Tissue Iron Distribution in Anemic Patients with End-Stage Kidney Disease: Results of a Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Measurements and Classifications
2.3. Procedures of Magnetic Resonance Imaging
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Tissue Iron Distribution Measured by MRI R2* Sequence
3.3. Peripheral Iron Measurements Do Not Reflect Tissue Iron Distribution Appropriately
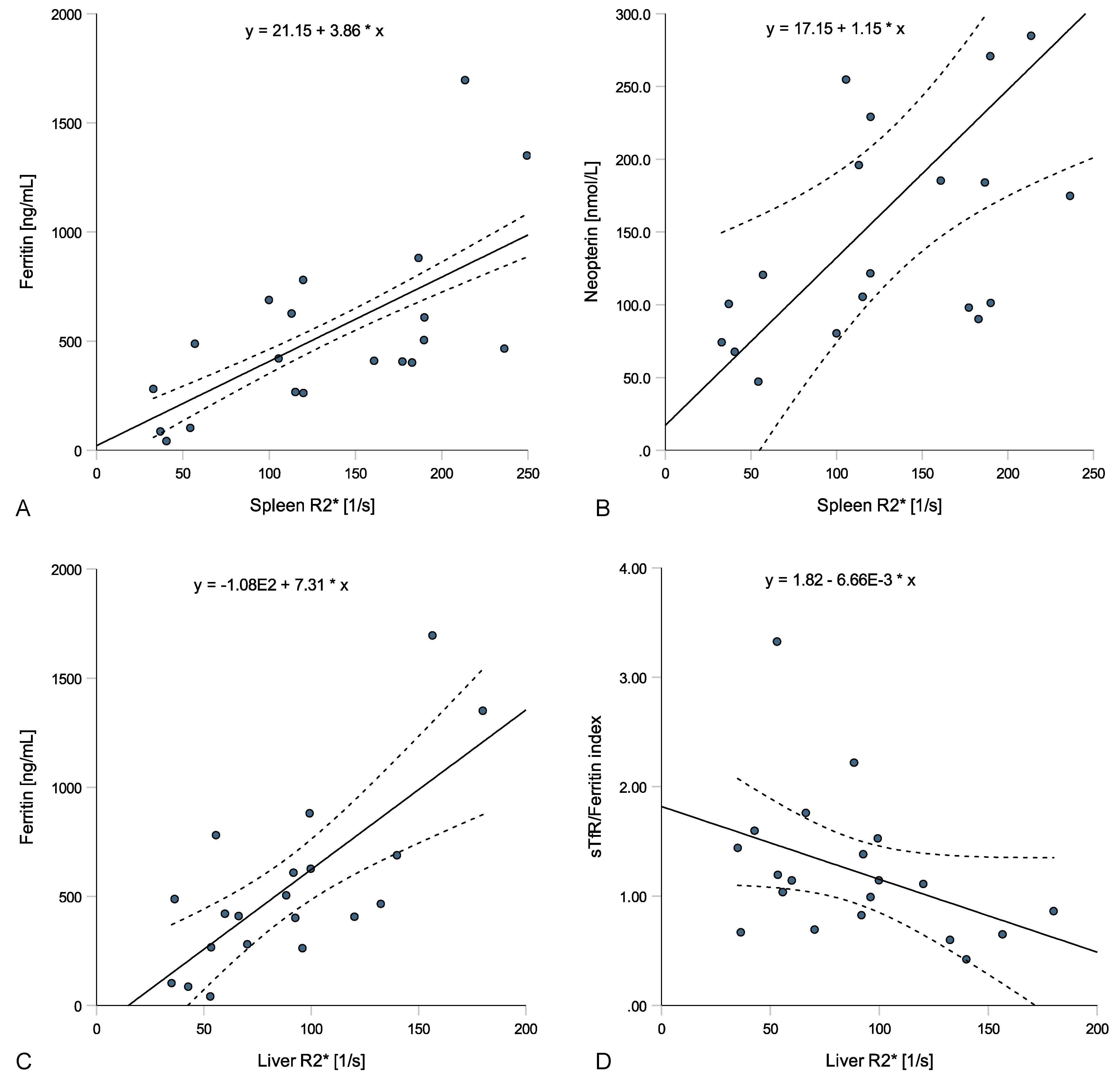
3.4. Tissue Iron Distribution, Serum Iron Homeostasis and Inflammatory Biomarkers
3.5. Iron Supplementation, ESA Therapy and Tissue Iron Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreno, F.; Gomez, J.M.L.; Sanz-Guajardo, D.; Jofre, R.; Valderrábano, F.; Spanish Cooperative Renal Patients Quality of Life Study Group4. Quality of life in dialysis patients. A Spanish multicentre study. Nephrol. Dial. Transplant. 1996, 11, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Astor, B.C.; Coresh, J.; Heiss, G.; Pettitt, D.; Sarnak, M.J. Kidney function and anemia as risk factors for coronary heart disease and mortality: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2006, 151, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Trivedi, B.K.; Kalantar-Zadeh, K.; Anderson, J.E. Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int. 2006, 69, 560–564. [Google Scholar] [CrossRef] [PubMed]
- St Peter, W.L.; Guo, H.; Kabadi, S.; Gilbertson, D.T.; Peng, Y.; Pendergraft, T.; Li, S. Prevalence, treatment patterns, and healthcare resource utilization in Medicare and commercially insured non-dialysis-dependent chronic kidney disease patients with and without anemia in the United States. BMC Nephrol. 2018, 19, 67. [Google Scholar] [CrossRef]
- Inker, L.A.; Grams, M.E.; Levey, A.S.; Coresh, J.; Cirillo, M.; Collins, J.F.; Gansevoort, R.T.; Gutierrez, O.M.; Hamano, T.; Heine, G.H.; et al. Relationship of Estimated GFR and Albuminuria to Concurrent Laboratory Abnormalities: An Individual Participant Data Meta-analysis in a Global Consortium. Am. J. Kidney Dis. 2019, 73, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Portolés, J.; Martín, L.; Broseta, J.J.; Cases, A. Anemia in Chronic Kidney Disease: From Pathophysiology and Current Treatments, to Future Agents. Front. Med. 2021, 8, 642296. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-M.; Wu, C.-J.; Lin, S.-L. Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formos. Med. Assoc. 2018, 117, 955–963. [Google Scholar] [CrossRef]
- Babitt, J.L.; Lin, H.Y. Mechanisms of anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef]
- Wenger, R.H.; Hoogewijs, D. Regulated oxygen sensing by protein hydroxylation in renal erythropoietin-producing cells. Am. J. Physiol. Ren. Physiol. 2010, 298, F1287–F1296. [Google Scholar] [CrossRef]
- Dallalio, G.; Law, E.; Means, R.T., Jr. Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrations. Blood 2006, 107, 2702–2704. [Google Scholar] [CrossRef]
