Use of HugoTM RAS in General Surgery: The First 70 Cases at a German Centre and a Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Own Patients
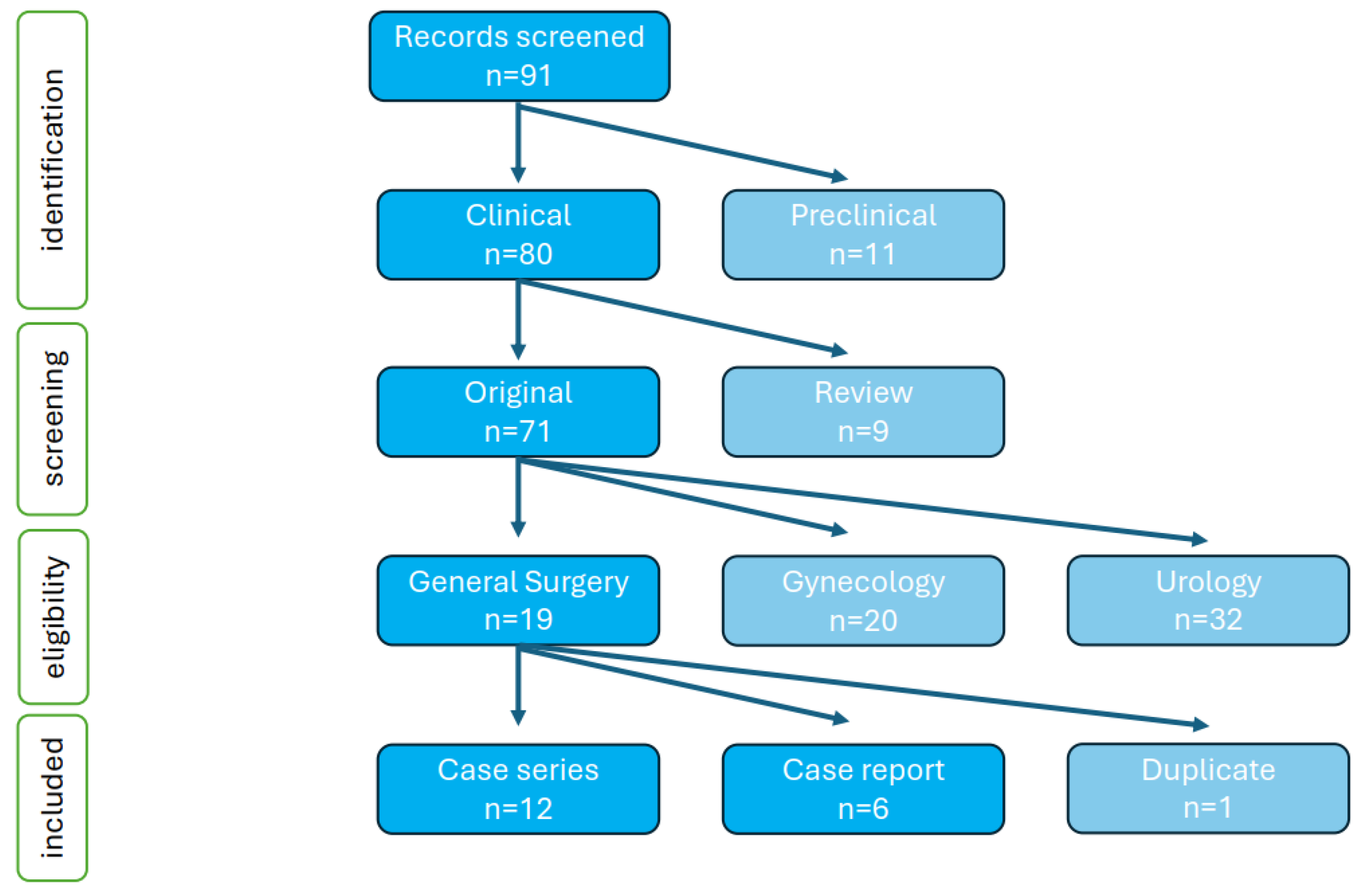
2.2. Systematic Review of the Literature
3. Results
3.1. Own Patients and Procedures
3.2. Patients and Procedures Reported in the Literature
3.3. Combined Data on Most Common Surgical Procedures
3.3.1. Lower GI surgery
3.3.2. Upper GI surgery
3.3.3. Cholecystectomy
3.3.4. Hernia Repair
3.3.5. Adrenals and Spleen
3.3.6. Technical Performance of Device
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marino, F.; Moretto, S.; Rossi, F.; Gandi, C.; Gavi, F.; Bientinesi, R.; Campetella, M.; Russo, P.; Bizzarri, F.P.; Scarciglia, E.; et al. Robot-Assisted Radical Prostatectomy Performed with the Novel Hugo™ RAS System: A Systematic Review and Pooled Analysis of Surgical, Oncological, and Functional Outcomes. J. Clin. Med. 2024, 13, 2551. [Google Scholar] [CrossRef] [PubMed]
- Prata, F.; Ragusa, A.; Tedesco, F.; Pira, M.; Iannuzzi, A.; Fantozzi, M.; Civitella, A.; Scarpa, R.M.; Papalia, R. Trifecta outcomes of robot-assisted partial nephrectomy using the new HugoTM RAS system versus laparoscopic partial nephrectomy. J. Clin. Med. 2024, 13, 2138. [Google Scholar] [CrossRef] [PubMed]
- Gioe, A.; Monterossi, G.; Alletti, S.G.; Panico, G.; Campagna, G.; Costatini, B.; Naldini, A.; Anchora, L.P.; Oliva, R.; Mastrovito, S.; et al. The new robotic system HUGO RAS for gynecologic surgery: First European experience from Gemelli Hospital. Int. J. Gynaecol. Obstet. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Belyaev, O.; Fahlbusch, T.; Slobodkin, I.; Uhl, W. Safety and feasibility of cholecystectomy with the HugoTM RAS: Proof of setup guides and first-in-human German experience. Visc. Med. 2023, 39, 76–86. [Google Scholar] [CrossRef]
