Neurasites®—A Standardized Plant Extract in the Treatment and Prevention of Migraine Attacks
Abstract
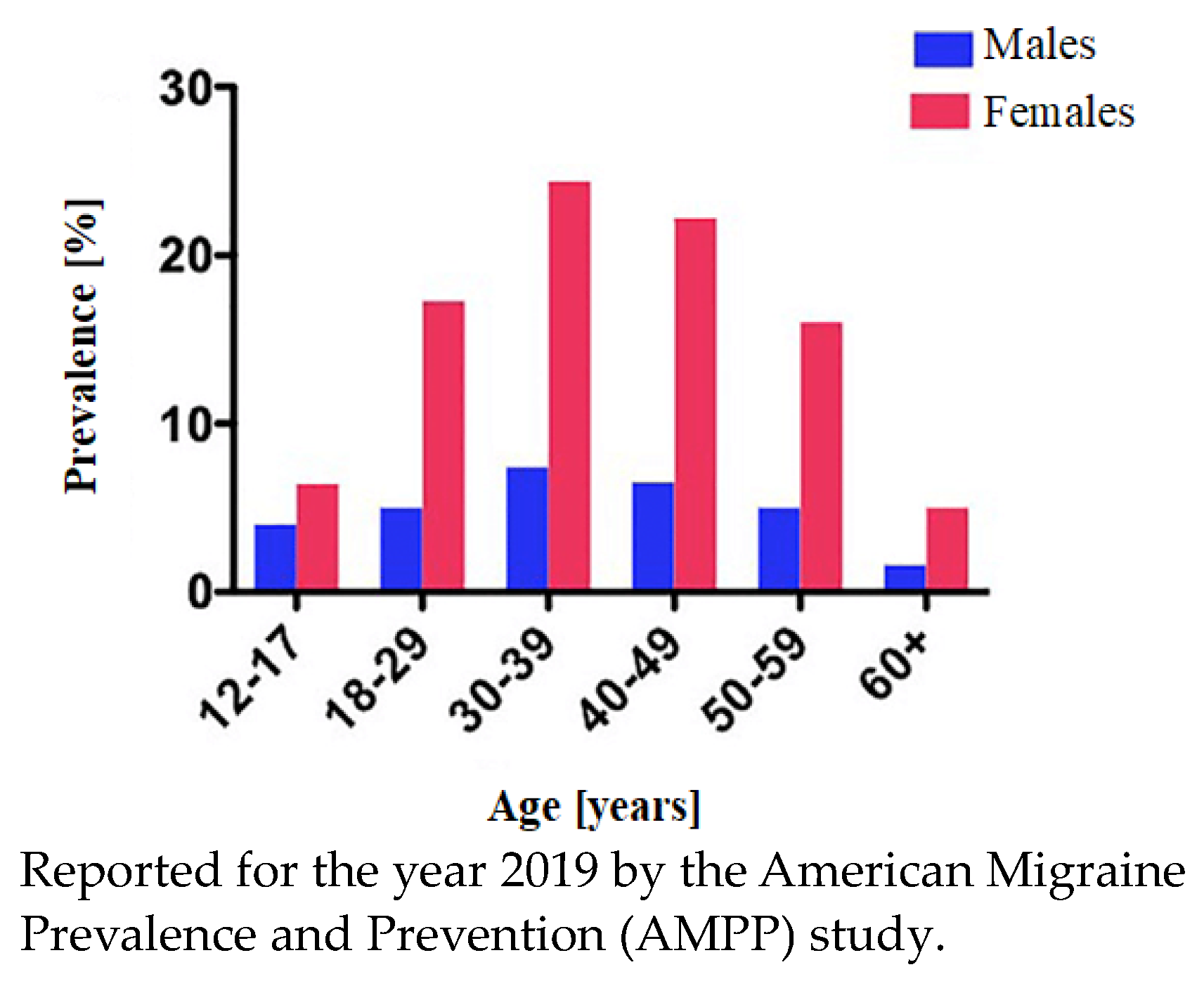
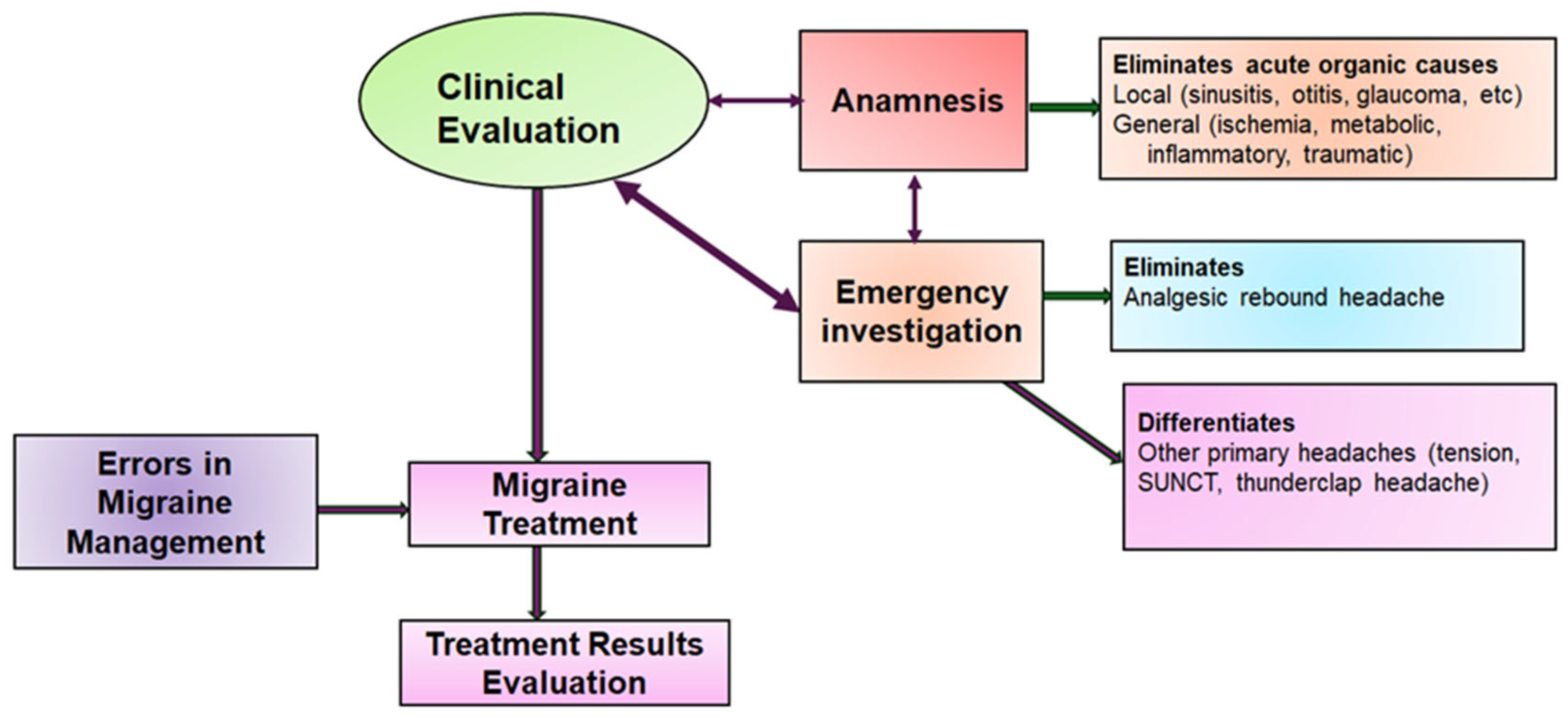
1. Introduction
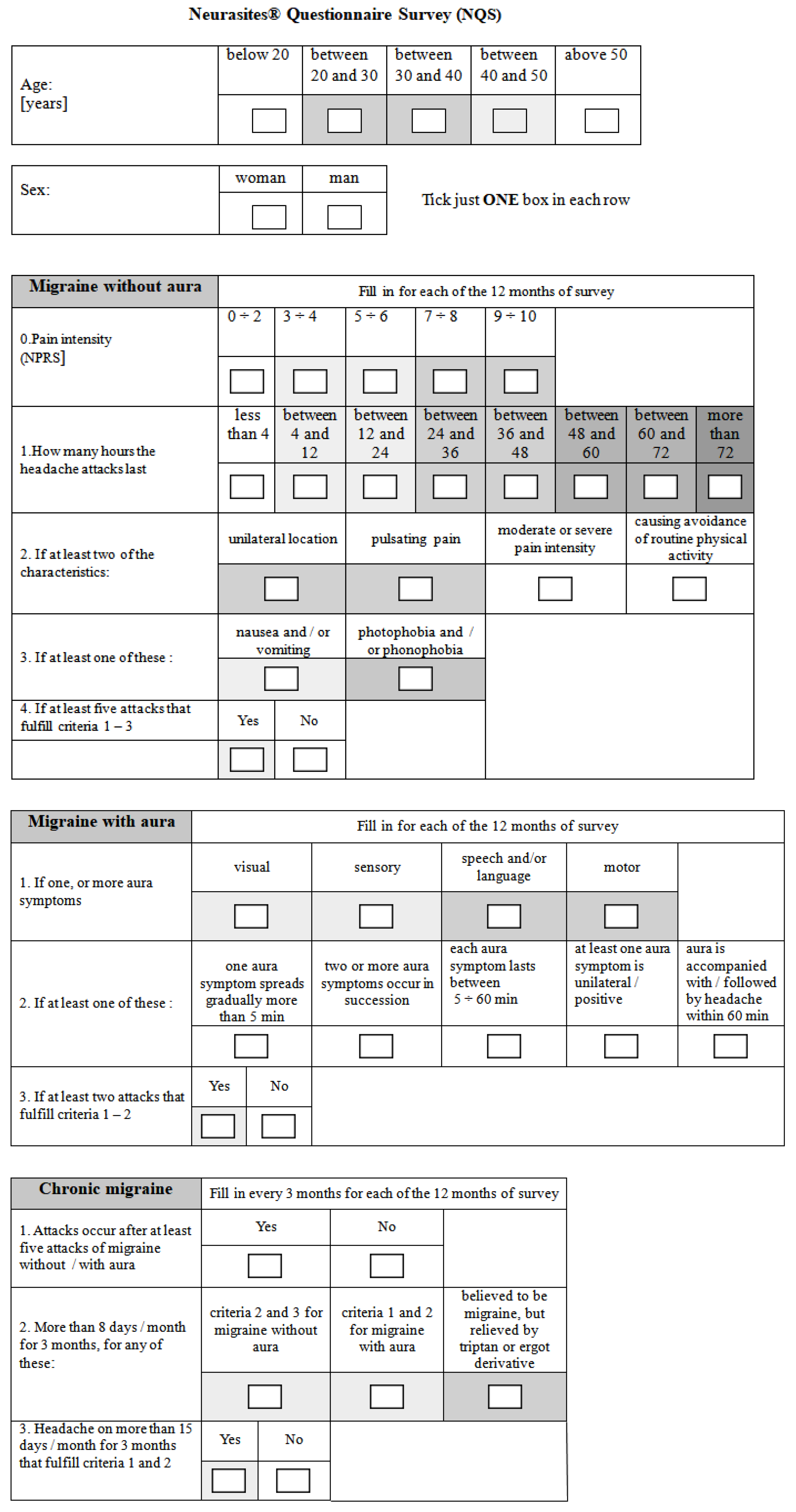
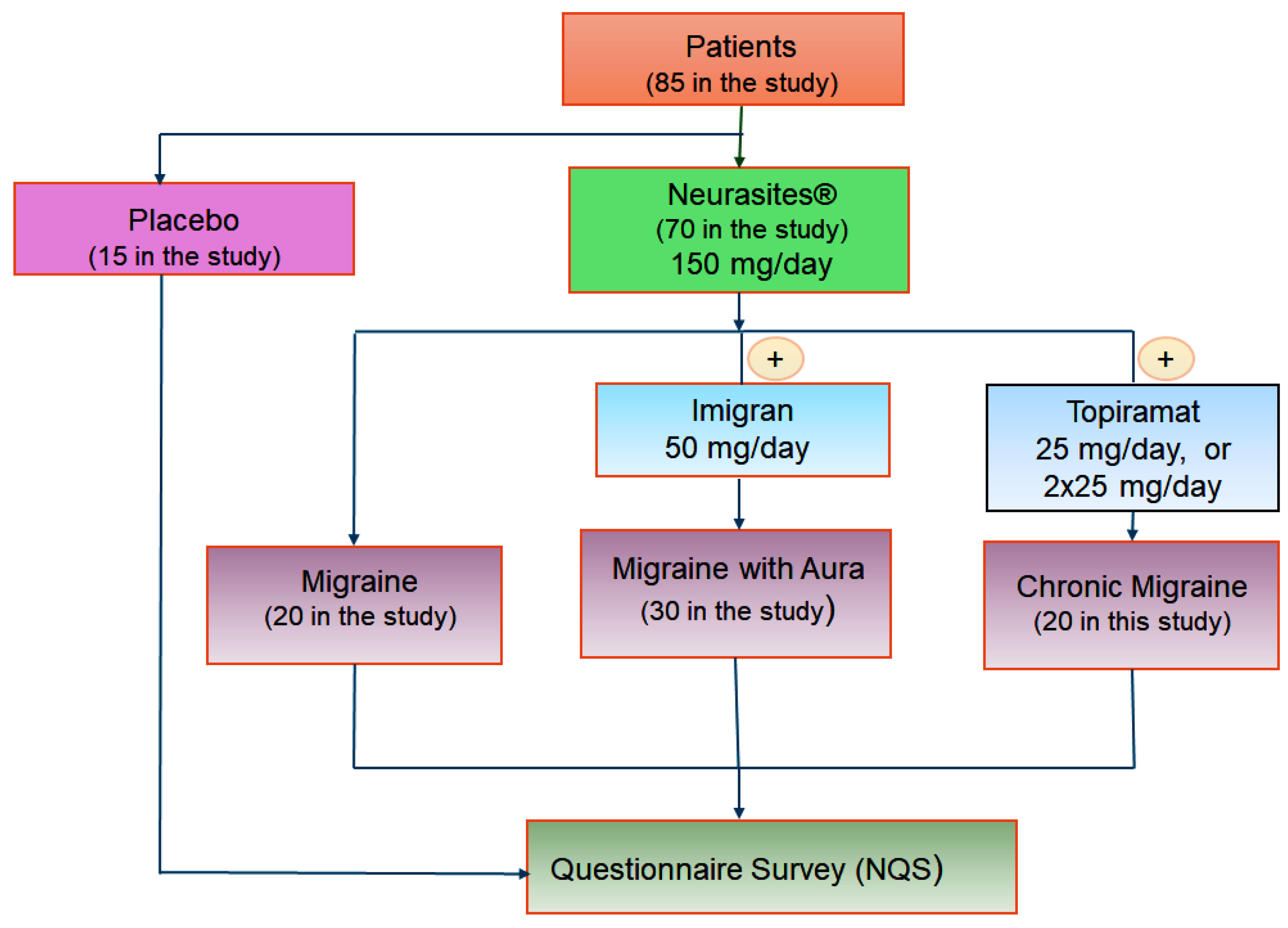
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eigenbrodt, A.K.; Ashina, H.; Khan, S.; Diener, H.C.; Mitsikostas, D.D.; Sinclair, A.J.; Pozo-Rosich, P.; Martelletti, P.; Ducros, A.; Lantéri-Minet, M.; et al. Diagnosis and management of migraine in ten steps. Nat. Rev. Neurol. 2021, 17, 501–514. [Google Scholar] [CrossRef]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension- type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.K.; Olesen, J. Migraine with aura and migraine without aura: An epidemiological study. Cephalalgia 1992, 12, 221–228. [Google Scholar] [CrossRef]
- Hansen, J.M.; Lipton, R.B.; Dodick, D.W.; Silberstein, S.D.; Saper, J.R.; Aurora, S.K.; Goadsby, P.J.; Charles, A. Migraine headache is present in the aura phase: A prospective study. Neurology 2012, 79, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Mangrum, R.; Gerstein, M.T.; Hall III, C.J.; Buse, D.C.; Houts, C.R.; McGinley, J.S.; McCarrier, K.P.; Lipton, R.B.; Wirth, R.J. Priority acute and