- Libregts, S.F.; Gutiérrez, L.; de Bruin, A.M.; Wensveen, F.M.; Papadopoulos, P.; van Ijcken, W.; Ozgür, Z.; Philipsen, S.; Nolte, M.A. Chronic IFN-γ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood 2011, 118, 2578–2588. [Google Scholar] [CrossRef] [PubMed]
- Mitlyng, B.L.; Singh, J.A.; Furne, J.K.; Ruddy, J.; Levitt, M.D. Use of breath carbon monoxide measurements to assess erythrocyte survival in subjects with chronic diseases. Am. J. Hematol. 2006, 81, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Theurl, I.; Hilgendorf, I.; Nairz, M.; Tymoszuk, P.; Haschka, D.; Asshoff, M.; He, S.; Gerhardt, L.M.; Holderried, T.A.; Seifert, M.; et al. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat. Med. 2016, 22, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Bock, A.; Carrera, F.; Eckardt, K.U.; Gaillard, C.; Van Wyck, D.; Roubert, B.; Cushway, T.; Roger, S.D. The FIND-CKD study--a randomized controlled trial of intravenous iron versus oral iron in non-dialysis chronic kidney disease patients: Background and rationale. Nephrol. Dial. Transpl. 2014, 29, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Batchelor, E.K.; Kapitsinou, P.; Pergola, P.E.; Kovesdy, C.P.; Jalal, D.I. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J. Am. Soc. Nephrol. 2020, 31, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Theurl, I.; Aigner, E.; Theurl, M.; Nairz, M.; Seifert, M.; Schroll, A.; Sonnweber, T.; Eberwein, L.; Witcher, D.R.; Murphy, A.T.; et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: Diagnostic and therapeutic implications. Blood 2009, 113, 5277–5286. [Google Scholar] [CrossRef]
- Weiss, G.; Theurl, I.; Eder, S.; Koppelstaetter, C.; Kurz, K.; Sonnweber, T.; Kobold, U.; Mayer, G. Serum hepcidin concentration in chronic haemodialysis patients: Associations and effects of dialysis, iron and erythropoietin therapy. Eur. J. Clin. Investig. 2009, 39, 883–890. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Sonnweber, T.; Theurl, I.; Seifert, M.; Schroll, A.; Eder, S.; Mayer, G.; Weiss, G. Impact of iron treatment on immune effector function and cellular iron status of circulating monocytes in dialysis patients. Nephrol. Dial. Transplant. 2010, 26, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; White, C.; Anker, S.D.; Bhandari, S.; Farrington, K.; Kalra, P.A.; McMurray, J.J.V.; Murray, H.; Tomson, C.R.V.; Wheeler, D.C.; et al. Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. N. Engl. J. Med. 2019, 380, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Freburger, J.K.; Ellis, A.R.; Wang, L.; Butler, A.M.; Kshirsagar, A.V.; Winkelmayer, W.C.; Brookhart, M.A. Comparative Effectiveness of Iron and Erythropoiesis-Stimulating Agent Dosing on Health-Related Quality of Life in Patients Receiving Hemodialysis. Am. J. Kidney Dis. 2016, 67, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Babitt, J.L.; Eisenga, M.F.; Haase, V.H.; Kshirsagar, A.V.; Levin, A.; Locatelli, F.; Małyszko, J.; Swinkels, D.W.; Tarng, D.C.; Cheung, M.; et al. Controversies in optimal anemia management: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021, 99, 1280–1295. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, L.E.K.; Thomas, W.; Glen, J.; Padhi, S.; Pordes, B.A.J.; Wonderling, D.; Connell, R.; Stephens, S.; Mikhail, A.I.; Fogarty, D.G.; et al. Diagnosis and Management of Iron Deficiency in CKD: A Summary of the NICE Guideline Recommendations and Their Rationale. Am. J. Kidney Dis. 2016, 67, 548–558. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.V.; Hoffmann, A.; Fischer, C.; Petzer, V.; Asshoff, M.; Theurl, I.; Tymoszuk, P.; Seifert, M.; Brigo, N.; Hilbe, R.; et al. Comparative analysis of oral and intravenous iron therapy in rat models of inflammatory anemia and iron deficiency. Haematologica 2023, 108, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Bircher, A.J.; Eckardt, K.U.; Obrador, G.T.; Pollock, C.A.; Stenvinkel, P.; Swinkels, D.W.; Wanner, C.; Weiss, G.; Chertow, G.M. Iron management in chronic kidney disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016, 89, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.E.; Ponka, P. Iron Overload in Human Disease. N. Engl. J. Med. 2012, 366, 348–359. [Google Scholar] [CrossRef]
- Nashwan, A.J.; Yassin, M.A.; Abd-Alrazaq, A.; Shuweihdi, F.; Othman, M.; Abdul Rahim, H.F.; Shraim, M. Hepatic and cardiac iron overload quantified by magnetic resonance imaging in patients on hemodialysis: A systematic review and meta-analysis. Hemodial. Int. 2023, 27, 3–11. [Google Scholar] [CrossRef]
- Ribeiro Júnior, R.F.; Marques, V.B.; Nunes, D.O.; Stefanon, I.; Dos Santos, L. Chronic iron overload induces functional and structural vascular changes in small resistance arteries via NADPH oxidase-dependent O2− production. Toxicol. Lett. 2017, 279, 43–52. [Google Scholar] [CrossRef]
- World Health, O. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Mcmurray, J.; Parfrey, P.S.; Adamson, J.W.; Aljama, P.; Berns, J.S.; Bohlius, J.; Drüeke, T.B.; Finkelstein, F.O.; Fishbane, S.; Ganz, T.; et al. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Off. J. Int. Soc. Nephrol. 2012, 2, 279–335. [Google Scholar] [CrossRef]
- Henninger, B.; Zoller, H.; Rauch, S.; Finkenstedt, A.; Schocke, M.; Jaschke, W.; Kremser, C. R2* relaxometry for the quantification of hepatic iron overload: Biopsy-based calibration and comparison with the literature. Rofo 2015, 187, 472–479. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Gatehouse, P.D.; Smith, G.C.; Mohiaddin, R.H.; Pennell, D.J.; Firmin, D.N. Myocardial T measurements in iron-overloaded thalassemia: An in vivo study to investigate optimal methods of quantification. Magn. Reson. Med. 2008, 60, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Henninger, B.; Alustiza, J.; Garbowski, M.; Gandon, Y. Practical guide to quantification of hepatic iron with MRI. Eur. Radiol. 