The Non-Invasive Detection of Pulmonary Exacerbations in Disorders of Mucociliary Clearance with Breath Analysis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Criteria
2.2. Selection Process
2.3. Data Extraction and Synthesis
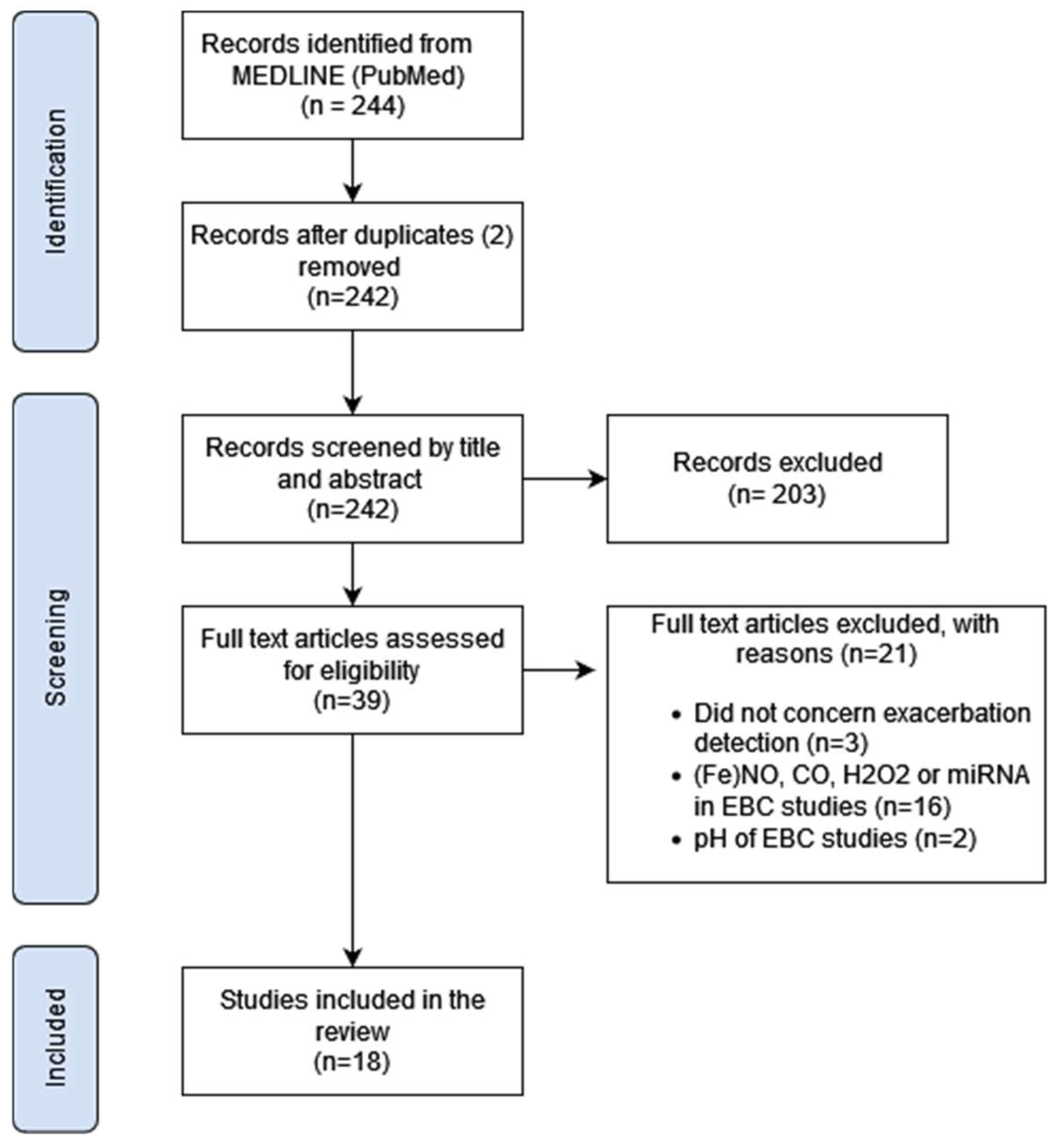
3. Results
3.1. Study Selection
3.2. Description of Included Studies
3.3. The Definition of an Exacerbation
3.4. Exacerbation Detection with VOCs in Exhaled Breath Using Mass Spectrometry
3.5. Exacerbation Detection with VOCs in Exhaled Breath Using eNose
3.6. Exacerbation Detection with Biomarkers in Exhaled Breath Condensate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Waters, V.; Stanojevic, S. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur. Respir. J. 2012, 40, 61–66. [Google Scholar] [CrossRef]
- Lucas, J.S.; Gahleitner, F. Pulmonary exacerbations in patients with primary ciliary dyskinesia: An expert consensus definition for use in clinical trials. ERJ Open Res. 2019, 5, 00147–2018. [Google Scholar] [CrossRef] [PubMed]
- Bilton, D.; Canny, G. Pulmonary exacerbation: Towards a definition for use in clinical trials. Report from the EuroCareCF Working Group on outcome parameters in clinical trials. J. Cyst. Fibros. 2011, 10 (Suppl. 2), S79–S81. [Google Scholar] [CrossRef]
- Treggiari, M.M.; Rosenfeld, M. Early anti-pseudomonal acquisition in young patients with cystic fibrosis: Rationale and design of the EPIC clinical trial and observational study. Contemp. Clin. Trials 2009, 30, 256–268. [Google Scholar] [CrossRef]
- Chang, A.B.; Fortescue, R. European Respiratory Society guidelines for the management of children and adolescents with bronchiectasis. Eur. Respir. J. 2021, 58, 2002990. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Zachariewicz, A. European Respiratory Society statement for defining respiratory exacerbations in children and adolescents with bronchiectasis for clinical trials. Eur. Respir. J. 2022, 60, 2200300. [Google Scholar] [CrossRef]
- Landini, N.; Ciet, P. Management of respiratory tract exacerbations in people with cystic fibrosis: Focus on imaging. Front. Pediatr. 2023, 10, 1084313. [Google Scholar] [CrossRef] [PubMed]
- Issit, T.; Wiggins, L. Volatile compounds in human breath: Critical review and meta-analysis. J. Breath Res. 2022, 16, 024001. [Google Scholar] [CrossRef]
- Brinkman, P.; Maitland-van der Zee, A.H. Breathomics and treatable traits for chronic airway diseases. Curr. Opin. Pulm. Med. 2019, 25, 94–100. [Google Scholar] [CrossRef]
- Horváth, I.; Hunt, J. Exhaled breath condensate: Methodological recommendations and unresolved question. Eur. Respir. J. 2005, 26, 523–548. [Google Scholar] [CrossRef] [PubMed]
- Kos, R.; Brinkman, P. Targeted exhaled breath analysis for detection of Pseudomonas aeruginosa in cystic fibrosis patients. J. Cyst. Fibros. 2022, 21, e28–e34. [Google Scholar] [CrossRef] [PubMed]