preventive migraine treatment benefits: Results of the Migraine Clinical Outcome Assessment System (MiCOAS) qualitative study of people living with migraine. Headache J. Head Face Pain 2023, 63, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassany, L.; Lyons, H.S.; Boucherie, D.M.; Farham, F.; Lange, K.S.; Marschollek, K.; Onan, D.; Pensato, U.; Storch, E.; Torrente, A.; et al. The sense of stopping migraine prophylaxis. J. Headache Pain 2023, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Tzankova, V.; Becker, W.J.; Chan, T.L. Pharmacologic prevention of migraine. CMAJ 2023, 195, E187–E192. [Google Scholar] [CrossRef] [PubMed]
- Silva-Néto, R.P.; Jevoux, C.; Krymchantowski, A. Preventive and abortive treatment of migraine with traditional Drugs. The state of the art. Headache Med. 2023, 14, 133–143. [Google Scholar] [CrossRef]
- Lampl, C.; MaassenVanDenBrink, A.; Deligianni, C.I.; Gil-Gouveia, R.; Jassal, T.; Sanchez-del-Rio, M.; Reuter, U.; Uluduz, D.; Versijpt, J.; Zeraatkar, D.; et al. The comparative effectiveness of migraine preventive drugs: A systematic review and network meta-analysis. J. Headache Pain 2023, 24, 56. [Google Scholar] [CrossRef]