2020, 30, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Kucybała, I.; Ciuk, S.; Tęczar, J. Spleen enlargement assessment using computed tomography: Which coefficient correlates the strongest with the real volume of the spleen? Abdom. Radiol. 2018, 43, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Schwenzer, N.F.; Machann, J.; Haap, M.M.; Martirosian, P.; Schraml, C.; Liebig, G.; Stefan, N.; Häring, H.U.; Claussen, C.D.; Fritsche, A.; et al. T2* relaxometry in liver, pancreas, and spleen in a healthy cohort of one hundred twenty-nine subjects-correlation with age, gender, and serum ferritin. Investig. Radiol. 2008, 43, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, E.P.; Basty, N.; Whitcher, B.; Liu, Y.; Bell, J.D.; Cohen, R.L.; Cule, M.; Thomas, E.L. Analysis of MRI-derived spleen iron in the UK Biobank identifies genetic variation linked to iron homeostasis and hemolysis. Am. J. Hum. Genet. 2022, 109, 1092–1104. [Google Scholar] [CrossRef]
- Kromrey, M.L.; Röhnert, A.; Blum, S.; Winzer, R.; Hoffman, R.T.; Völzke, H.; Kacprowski, T.; Kühn, J.P. Whole-body R2∗ mapping to quantify tissue iron in iron storage organs: Reference values and a genotype. Clin. Radiol. 2021, 76, 863.e11–863.e17. [Google Scholar] [CrossRef]
- Coyne, D.W. Iron Overload in Dialysis Patients: Rust or Bust? Kidney Int. Rep. 2017, 2, 995–997. [Google Scholar] [CrossRef]
- Rostoker, G.; Vaziri, N.D.; Fishbane, S. Iatrogenic Iron Overload in Dialysis Patients at the Beginning of the 21st Century. Drugs 2016, 76, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Kohgo, Y.; Ikuta, K.; Ohtake, T.; Torimoto, Y.; Kato, J. Body iron metabolism and pathophysiology of iron overload. Int. J. Hematol. 2008, 88, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Fuchs, D.; Kurz, K.; Weiss, G. Physiology and Inflammation Driven Pathophysiology of Iron Homeostasis—Mechanistic Insights into Anemia of Inflammation and Its Treatment. Nutrients 2021, 13, 3732. [Google Scholar] [CrossRef] [PubMed]
- Styczyński, J.; Słomka, A.; Łęcka, M.; Albrecht, K.; Romiszewski, M.; Pogorzała, M.; Kubicka, M.; Kuryło-Rafińska, B.; Tejza, B.; Gadomska, G.; et al. Soluble Hemojuvelin and Ferritin: Potential Prognostic Markers in Pediatric Hematopoietic Cell Transplantation. Cancers 2023, 15, 1041. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G. Anemia of Chronic Disorders: New Diagnostic Tools and New Treatment Strategies. Semin. Hematol. 2015, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ghoti, H.; Rachmilewitz, E.A.; Simon-Lopez, R.; Gaber, R.; Katzir, Z.; Konen, E.; Kushnir, T.; Girelli, D.; Campostrini, N.; Fibach, E.; et al. Evidence for tissue iron overload in long-term hemodialysis patients and the impact of withdrawing parenteral iron. Eur. J. Haematol. 2012, 89, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Tharmaraj, D.; Kerr, P.G. Haemolysis in haemodialysis. Nephrology 2017, 22, 838–847. [Google Scholar] [CrossRef]