- McGrath, L.T.; Patrick, R. Breath isoprene during acute respiratory exacerbation in cystic fibrosis. Eur. Respir. J. 2000, 16, 1065–1069. [Google Scholar] [CrossRef]
- Barker, M.; Hengst, M. Volatile organic compounds in the exhaled breath of young patients with cystic fibrosis. Eur. Respir. J. 2006, 27, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Van Horck, M.; Smolinska, A. Exhaled volatile organic compounds detect pulmonary exacerbations early in children with cystic fibrosis: Results of a 1 year observational pilot study. J. Breath Res. 2021, 15, 026012. [Google Scholar] [CrossRef]
- Woollam, M.; Siegel, A.P. Preliminary method for profiling volatile organic compounds in breath that correlate with pulmonary function and other clinical traits of subjects diagnosed with cystic fibrosis: A pilot study. J. Breath Res. 2022, 16, 027103. [Google Scholar] [CrossRef]
- Paff, T.; van der Schee, M.P. Exhaled molecular profiles in the assessment of cystic fibrosis and primary ciliary dyskinesia. J. Cyst. Fibros. 2013, 12, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Joensen, O.; Paff, T. Exhaled breath analysis using electronic nose in cystic fibrosis and primary ciliary dyskinesia patients with chronic pulmonary infections. PLoS ONE 2014, 9, e115584. [Google Scholar] [CrossRef] [PubMed]
- Enderby, B.; Smith, D. Hydrogen cyanide as a biomarker for Pseudomonas aeruginosa in the breath of children with cystic fibrosis. Pediatr. Pulmonol. 2009, 44, 142–147. [Google Scholar] [CrossRef]
- Zang, X.; Monge, M.E. Feasibility of Early Detection of Cystic Fibrosis Acute Pulmonary Exacerbations by Exhaled Breath Condensate Metabolomics: A Pilot Study. J. Proteome Res. 2017, 16, 550–558. [Google Scholar] [CrossRef]
- Zang, X.; Monge, M.E. Early Detection of Cystic Fibrosis Acute Pulmonary Exacerbations by Exhaled Breath Condensate Metabolomics. J. Proteome Res. 2020, 19, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lucca, F.; Da Dalt, L. Asymmetric dimethylarginine and related metabolites in exhaled breath condensate of children with cystic fibrosis. Clin. Respir. J. 2018, 12, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Esther, C.R., Jr.; Boysen, G. Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L987–L993. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carpagnano, G.E.; Barnes, P.J. Increased leukotriene B4 and interleukin-6 in exhaled breath condensate in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 167, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Bodini, A.; D’Orazio, C. IL-8 and pH values in exhaled condensate after antibiotics in cystic fibrosis children. Int. J. Immunopathol. Pharmacol. 2007, 20, 467–472. [Google Scholar] [CrossRef]
- Robroeks, C.M.; Rosias, P.P. Biomarkers in exhaled breath condensate indicate presence and severity of cystic fibrosis in children. Pediatr. Allergy Immunol. 2008, 19, 652–659. [Google Scholar] [CrossRef]