- Bentivegna, E.; Onan, D.; Martelletti, P. Unmet needs in preventive treatment of migraine. Neurol. Ther. 2023, 12, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Puledda, F.; Silva, E.M.; Suwanlaong, K.; Goadsby, P.J. Migraine: From pathophysiology to treatment. J. Neurol. 2023, 270, 3654–3666. [Google Scholar] [CrossRef] [PubMed]
- Puledda, F.; Tassorelli, C.; Diener, H.C. New migraine drugs. Cephalalgia 2023, 43, 3331024221144784. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Rimegepant: A review in the acute treatment and preventive treatment of migraine. CNS Drugs 2023, 37, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Afra, J.; Frese, A.; Goadsby, P.J.; Linde, M.; May, A.; Sándor, P.S. European Federation of Neurological Societies. EFNS guideline on the drug treatment of migraine—Revised report of an EFNS task force. Eur. J. Neurol. 2009, 16, 968–981. [Google Scholar] [CrossRef]
- Tzankova, V.; Becker, W.J.; Chan, T.L.H. Diagnosis and acute management of migraine. CMAJ 2023, 195, E153–E158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Headache Classification Committee of the International Headache Society (IHS): The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [CrossRef]
- Lipton, R.B.; Dodick, D.; Sadovsky, R.E.A.A.; Kolodner, K.; Endicott, J.; Hettiarachchi, J.; Harrison, W. A selfadministered screener for migraine in primary care: The ID Migraine validation study. Neurology 2003, 61, 375–382. [Google Scholar] [CrossRef]