- Raffaelli, M.; Gallucci, P.; Voloudakis, N.; Pennestri, F.; De Cicco, R.; Arcuri, G.; De Crea, C.; Bellantone, R. The new robotic platform HugoTM RAS for lateral transabdominal adrenalectomy: A first world report of a series of five cases. Updates Surg. 2023, 75, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, P.P.; Salaj, A.; Rocco, B.; Formisano, G. First worldwide report on Hugo RAS™ surgical platform in right and left colectomy. Updates Surg. 2023, 75, 775–780. [Google Scholar] [CrossRef]
- Gangemi, A.; Bernante, P.; Rottoli, M.; Pasquali, F.; Poggioli, G. Surgery of the alimentary tract for benign and malignant disease with the novel robotic platform HugoTM RAS. A first world report of safety and feasibility. Int. J. Med. Robot. 2023, 19, e2544. [Google Scholar] [CrossRef] [PubMed]
- Quijano, Y.; Vicente, E.; Ferri, V.; Naldini, C.; Pizzuti, G.; Caruso, R. Robot-assisted Nissen fundoplication with the new HUGOtm Robotic assisted system: First worldwide report with system description, docking settings and video. Int. J. Surg. Case Rep. 2023, 106, 108178. [Google Scholar] [CrossRef]
- Vicente, E.; Quijano, Y.; Ferri, V.; Caruso, R. Robot-assisted cholecystectomy with the new HUGOtm robotic-assisted system: First worldwide report with system description, docking settings, and video. Updates Surg. 2023, 75, 2039–2042. [Google Scholar] [CrossRef]
- Mintz, Y.; Pikarsky, A.J.; Brodie, R.; Elazary, R.; Helou, B.; Marom, G. Robotic inguinal hernia repair with the new Hugo RASTM system: First worldwide case series report. Minim. Invasive Ther. Allied Technol. 2023, 32, 300–306. [Google Scholar] [CrossRef]