- Colombo, C.; Faelli, N. Analysis of inflammatory and immune response biomarkers in sputum and exhaled breath condensate by a multi-parametric biochip array in cystic fibrosis. Int. J. Immunopathol. Pharmacol. 2011, 24, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Van Horck, M.; Alonso, A. Biomarkers in Exhaled Breath Condensate Are Not Predictive for Pulmonary Exacerbations in Children with Cystic Fibrosis: Results of a One-Year Observational Study. PLoS ONE 2016, 11, e0152156. [Google Scholar] [CrossRef]
- Toprak Kanik, E.; Yilmaz, O. Relevance between clinical status and exhaled molecules related to neutrophilic inflammation in pediatric cystic fibrosis. J. Breath Res. 2020, 14, 046007. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; Alexis, N.E. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur. Respir. J. 2008, 31, 949–956. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Part 1: VOCs in exhaled breath measured with GC-MS | |||||||
| Study population | Results | ||||||
| Author, year | Design | Disease | Adult/Paediatric | Total [n PEx] | Technique | Biomarkers included in the model | Main result |
| McGrath, L.T., 2000 [13] | Longitudinal cohort | CF | Adult | 12 (12) | GC-MS | Isoprene | Significant increase after treatment of PEx |
| Barker, M., 2006 [14] | Case–control: CF with vs. without PEx | CF | Both | 20 (5) | GC-MS | Pentane | Significantly increased during Pex |
| Enderby, B., 2009 [19] | Longitudinal cohort | CF | Paediatric (≥7 years) | 16 (not stated) | SIFT-MS | Hydrogen Cyanide | No significant changes during PEx |
| Van Horck, M., 2021 [15] | Longitudinal cohort | CF | Paediatric | 49 (31) | GC-tof-MS | Hydrocarbons: C8H18, C9H20, 2,4-dimethyl-1-heptene, pentadecane. Aromatic compounds: 1,3-dimethylbenene, p-benzoquinone. Other: Camphene, Tetradecanal, 3-methyl-2-butanone | Sens: 79% Spec: 78% AUROCC: 0.88 |
| Woollam, M., 2022 [16] | Cross-sectional cohort | CF | Paediatric (>8 years) | 18 (7) | SPME GC-MS | Hydrocarbon: 3,7–dimethyldecane | Sens: 100% Spec: 73% AUROCC: 0.91 |
| Part 2: VOCs in exhaled breath measured with eNose | |||||||
| Study population | Results | ||||||
| First author, year | Design | Disease | Adult/Paediatric | Total [n PEx] | Technique | Main result | Main result |
| Paff, T., 2013 [17] | Cross-sectional case–control | CF and PCD | Paediatric | 50 (13) | eNose | CF (n = 25) Sens: 89% Spec: 56% AUROCC: 0.76 | PCD (n = 25) Sens: 100% Spec: 90% AUROCC: 0.90 |
| Joensen, O., 2014 [18] | Cross-sectional case–control | CF and PCD | Both | 85 (14) | eNose | CF (n = 64) Sens: 90% Spec: 50% AUROCC: 0.69 | PCD (n = 21) No significant differences found |
| Part 3: Biomarkers in exhaled breath condensate | |||||||
| Study population | Results | ||||||
| First author, year | Design | Disease | Adult/Paediatric | Total [n PEx] | Technique | Biomarkers included in the model | Main result |