- Lipton, R.B.; Bigal, M.E. Migraine: Epidemiology, impact, and risk factors for progression. Headache 2005, 45 (Suppl. S1), S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Willis-Parker, M.; Lundberg, G.P. Migraine headache: Is it only a neurological disorder? Links between migraine and cardiovascular disorders. Trends Cardiovasc. Med. 2019, 30, 424–430. [Google Scholar] [CrossRef]
- Steiner, T.J.; Jensen, R.; Katsarava, Z.; Linde, M.; MacGregor, E.A.; Osipova, V.; Paemeleire, K.; Olesen, J.; Peters, M.; Martelletti, P. Aids to management of headache disorders in primary care (2nd edition). J. Headache Pain 2019, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Kirthi, V.; Derry, S.; Moore, R.A. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst. Rev. 2013, 4, CD008041. [Google Scholar] [CrossRef] [PubMed]
- Rabbie, R.; Derry, S.; Moore, R.A. Ibuprofen with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst. Rev. 2013, 4, CD008039. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Zanchin, G.; Geraud, G.A.; De Klippel, N.; Diaz-Insa, S.; Gobel, H.; Cunha, L.; Ivanoff, N.; Falques, M.; Fortea, J. Early vs. non- early intervention in acute migraine—‘Act when Mild (AwM)’. A doubleblind, placebo-controlled trial of almotriptan. Cephalalgia 2008, 28, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Lantéri- Minet, M.; Mick, G.; Allaf, B. Early dosing and efficacy of triptans in acute migraine treatment: The TEMPO study. Cephalalgia 2012, 32, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Dodick, D.W.; Ailani, J.; Lu, K.; Finnegan, M.; Szegedi, A.; Trugman, J.M. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: The ACHIEVE II randomized clinical trial. JAMA 2019, 322, 1887–1898. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Wietecha, L.A.; Dennehy, E.B.; Kuca, B.; Case, M.G.; Aurora, S.K.; Gaul, C. Phase 3 randomized, placebocontrolled, double- blind study of lasmiditan for acute treatment of migraine. Brain 2019, 142, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.; Silberstein, S.D.; Freitag, F.; Dodick, D.W.; Argoff, C.; Ashman, E. Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012, 78, 1346–1353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Utterback, G.; Zacharias, R.; Timraz, S.; Mershman, D. Butterbur extract: Prophylactic treatment for childhood migraines. Complement. Ther. Clin. Pract. 2014, 20, 61–64. [Google Scholar] [CrossRef]