- Klei, T.R.L.; Meinderts, S.M.; van den Berg, T.K.; van Bruggen, R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front. Immunol. 2017, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Okumiya, T.; Sugiura, T.; Takahashi, N.; Yamamoto, Y.; Kikuchi, S.; Fujii, K.; Otagaki, M.; Shiojima, I. Shortened red blood cell age in patients with end-stage renal disease who were receiving haemodialysis: A cross-sectional study. BMC Nephrol. 2020, 21, 418. [Google Scholar] [CrossRef]
- Sato, Y.; Mizuguchi, T.; Shigenaga, S.; Yoshikawa, E.; Chujo, K.; Minakuchi, J.; Kawashima, S. Shortened red blood cell lifespan is related to the dose of erythropoiesis-stimulating agents requirement in patients on hemodialysis. Ther. Apher. Dial. 2012, 16, 522–528. [Google Scholar] [CrossRef]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef] [PubMed]
- Beshara, S.; Sörensen, J.; Lubberink, M.; Tolmachev, V.; Långström, B.; Antoni, G.; Danielson, B.G.; Lundqvist, H. Pharmacokinetics and red cell utilization of 52Fe/59Fe-labelled iron polymaltose in anaemic patients using positron emission tomography. Br. J. Haematol. 2003, 120, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Theurl, I.; Mattle, V.; Seifert, M.; Mariani, M.; Marth, C.; Weiss, G. Dysregulated monocyte iron homeostasis and erythropoietin formation in patients with anemia of chronic disease. Blood 2006, 107, 4142–4148. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Ulmer, H.; Kaser, A.; Weiss, G. Role of IL-10 for induction of anemia during inflammation. J. Immunol. 2002, 169, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Plaikner, M.; Schroll, A.; Burkert, F.R.; Seiwald, S.; Fauser, J.; Petzer, V.; Bellmann-Weiler, R.; Fritsche, G.; Tancevski, I.; et al. Tissue iron distribution in patients with anemia of inflammation: Results of a pilot study. Am. J. Hematol. 2023, 98, 890–899. [Google Scholar] [CrossRef]
- Maximova, N.; Gregori, M.; Boz, G.; Simeone, R.; Zanon, D.; Schillani, G.; Zennaro, F. MRI-based evaluation of multiorgan iron overload is a predictor of adverse outcomes in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Oncotarget 2017, 8, 79650–79661. [Google Scholar] [CrossRef]
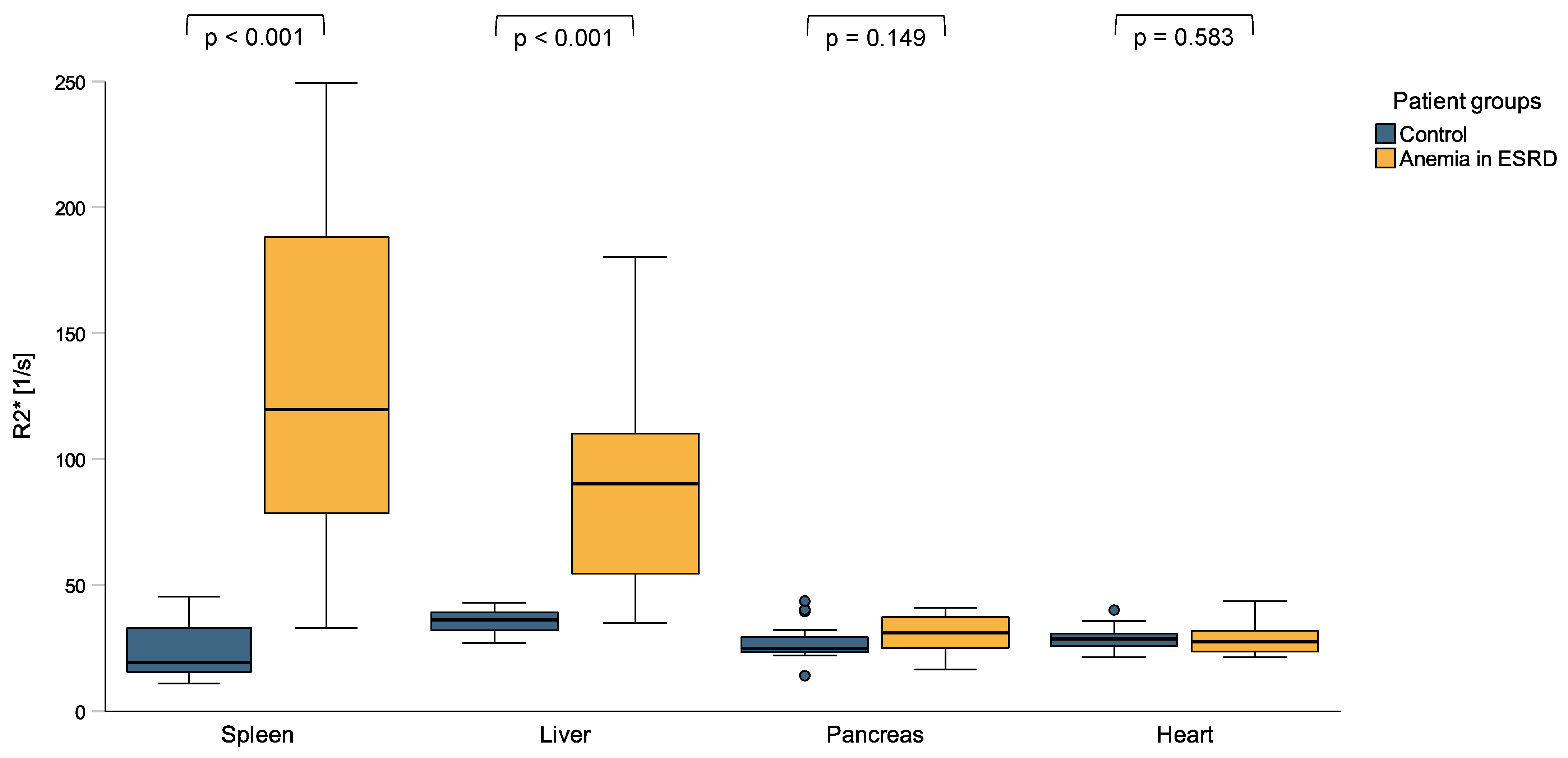

| Controls | ESKD Patients | Sig. | |
|---|---|---|---|
| n = 20 | n = 20 | ||
| Median (IQR) or % | Median (IQR) or % | p-Value | |
| Demographic and clinical characteristics | |||
| Age [years] | 30 (26–51) | 63 (49–76) | <0.001 |
| Sex [male] | 50.0% | 80.0% | 0.047 |
| BMI [kg/m2] | 21.7 (20.3–22.5) | 23.9 (21.9–27.4) | 0.006 |
| Comorbidities | |||
| Arterial hypertension | 5.0% | 75.0% | <0.001 |
| Hyperlipidemia | 0.0% | 35.0% | 0.004 |
| Diabetes mellitus | 10.0% | 7.7% | 0.945 |