| Carpagnano, G.E., 2003 [24] | Longitudinal cohort | CF | Adult | 20 (20) | Immunoassay | LTB4 and IL-6 | Significant decrease after treatment of PEx |
| Bodini, A., 2007 [25] | Longitudinal cohort | CF | Paediatric | 15 (15) | Immunoassay | IL-8 | Significant decrease after treatment of PEx |
| Robroeks, CM., 2008 [26] | Cross-sectional cohort | CF | Paediatric | 48 (6) | Immunoassay | 8-isoprostane and nitrite | Sens: 40% Spec: 97% AUROCC: 0.84 |
| Esther, C.R., 2008 [30] | Longitudinal cohort | CF | Paediatric | 14 (14) | Luminometry | ATP | Significant decrease after treatment of PEx |
| Esther, C.R., 2009 [23] | Longitudinal cohort | CF | Paediatric | 26 (26) | UPLC-MS | Purine to urea ratio | No significant differences |
| Colombo, C., 2011 [27] | Longitudinal cohort | CF | Both (>12 years) | 24 (24) | Immunoassay | Cytokines and growth factors (e.g., IL-6, IL-8, IL-10, TNF-α, VEGF, IFN-y) | No significant differences |
| Van Horck, M., 2016 [28] | Cross-sectional cohort | CF | Paediatric | 49 (32) | Immunoassay | IL-6, IL-8, TNF-α, MIF | Sens: 70% Spec: 50% AUROCC: 0.62 |
| Zang, X., 2017 [20] | Cross-sectional cohort | CF | Both | 26 (9) | UPLC-MS | Pyroglutamic acid and 4-hydroxycyclohexylcarboxylic acid | Sens: 77.8% Spec: 88.2% Accuracy 84.6% |
| Lucca, F., 2018 [22] | Longitudinal cohort | CF | Paediatric | 34 (13) | UPLC-MS | Asymmetric dimethylarginine (ADMA) and related amino acids | No significant differences |
| Zang, X., 2020 [21] | Cross-sectional cohort | CF | Both | 138 (41) | UPLC-MS | Paediatric patients Higher in PEx: lactic acid, pyroglutamic acid, dihydrothymine, C5H9NO3, prolylhydroxyproline, C9H10O3 and C7H8O4S Lower in PEx: acetic acid and 3-methylglutaconic acid Adult patients Higher in PEx: 4-hydroxy-cyclohexylcarboxylic acid, nonanedioic acid, sebacic acid, y-butyrolactone, levulinic acid, C9H10O3 and C6H10O6 Lower in PEx: acetic acid and C8H11NO3 | Sens: 83.3% Spec: 91.7% Accuracy 88.9% Sens: 76.2% Spec: 83.7% Accuracy 81.3% |
| Toprak, Kanik E., 2020 [29] | Case–control: CF with and without PEx in past year | CF | Paediatric | 30 (10) | Immunoassay | IL-8, IL-17, LTB4, E-cadherin and neutrophil elastase | No significant differences |
| Author, Year | The Definition of a Pulmonary Exacerbation |
|---|---|
| McGrath, L.T., 2000 [13] | Increase in respiratory symptoms AND >10% decrease in FEV1 compared to previous year AND Decision to treat with intravenous antibiotics |
| Carpagnano, G.E., 2003 [24] | Increase in respiratory symptoms >10% decrease in FEV1 compared to previous year Signs of infection (fever, increase CRP or leukocytosis) Bacterial colonisation of sputum |
| Barker, M., 2006 [14] | Opinion of clinician: PEx in need of intravenous antibiotics |
| Bodini, A., 2007 [25] | ‘Conventional criteria’: clinical symptoms, radiology, >10% decrease in FEV1, increased CRP and leukocytosis |
| Robroeks, CM., 2008 [26] | Increase in respiratory symptoms AND/OR >10% decrease in FEV1 or FVC from baseline |