- Kandil, M.; Jaber, S.; Desai, D.; Nuñez Cruz, S.; Lomotan, N.; Ahmad, U.; Cirone, M.; Burkins, J.; McDowell, M. MAGraine: Magnesium compared to conventional therapy for treatment of migraines. Am. J. Emerg. Med. 2021, 39, 28–33. [Google Scholar] [CrossRef]
- de Vries, T.; Villalón, C.M.; Maassen Van Den Brink, A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol. Ther. 2020, 211, 107528. [Google Scholar] [CrossRef] [PubMed]
- Dahri, M.; Tarighat-Esfanjani, A.; Asghari-Jafarabadi, M.; Hashemilar, M. Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr. Neurosci. 2019, 22, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Nowaczewska, M.; Wiciński, M.; Osiński, S.; Kaźmierczak, H. The role of vitamin D in primary headache–from potential mechanism to treatment. Nutrients 2020, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Buse, D.C.; Al Jumah, M.; Westergaard, M.L.; Jensen, R.H.; Reed, M.L.; Prilipko, L.; Mennini, F.S.; Láinez, M.J.A.; Ravishankar, K.; et al. The headache under-response to treatment (HURT) questionnaire, an outcome measure to guide follow-up in primary care: Development, psychometric evaluation and assessment of utility. J. Headache Pain 2018, 19, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online:https://www.healthline.com/health/pain-scale#types (accessed on 10 September 2022).