- Caruso, R.; Vicente, E.; Quijano, Y.; Ferri, V. New era of robotic surgery: First case in Spain of right hemicolectomy on Hugo RAS surgical platform. BMJ Case Rep. 2023, 16, e256035. [Google Scholar] [CrossRef]
- Raffaelli, M.; Greco, F.; Pennestri, F.; Gallucci, P.; Ciccoritti, L.; Salvi, G.; Procopio, P.F.; Voloudakis, N. Robotic-assisted Roux-en-Y gastric bypass with the novel platform HugoTM RAS: Preliminary experience in 15 patients. Updates Surg. 2024, 76, 179–185. [Google Scholar] [CrossRef]
- Jebakumar, S.G.S.; Swain, S.K.; Munikrishnan, V.; Jayapal, L.; Kumar, R.S.; Baskaran, A.; Tasgaonkar, S.; Srivatsan, S. Robotic hernia repair with the novel HUGO robot system—An initial experience from a tertiary centre. J. Min. Access Surg. 2024. [Google Scholar] [CrossRef]
- Caputo, D.; Farolfi, T.; Molina, C.; Coppola, R. Full robotic cholecystectomy: First worldwide experiences with HUGO RAS surgical platform. ANZ J. Surg. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Salem, S.A.; Marom, G.; Shein, G.S.; Fishman, Y.; Helou, B.; Brodie, R.; Elazary, R.; Pikarsky, A.J.; Mintz, Y. Robotic Heller’s myotomy using the new HugoTM RAS system: First worldwide report. Surg. Endosc. 2024, 38, 1180–1190. [Google Scholar] [CrossRef]
- Caputo, D.; Cammarata, R.; Farolfi, T.; Coppola, R.; La Vaccara, V. First worldwide report on rectal resections with HugoTM surgical system: Description of docking angles and tips for an effective setup. ANZ J. Surg. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Romero-Marcos, J.M.; Sampson-Davila, J.G.; Cuenca-Gomez, C.; Altet-Torne, J.; Gonzalez-Abos, S.; Ojeda-Jimenez, I.; Galaviz-Sosa, M.L.; Delgado-Rivilla, S. Colorectal procedures with the novel HugoTM RAS system: Training process and case series report from a non-robotic surgical team. Surg. Endosc. 2024, 38, 2160–2168. [Google Scholar] [CrossRef]
- Wen, C.Y.; Lin, S.Y.; Wang, C.J.; Wu, J.C.H. Robot-assisted transabdominal preperitoneal approach for inguinal hernioplasty using Hugo RAS system: The first case report in Asia. Asian J. Surg. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Formisano, G.; Ferraro, L.; Salaj, A.; Bianchi, P.P. First report of robotic retromuscular incisional hernia repair with Hugo Ras™ surgical system. Updates Surg. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Quesada, N.; Irarrazaval, M.J.; Chen, D.C.; Grimoldi, M.; Pimentel, F.; Crovari, F. Robotic transversus abdominis release using HUGO RAS system: Our initial experience. Surg. Endosc. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Toyota, M.; Miyo, M.; Okuya, K.; Ito, T.; Akizuki, E.; Noda, A.; Ogawa, T.; Ishii, M.; Miura, R.; Ichihara, M.; et al. Cylindrical abdominoperineal resection for rectal cancer using the Hugo RAS system: The first ever case report for rectal cancer. Asian J. Endosc. Surg. 2024, 17, e13321. [Google Scholar] [CrossRef] [PubMed]
- Menendez, R.B.; Rojo, E.G.; Palacios, V.H.; Ochoa, J.A.F.; Quintas, J.J.; Mateos, F.L.; Fraile, A.; Manfredi, C.; Belli, S.; Bozzini, G.; et al. Da Vinci vs. Hugo RAS for robot-assisted radical prostatectomy: A prospective comparative single-center study. World J. Urol. 2024, 42, 336. [Google Scholar] [CrossRef] [PubMed]