| Coronary artery disease | 10.0% | 45.0% | 0.013 |
| Chronic heart failure | 0.0% | 20.0% | 0.035 |
| Acute infection | 0.0% | 50.0% | <0.001 |
| Comedication | |||
| Iron supplementation | 0.0% | 70.0% | <0.001 |
| Dosage within last year [IE] | - | 1650 (0–4750) | - |
| Erythropoiesis-stimulating agents | 0.0% | 95.0% | <0.001 |
| Dosage within last year [mg] | - | 395,000 (296,000–705,000) | - |
| Laboratory parameters | |||
| Creatinine [mg/dL] | 0.84 (0.68–0.95) | 7.30 (5.47–9.35) | <0.001 |
| Aspartate aminotransferase [U/L] | 22 (18–28) | 16 (14–20) | <0.001 |
| Alanine transaminase [U/L] | 19 (15–23) | 12 (9–22) | 0.091 |
| Gamma-GT [U/L] | 16 (14–22) | 30 (16–58) | 0.013 |
| Alkaline phosphatase [U/L] | 64 (51–79) | 94 (75–148) | 0.004 |
| Lactate dehydrogenase [U/L] | 172 (153–192) | 190 (163–223) | 0.183 |
| NT-proBNP [ng/L] | 49 (49–111) | 5386 (2749–23,862) | <0.001 |
| CRP [mg/dL] | 0.10 (0.05–0.14) | 0.54 (0.18–1.82) | <0.001 |
| Interleukin 6 [ng/L] | 1.4 (1.4–3.4) | 6.3 (3.6–18.5) | <0.001 |
| Neopterin [nmol/L] | 6.9 (5.4–8.6) | 121.1 (94.2–212.6) | <0.001 |
| Blood count and iron parameters | |||
| Leucocytes [G/L] | 6.5 (5.7–8.1) | 5.8 (4.8–6.5) | 0.091 |
| Lymphocytes [G/L] | 2.18 (1.80–2.46) | 1.21 (0.97–1.61) | <0.001 |
| Erythrocytes [T/L] | 4.46 (3.54–4.91) | 3.28 (2.98–3.48) | <0.001 |
| Hemoglobin [g/L] | 129 (108–147) | 100 (93–108) | <0.001 |
| Hematocrit [L/L] | 0.379 (0.324–0.415) | 0.302 (0.274–0.320) | <0.001 |
| MCH [pg] | 29.9 (28.0–31.2) | 31.1 (30.1–32.0) | 0.052 |
| MCV [fL] | 85.2 (82.8–88.3) | 91.9 (90.3–96.7) | <0.001 |
| MCHC [g/L] | 344 (335–349) | 331 (322–346) | 0.183 |
| Red cell distribution width [%] | 12.8 (12.2–14.4) | 14.0 (13.3–15.2) | 0.015 |
| Platelets [G/L] | 277 (243–360) | 176 (151–239) | <0.001 |
| Reticulocytes [G/L] | 57.0 (49.4–69.9) | 49.6 (35.0–89.4) | 0.461 |
| Reticulocyte Hb [pg] | 34 (31–35) | 35 (31–37) | 0.369 |
| Serum iron [µmol/L] | 10.5 (6.5–15.7) | 9.4 (7.3–15.4) | 0.989 |
| Ferritin [ng/mL] | 32 (20–115) | 444 (274–658) | <0.001 |
| Transferrin [mg/dL] | 282 (232–325) | 178 (161–191) | <0.001 |
| Transferrin saturation [%] | 17 (9–24) | 21 (17–30) | 0.043 |
| sTfR [mg/L] | 3.3 (2.8–4.2) | 2.9 (2.2–3.4) | 0.121 |
| sTfR/Ferritin index | 1.90 (1.44–2.95) | 1.13 (0.76–1.48) | <0.001 |
| Hepcidin [µg/L] | 3.1 (1.5–10.8) | 82.0 (28.5–82.0) | <0.001 |
| Folic acid [µg/L] | 7.5 (6.0–10.3) | 6.4 (3.5–15.6) | 0.425 |
| Vitamin B12 [ng/L] | 301 (216–427) | 350 (275–534) | 0.134 |
| MRI detections | |||
| Spleen R2* [s−1] | 19.3 (15.5–33.0) | 119.8 (78.5–188.2) | <0.001 |
| Spleen PDFF [%] | 1.8 (1.1–2.2) | 3.4 (1.7–5.7) | 0.005 |
| Spleen volume [cm3] | 223.7 (180.1–292.8) | 351.8 (239.6–410.3) | 0.001 |
| Liver R2* [s−1] | 36.1 (31.9–39.1) | 90.1 (54.5–110.0) | <0.001 |
| Liver PDFF [%] | 1.7 (0.9–3.0) | 2.3 (1.8–4.1) | 0.086 |