| Esther, C.R., 2008 [30] | Opinion of clinician: PEx in need of intravenous antibiotics |
| Esther, C.R., 2009 [23] | |
| Enderby, B., 2009 [19] | Not defined in the article |
| Colombo, C., 2011 [27] | Increase in respiratory symptoms Decrease in FEV1 compared with previous best Weight loss and fever |
| Paff, T., 2013 [17] | Additional antibiotic treatment due to respiratory symptoms, >10% decrease in pulmonary function or radiographic changes |
| Joensen, O., 2014 [18] | Additional antibiotic treatment due to respiratory symptoms, >10% decrease in pulmonary function or radiographic changes |
| Zang, X., 2017 [20] | Increase in respiratory symptoms and/or changes in physical examination of the lungs >10% decrease in FEV1 According to clinician in need of hospitalisation for treatment of PEx |
| Zang, X., 2020 [21] | |
| van Horck, M., 2016 [28] | EPIC trial criteria: ≥5 days of respiratory symptoms, >10% decrease in FEV1, radiographic changes AND/OR Opinion of clinician: PEx in need of therapeutic antibiotics |
| van Horck, M., 2021 [15] | |
| Lucca, F., 2018 [22] | Definition of EuroCareCF working group. Two of the following: Respiratory symptoms OR >10% decrease in FEV1 OR Radiographic changes |
| Toprak Kanik, E., 2020 [29] | Increase in respiratory symptoms or signs Radiographic changes >10% decrease in spirometry |
| Woollam, M., 2022 [16] | Opinion of clinician: PEx in need of therapeutic antibiotics AND/OR >10% decrease in FEV1 from baseline |
| Main findings |
|
| Advice for future research |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nessen, E.; Toussaint, B.; Israëls, J.; Brinkman, P.; Maitland-van der Zee, A.-H.; Haarman, E. The Non-Invasive Detection of Pulmonary Exacerbations in Disorders of Mucociliary Clearance with Breath Analysis: A Systematic Review. J. Clin. Med. 2024, 13, 3372. https://doi.org/10.3390/jcm13123372
Nessen E, Toussaint B, Israëls J, Brinkman P, Maitland-van der Zee A-H, Haarman E. The Non-Invasive Detection of Pulmonary Exacerbations in Disorders of Mucociliary Clearance with Breath Analysis: A Systematic Review. Journal of Clinical Medicine. 2024; 13(12):3372. https://doi.org/10.3390/jcm13123372
Chicago/Turabian StyleNessen, Emma, Belle Toussaint, Joël Israëls, Paul Brinkman, Anke-Hilse Maitland-van der Zee, and Eric Haarman. 2024. "The Non-Invasive Detection of Pulmonary Exacerbations in Disorders of Mucociliary Clearance with Breath Analysis: A Systematic Review" Journal of Clinical Medicine 13, no. 12: 3372. https://doi.org/10.3390/jcm13123372
APA StyleNessen, E., Toussaint, B., Israëls, J., Brinkman, P., Maitland-van der Zee, A.-H., & Haarman, E. (2024). The Non-Invasive Detection of Pulmonary Exacerbations in Disorders of Mucociliary Clearance with Breath Analysis: A Systematic Review. Journal of Clinical Medicine, 13(12), 3372. https://doi.org/10.3390/jcm13123372