- Rojo, E.G.; Palacios, V.H.; Menendez, R.B.; Ochoa, J.A.F.; Quintas, J.J.; Mateos, F.L.; Touijer, K.; Romero, O.J. Da Vinci and Hugo RAS platforms for robot-assisted partial nephrectomy: A preliminary prospective comparative analysis of the outcomes. Minerva Urol. Nephrol. 2024; online ahead of print. [Google Scholar] [CrossRef]
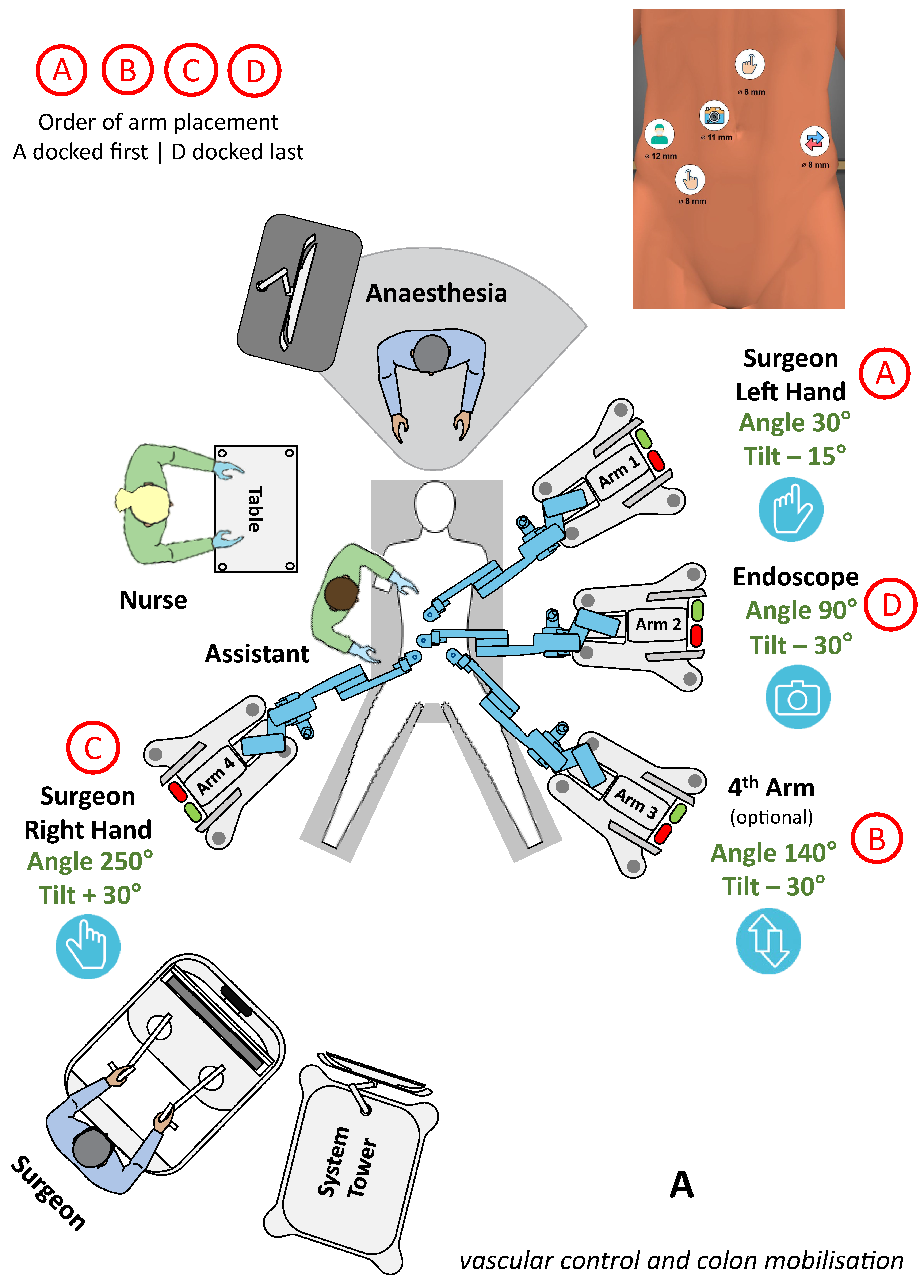
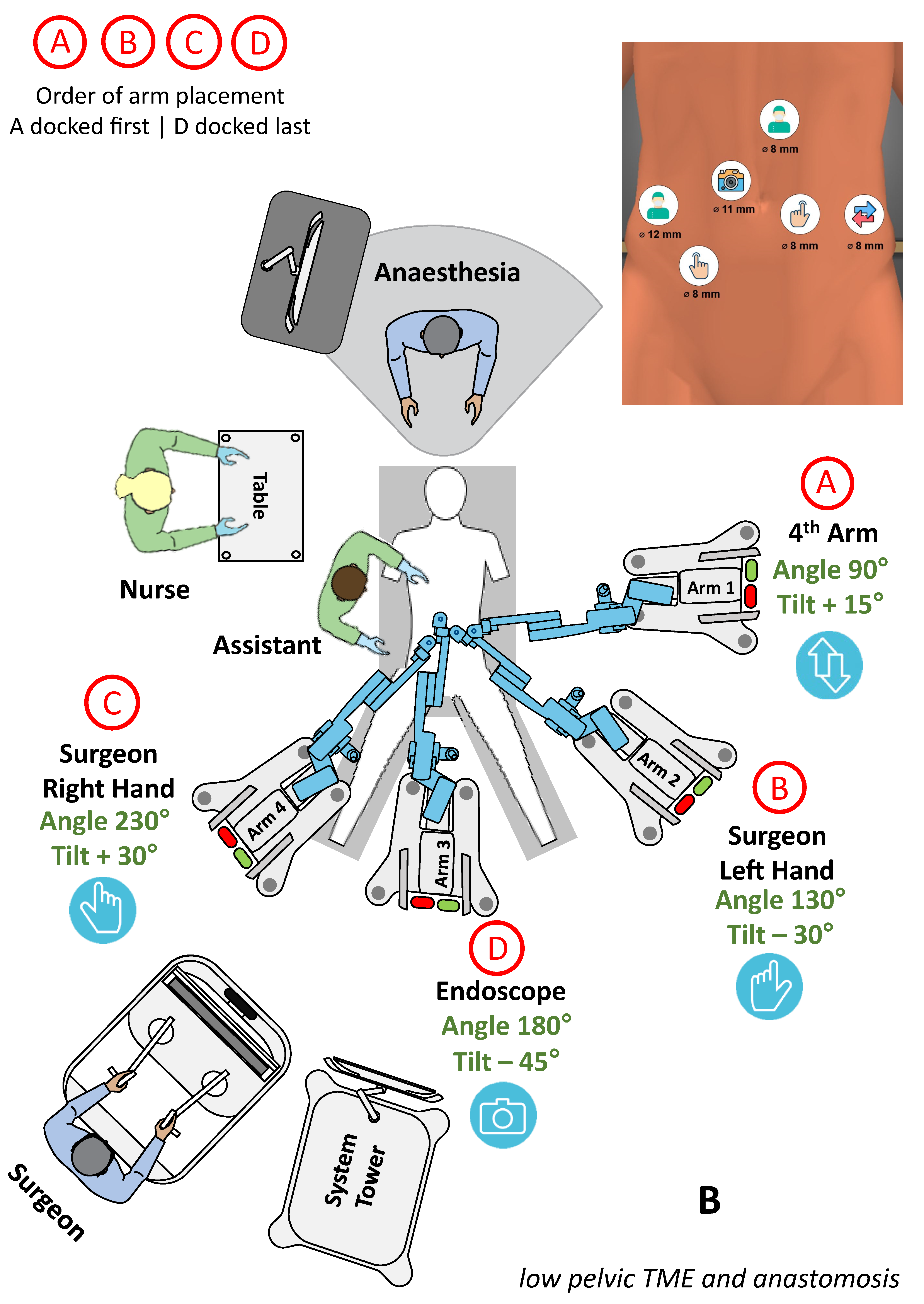
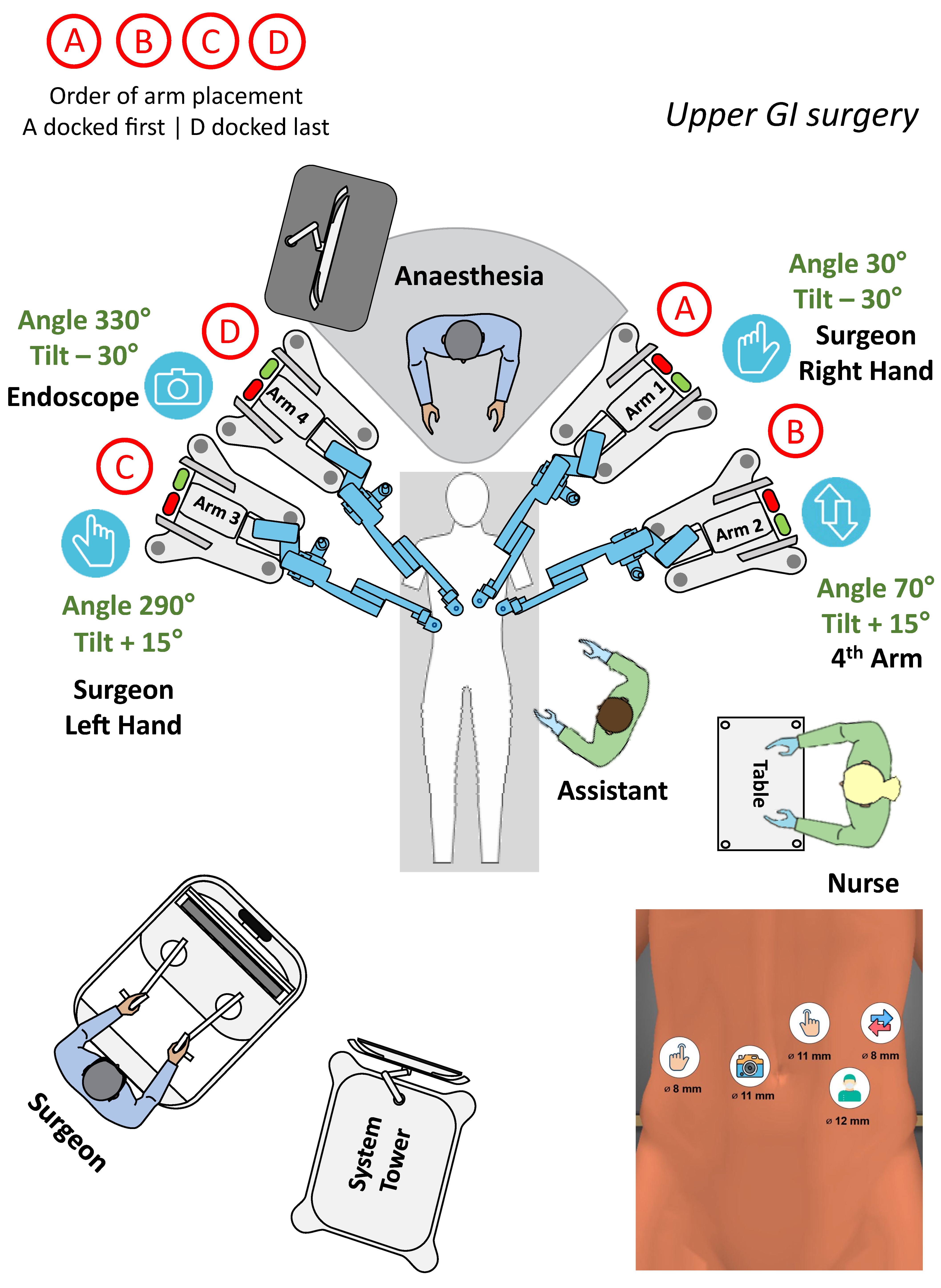
| Surgery | Diagnosis | Patients and Sex Distribution (F/M) | Age [Years] | BMI [kg/m2] | Dock Time [min] | Console Time [min] | OP Time [min] | Morbidity Major/Minor | Stay [Days] |
|---|---|---|---|---|---|---|---|---|---|
| Cholecystectomy | Lithiasis | 32 (23 F/9 M) | 62 (16–86) | 28 (19–35) | 10 (3–15) | 45 (21–172) | 72 (57–182) | 0/0 | 2 (1–5) |
| Sigmoidectomy | Diverticula | 11 (6 F/5 M) | 66 (52–77) | 25 (21–37) | 10 (7–18) | 120 (70–183) | 189 (115–249) | 0/1 | 7 (5–12) |
| Rectum resection | Cancer | 8 (2 F/6 M) | 66 (41–85) | 27 (21–34) | 12 (7–15) | 233 (120–332) | 325 (290–420) | 2/2 | 10 (7–34) |
| Right colectomy | Cancer | 8 (5 F/3 M) | 67 (61–87) | 27 (21–30) | 12 (10–15) | 180 (85–316) | 219 (193–280) | 1/1 | 7 (6–14) |
| Hiatoplasty | Hiatal hernia | 3 (2 F/1 M) | 47 (24–51) | 27 (21–36) | 10 (6–12) | 151 (120–169) | 197 (163–211) | 0/0 | 6 (5–7) |
| Hartmann reversal | Colostomy | 2 (1 F/1 M) | 50 (33–67) | 27 (24–30) | 13 (12–14) | 142 (120–164) | 278 (264–290) | 0/0 | 7 (7–7) |
| Cyst deroofing | Splenic cyst | 2 (1 F/1 M) | 55 (44–66) | 38 (36–40) | 15 (13–17) | 90 (81–100) | 130 (117–143) | 0/0 | 3 (3–3) |
| Left colectomy | Cancer | 1 (M) | 68 | 23 | 12 | 155 | 228 | 0/0 | 6 |
| Rectum exstirpation | Cancer | 1 (F) | 65 | 30 | 15 | 240 | 360 | 0/0 | 13 |