| Pancreas R2* [s−1] | 195.3 (184.0–216.5) | 258.3 (211.5–297.0) | 0.149 |
| Heart R2* [s−1] | 24.9 (23.2–29.9) | 31.0 (25.1–37.3) | 0.583 |
| Univariate Regression Analysis # | Multivariate Regression Analysis # | |||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Prediction of spleen R2* | ||||
| Ferritin | 0.605 (0.352–0.857) | <0.001 | 0.580 (0.345–0.815) | <0.001 |
| Transferrin | −1.816 (−3.240–−0.393) | 0.016 | 0.214 (−1.109–1.536) | 0.733 |
| Transferrin saturation | −0.121 (−0.551–0.308) | 0.558 | ||
| Hepcidin | 0.413 (0.119–0.706) | 0.009 | −0.223 (−0.630–0.184) | 0.258 |
| sTfR | 0.249 (−0.673–1.169) | 0.576 | ||
| sTfR-Ferritin index | −0.231 (−1.018–0.555) | 0.542 | ||
| C-reactive protein | 0.177 (−0.035–0.388) | 0.096 | ||
| Interleukin 6 | 0.322 (−0.072–0.715) | 0.102 | ||
| Neopterin | 0.644 (0.217–1.072) | 0.006 | 0.530 (0.342–0.718) | <0.001 |
| Prediction of liver R2* | ||||
| Ferritin | 0.422 (0.234–0.609) | <0.001 | 0.633 (0.263–1.004) | 0.003 |
| Transferrin saturation | −1.085 (−2.170–0.000) | 0.050 | ||
| TfS | 0.376 (0.071–0.824) | 0.094 | ||
| Hepcidin | 0.236 (0.007–0.466) | 0.044 | 0.409 (−0.381–1.198) | 0.283 |
| sTfR | −0.198 (−0.854–0.456) | 0.532 | ||
| sTfR-Ferritin index | −0.428 (−0.950–0.093) | 0.101 | ||
| C-reactive protein | 0.177 (0.040–0.313) | 0.014 | −0.223 (−0.563–0.116) | 0.179 |
| Interleukin 6 | 0.217 (−0.068–0.501) | 0.126 | ||
| Neopterin | 0.420 (0.099–0.742) | 0.014 | 0.351 (−0.131–0.833) | 0.140 |
| No iron Supplementation | Iron Supplementation | Sig. | |
|---|---|---|---|
| n = 6 | n = 14 | ||
| Median (IQR) or % | Median (IQR) or % | p-Value | |
| Demographic and clinical characteristics | |||
| Age [years] | 66 (63–73) | 62 (48–76) | 0.444 |
| Sex [male] | 83.3% | 78.6% | 0.807 |
| BMI [kg/m2] | 28.5 (22.2–32.6) | 23.6 (21.8–25.0) | 0.106 |
| Laboratory parameters | |||
| Creatinine [mg/dL] | 8.32 (7.53–9.00) | 6.08 (5.45–9.69) | 0.109 |
| Aspartate aminotransferase [U/L] | 16 (14–18) | 16 (13–22) | 0.841 |
| Alanine transaminase [U/L] | 12 (9–32) | 13 (9–21) | 0.904 |
| Gamma-GT [U/L] | 47 (22–59) | 28 (15–56) | 0.353 |
| Alkaline phoshatase [U/L] | 76 (63–84) | 125 (87–186) | 0.051 |
| Lactate dehydrogenase [U/L] | 193 (161–229) | 190 (165–216) | 0.904 |
| NT-proBNP [ng/L] | 23,862 (14,809–35,860) | 2862 (2375–5597) | 0.012 |
| CRP [mg/dL] | 0.80 (0.05–2.60) | 0.54 (0.19–1.40) | 0.841 |
| Interleukine 6 [ng/L] | 13.9 (3.4–53.7) | 5.6 (3.7–10.4) | 0.397 |
| Neopterin [nmol/L] | 121.1 (105.5–185.3) | 138.1 (90.2–229.2) | 0.968 |
| Blood count and iron parameters | |||
| Leucocytes [G/L] | 6.3 (5.6–6.5) | 5.7 (4.6–6.3) | 0.444 |
| Lymphocytes [G/L] | 1.24 (0.99–1.65) | 1.21 (0.95–1.58) | 0.841 |
| Erythrocytes [T/L] | 3.01 (2.95–3.33) | 3.30 (2.98–3.55) | 0.239 |
| Hemoglobin [g/L] | 9.6 (9.0–9.8) | 10.4 (9.5–11.0) | 0.051 |
| Hematocrit [L/L] | 0.284 (0.258–0.300) | 0.313 (0.286–0.321) | 0.076 |
