins and Their Relationship with Morphofunctional Alterations of the Kidney: A Systematic Review and Meta-Analysis of Prevalence. Journal of Clinical Medicine. 2024; 13(13):3689. https://doi.org/10.3390/jcm13133689
Chicago/Turabian StyleValenzuela Fuenzalida, Juan Jose, Karla Vera-Tapia, Camila Urzúa-Márquez, Javiera Yáñez-Castillo, Martín Trujillo-Riveros, Zmilovan Koscina, Mathias Orellana-Donoso, Pablo Nova-Baeza, Alejandra Suazo-Santibañez, Juan Sanchis-Gimeno, and et al. 2024. "Anatomical Variants of the Renal Veins and Their Relationship with Morphofunctional Alterations of the Kidney: A Systematic Review and Meta-Analysis of Prevalence" Journal of Clinical Medicine 13, no. 13: 3689. https://doi.org/10.3390/jcm13133689
APA StyleValenzuela Fuenzalida, J. J., Vera-Tapia, K., Urzúa-Márquez, C., Yáñez-Castillo, J., Trujillo-Riveros, M., Koscina, Z., Orellana-Donoso, M., Nova-Baeza, P., Suazo-Santibañez, A., Sanchis-Gimeno, J., Bruna-Mejias, A., & Gutiérrez Espinoza, H. (2024). Anatomical Variants of the Renal Veins and Their Relationship with Morphofunctional Alterations of the Kidney: A Systematic Review and Meta-Analysis of Prevalence. Journal of Clinical Medicine, 13(13), 3689. https://doi.org/10.3390/jcm13133689