| Gastric resection | Schwannoma | 1 (M) | 68 | 26 | 13 | 115 | 147 | 0/0 | 5 |
| Adrenalectomy | Adenoma | 1 (F) | 69 | 19 | 12 | 150 | 200 | 0/0 | 6 |
| First Author | Centre | Year | Surgery | Diagnosis | Patients | Age [Years] | BMI [kg/m2] | Dock Time [min] | Console Time [min] | OP Time [min] | Stay [Days] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Raffaelli et al. [5] | Italy–Rome | 2023 | Adrenalectomy | Various | 5 | 61 | 25 | 6 | 61 | 119 | 3 |
| Bianchi et al. [6] | Italy–Milan | 2023 | Colectomy right, left | Cancer | 3 | 72 | 26 | 8 | 260 | 350 | 6 |
| Quijano et al. [8] | Spain–Madrid | 2023 | Nissen fundoplication | GERD | 1 | 65 | - | 3 | - | 100 | 3 |
| Gangemi et al. [7] | Italy–Bologna | 2023 | Colectomy various | Various | 7 | 60 | 24 | 7 | 141 | 210 | 8 |
| Cholecystectomy | Lithiasis | 7 | 51 | 26 | 6 | 50 | 97 | 1 | |||
| Gastrectomy partial | Various | 2 | 53 | 26 | 5 | 140 | 186 | 8 | |||
| Nissen fundoplication | GERD | 1 | 67 | 26 | 6 | 150 | 175 | 4 | |||
| Belyaev et al. [4] | Germany–Bochum | 2023 | Cholecystectomy | Lithiasis | 14 | 61 | 27 | 10 | 77 | 95 | 2 |
| Vicente et al. [9] | Spain–Madrid | 2023 | Cholecystectomy | Lithiasis | 1 | 54 | - | 3 | - | 70 | 2 |
| Mintz et al. [10] | Israel–Jerusalem | 2023 | Hernia repair | Groin hernia | 10 | - | - | 10 | 50 | 75 | - |
| Caruso et al. [11] | Spain–Madrid | 2023 | Right colectomy | Cancer | 1 | - | 20 | 5 | - | 200 | 8 |
| Raffaelli et al. [12] | Italy–Rome | 2024 | Roux-Y gastric bypass | Obesity | 15 | 48 | 42 | 7 | 100 | 150 | 2 |
| Jebakumar et al. [13] | India–Chennai | 2024 | Hernia repair | Hernia various | 7 | - | - | 23 | - | 128 | 2 |