| MCH [pg] | 30.2 (29.5–31.3) | 31.3 (30.8–33.0) | 0.207 |
| MCV [fL] | 91.7 (89.8–91.9) | 92.8 (90.8–97.2) | 0.494 |
| MCHC [g/L] | 328 (321–349) | 331 (322–343) | 0.841 |
| Red cell distribution width [%] | 14.3 (13.4–15.0) | 13.9 (13.1–15.4) | 0.841 |
| Platelets [G/L] | 168 (136–263) | 184 (160–235) | 0.904 |
| Reticulocytes [G/L] | 89.4 (35.3–97.0) | 49.0 (34.7–65.0) | 0.312 |
| Reticulocyte Hb [pg] | 36 (32–37) | 34 (30–37) | 0.659 |
| Serum iron [µmol/L] | 9.1 (8.3–12.5) | 9.9 (7.2–17.6) | 0.904 |
| Ferritin [ng/mL] | 416 (267–488) | 486 (281–689) | 0.602 |
| Transferrin [mg/dL] | 191 (124–214) | 175 (162–185) | 0.602 |
| Transferrin saturation [%] | 25 (20–29) | 20 (17–30) | 0.718 |
| sTfR [mg/L] | 3.0 (2.9–3.0) | 2.8 (2.1–3.6) | 0.779 |
| sTfR/Ferritin index | 1.17 (1.04–1.44) | 1.05 (0.69–1.53) | 0.602 |
| Hepcidin [µg/L] | 82.0 (29.5–82.0) | 74.0 (27.5–82.0) | 0.718 |
| Folic acid [µg/L] | 13.7 (4.8–21.0) | 4.3 (3.3–8.7) | 0.106 |
| Vitamin B12 [ng/L] | 298 (234–551) | 408 (279–517) | 0.397 |
| MRI detections | |||
| Spleen R2* [s−1] | 110.4 (57.0–119.7) | 180.0 (99.9–190.0) | 0.207 |
| Spleen PDFF [%] | 3.0 (1.5–3.9) | 3.6 (2.0–5.8) | 0.779 |
| Spleen volume [cm3] | 379.1 (231.9–430.3) | 330.7 (246.9–396.6) | 0.659 |
| Liver R2* [s−1] | 54.5 (36.4–59.8) | 97.6 (88.4–132.4) | 0.003 |
| Liver PDFF [%] | 2.5 (1.6–2.9) | 2.1 (1.9–5.3) | 0.968 |
| Pancreas R2* [s−1] | 28.3 (24.2–36.1) | 32.4 (27.0–37.6) | 0.444 |
| Heart R2* [s−1] | 27.4 (25.6–27.8) | 27.0 (23.3–33.3) | 0.659 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanser, L.; Plaikner, M.; Fauser, J.; Petzer, V.; Denicolò, S.; Haschka, D.; Neuwirt, H.; Stefanow, K.; Rudnicki, M.; Kremser, C.; et al. Tissue Iron Distribution in Anemic Patients with End-Stage Kidney Disease: Results of a Pilot Study. J. Clin. Med. 2024, 13, 3487. https://doi.org/10.3390/jcm13123487
Lanser L, Plaikner M, Fauser J, Petzer V, Denicolò S, Haschka D, Neuwirt H, Stefanow K, Rudnicki M, Kremser C, et al. Tissue Iron Distribution in Anemic Patients with End-Stage Kidney Disease: Results of a Pilot Study. Journal of Clinical Medicine. 2024; 13(12):3487. https://doi.org/10.3390/jcm13123487
Chicago/Turabian StyleLanser, Lukas, Michaela Plaikner, Josia Fauser, Verena Petzer, Sara Denicolò, David Haschka, Hannes Neuwirt, Kiril Stefanow, Michael Rudnicki, Christian Kremser, and et al. 2024. "Tissue Iron Distribution in Anemic Patients with End-Stage Kidney Disease: Results of a Pilot Study" Journal of Clinical Medicine 13, no. 12: 3487. https://doi.org/10.3390/jcm13123487
APA StyleLanser, L., Plaikner, M., Fauser, J., Petzer, V., Denicolò, S., Haschka, D., Neuwirt, H., Stefanow, K., Rudnicki, M., Kremser, C., Henninger, B., & Weiss, G. (2024). Tissue Iron Distribution in Anemic Patients with End-Stage Kidney Disease: Results of a Pilot Study. Journal of Clinical Medicine, 13(12), 3487. https://doi.org/10.3390/jcm13123487