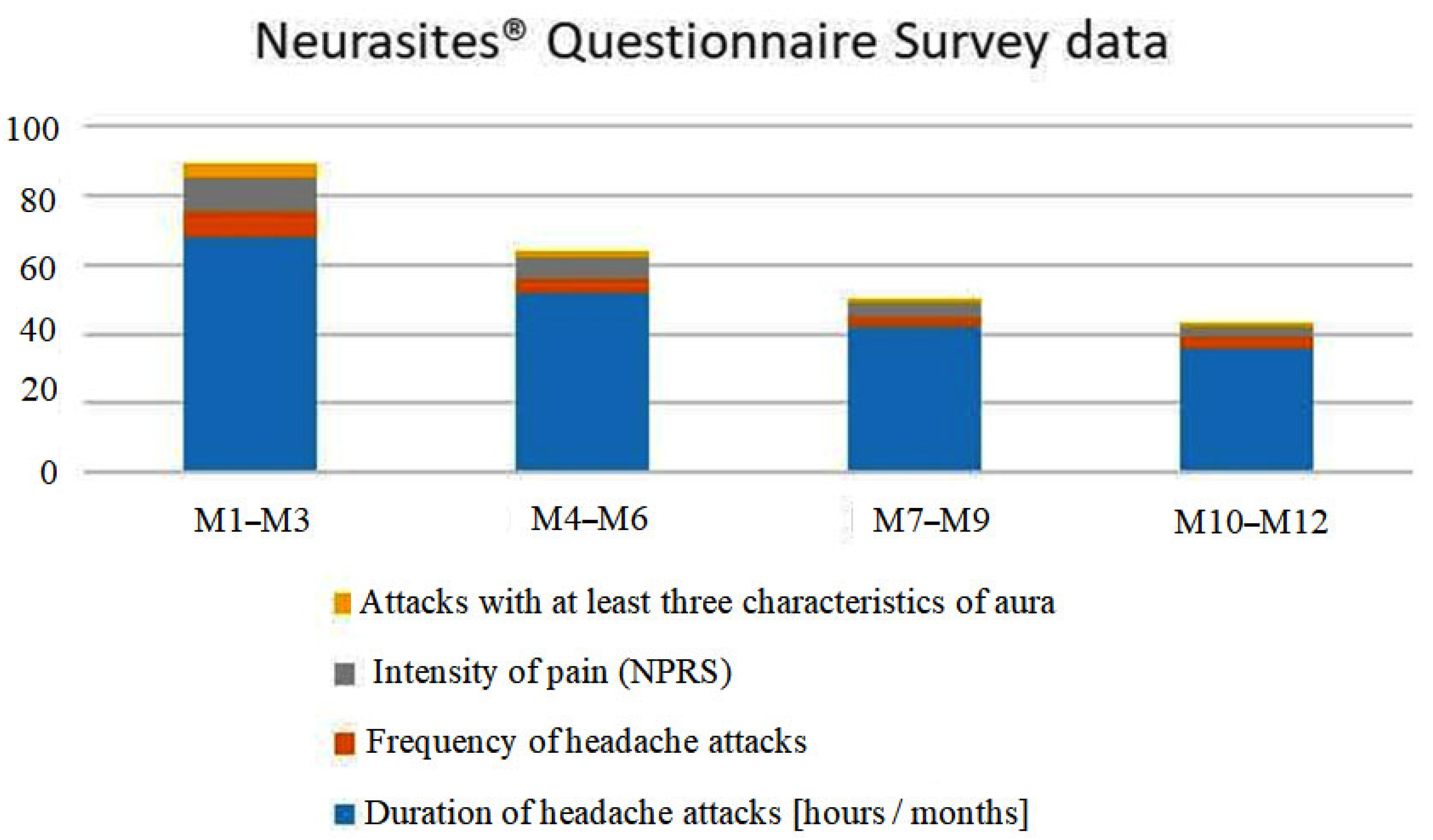
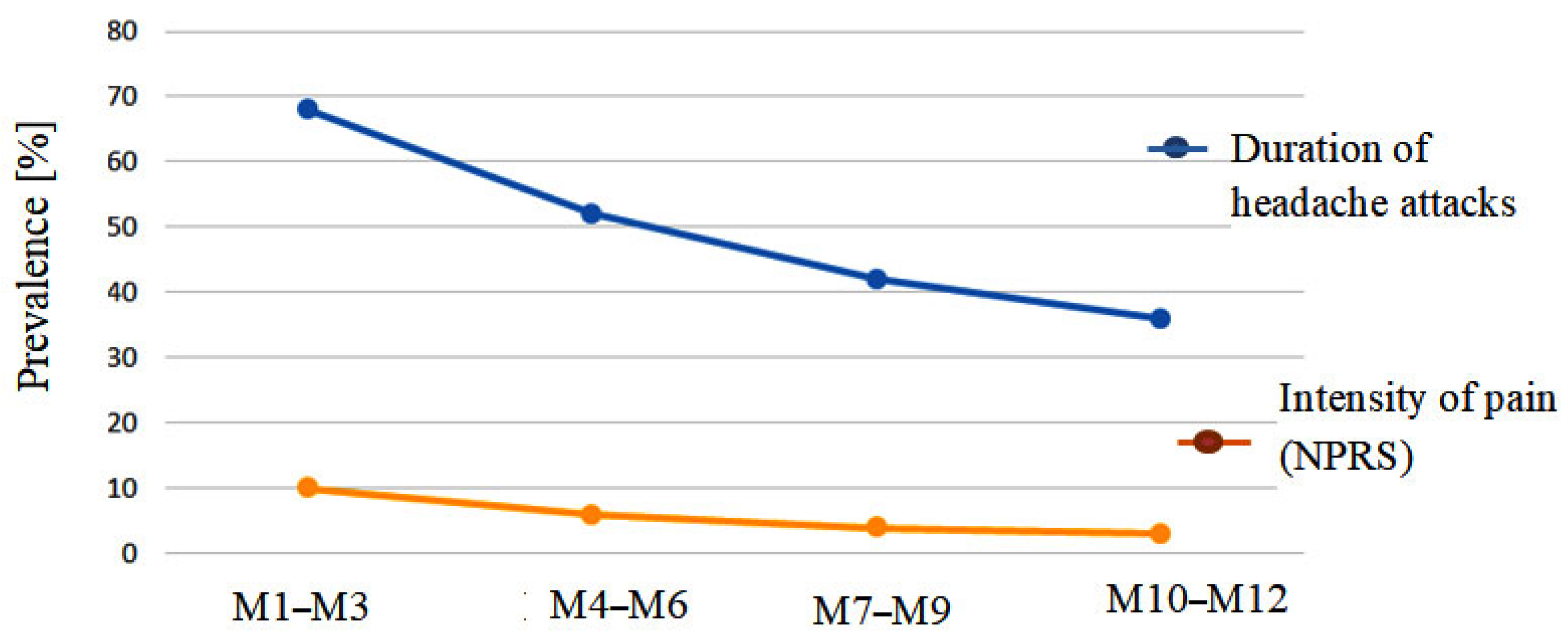
| Questionnaire Data | Months [M] | |||
|---|---|---|---|---|
| M1–M3 | M4–M6 | M7–M9 | M10–M12 | |
| Duration of headache attacks [hours/months] | 68 | 52 | 42 | 36 |
| Frequency of headache attacks | 7 | 4 | 3 | 3 |
| Intensity of pain (NPRS) | 10 | 6 | 4 | 3 |
| Attacks with at least three characteristics of aura | 4 | 2 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunguț, E.F.; Antal, C.; Turcu, S.; Varlas, V.-N.; Filipescu, A.G.; Balescu, I.; Bacalbașa, N.; Gorecki, G.-P. Neurasites®—A Standardized Plant Extract in the Treatment and Prevention of Migraine Attacks. J. Clin. Med. 2024, 13, 3364. https://doi.org/10.3390/jcm13123364
Lunguț EF, Antal C, Turcu S, Varlas V-N, Filipescu AG, Balescu I, Bacalbașa N, Gorecki G-P. Neurasites®—A Standardized Plant Extract in the Treatment and Prevention of Migraine Attacks. Journal of Clinical Medicine. 2024; 13(12):3364. https://doi.org/10.3390/jcm13123364
Chicago/Turabian StyleLunguț, Emilia Furdu, Claudia Antal, Suzana Turcu, Valentin-Nicolae Varlas, Alexandru George Filipescu, Irina Balescu, Nicolae Bacalbașa, and Gabriel-Petre Gorecki. 2024. "Neurasites®—A Standardized Plant Extract in the Treatment and Prevention of Migraine Attacks" Journal of Clinical Medicine 13, no. 12: 3364. https://doi.org/10.3390/jcm13123364
APA StyleLunguț, E. F., Antal, C., Turcu, S., Varlas, V.-N., Filipescu, A. G., Balescu, I., Bacalbașa, N., & Gorecki, G.-P. (2024). Neurasites®—A Standardized Plant Extract in the Treatment and Prevention of Migraine Attacks. Journal of Clinical Medicine, 13(12), 3364. https://doi.org/10.3390/jcm13123364