| Caputo et al. [14] | Italy–Rome | 2024 | Cholecystectomy | Lithiasis | 3 | 59 | 26 | 10 | 50 | 98 | 2 |
| Salem et al. [15] | Israel–Jerusalem | 2024 | Heller’s myotomy | Achalasia | 10 | 43 | 23 | 10 | - | 130 | 2 |
| Caputo et al. [16] | Italy–Rome | 2024 | Rectal resection | Cancer | 3 | 71 | 25 | 12 | 338 | 415 | 8 |
| Romero-Marcos et al. [17] | Spain–Barcelona | 2024 | Colorectal resections | Various | 10 | 67 | 28 | 14 | - | 200 | 3 |
| Wen et al. [18] | Taiwan–Changhua | 2024 | rTAPP | Groin hernia | 1 | 33 | - | - | - | - | 1 |
| Formisano et al. [19] | Italy–Milan | 2024 | rTAR | Incisional hernia | 1 | - | - | 15 | 75 | 95 | 2 |
| Quezada et al. [20] | Chile–Santiago | 2024 | Hernia rTAR | Ventral hernia | 10 | 61 | 31 | 50 | 243 | 373 | 3 |
| Toyota et al. [21] | Japan–Sapporo | 2024 | Rectum exstirpation | Rectal cancer | 1 | 68 | 28 | - | 163 | 450 | 13 |
| Features | Hugo RAS | DaVinci |
|---|---|---|
| Platform design | Modular with 4 independent arms | Single pivot tower |
| Ergonomics of surgeon console | Free head and neck movement | Constant head and neck strain |
| Team communication | Direct and easy through open design | Limited, no eye contact to rest of team |
| Hand controls | Laparoscopy-like pistol grips | Endo-wrist finger grips |
| Resolution and image quality | Excellent via dedicated 3D-glasses | Excellent via console binoculars |
| Side observers | 3D-glasses allow multiple observers | Dual console allows a single 3D-observer |
| Teaching | Difficult due to single console | Easy due to dual console |
| Instruments | Short shaft, single use, limited choice | Great variety of instruments, multiple use |
| Setup configuration | Individual setup for every patient | Automatic setup after targeting |
| Docking memory | Not available | Yes, procedure specific |
| Vessel sealing device and linear stapler | Wristed and straight, not yet certified | Wristed, available |
| Camera | 0° and 30°, 10 mm, no ICG | 0° and 30°, 8 mm, ICG |
| Proctorship | Limited number of proctors | Plenty of experienced proctors |
| Training program | Short, basic, still in development | Established variety of courses |
| Role of bed-side assistant | Crucial, active | Supporting, passive |
| Video recording, editing and sharing | Via Touch Surgery app with AI-analysis | Via Intuitive Hub |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belyaev, O.; Fahlbusch, T.; Slobodkin, I.; Uhl, W. Use of HugoTM RAS in General Surgery: The First 70 Cases at a German Centre and a Systematic Review of the Literature. J. Clin. Med. 2024, 13, 3678. https://doi.org/10.3390/jcm13133678
Belyaev O, Fahlbusch T, Slobodkin I, Uhl W. Use of HugoTM RAS in General Surgery: The First 70 Cases at a German Centre and a Systematic Review of the Literature. Journal of Clinical Medicine. 2024; 13(13):3678. https://doi.org/10.3390/jcm13133678
Chicago/Turabian StyleBelyaev, Orlin, Tim Fahlbusch, Illya Slobodkin, and Waldemar Uhl. 2024. "Use of HugoTM RAS in General Surgery: The First 70 Cases at a German Centre and a Systematic Review of the Literature" Journal of Clinical Medicine 13, no. 13: 3678. https://doi.org/10.3390/jcm13133678
APA StyleBelyaev, O., Fahlbusch, T., Slobodkin, I., & Uhl, W. (2024). Use of HugoTM RAS in General Surgery: The First 70 Cases at a German Centre and a Systematic Review of the Literature. Journal of Clinical Medicine, 13(13), 3678. https://doi.org/10.3390/jcm13133678






