Platelet microRNAs as Potential Novel Biomarkers for Antiplatelet Therapy with P2Y12 Inhibitors and Their Association with Platelet Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Sample Processing and miRNA Isolation
2.3. Quantitative Real-Time RT-PCR and Data Analyzes
2.4. Platelet Reactivity and Other Blood Parameters
2.5. Statistical Methods
3. Results
3.1. Population
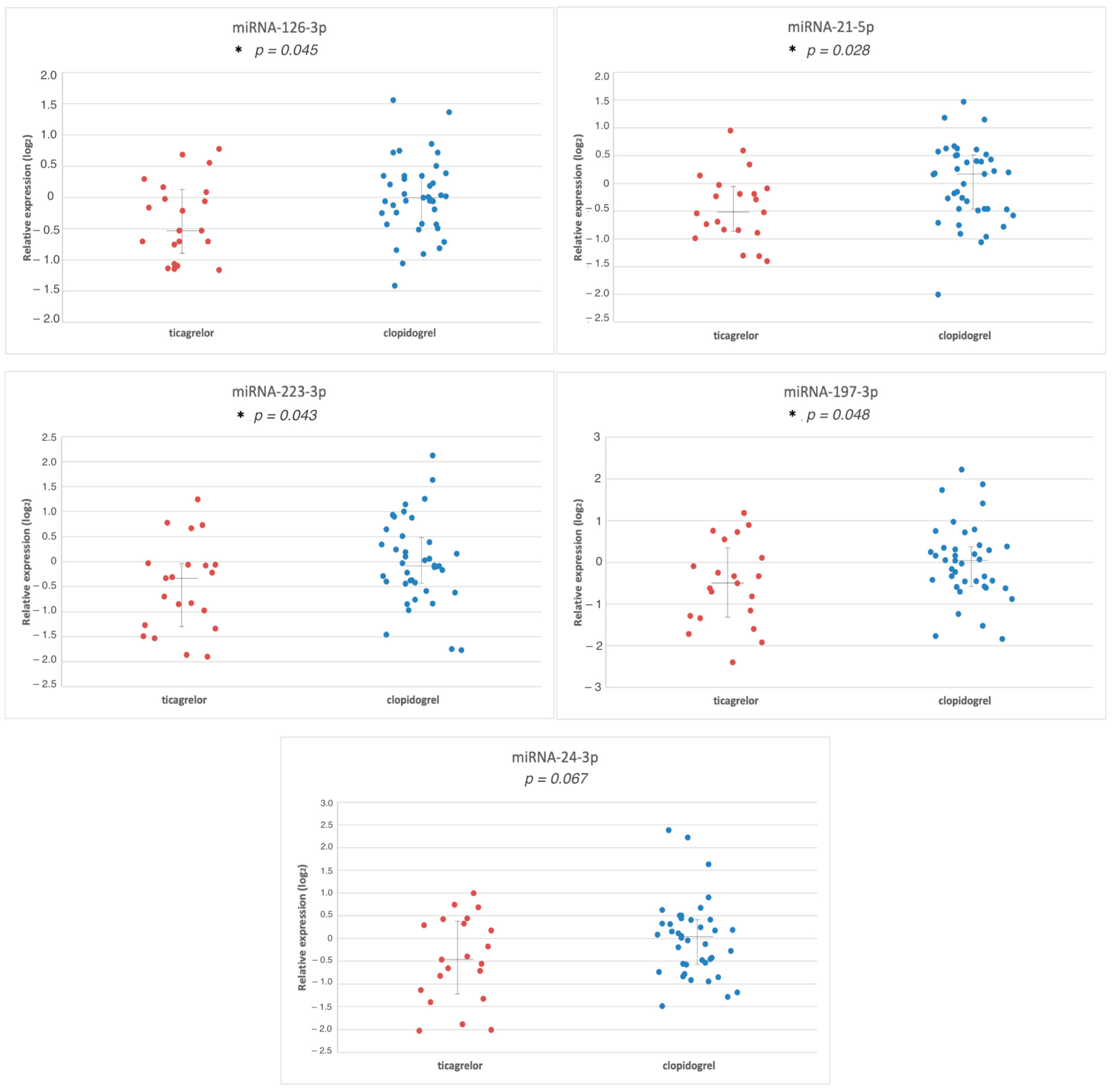
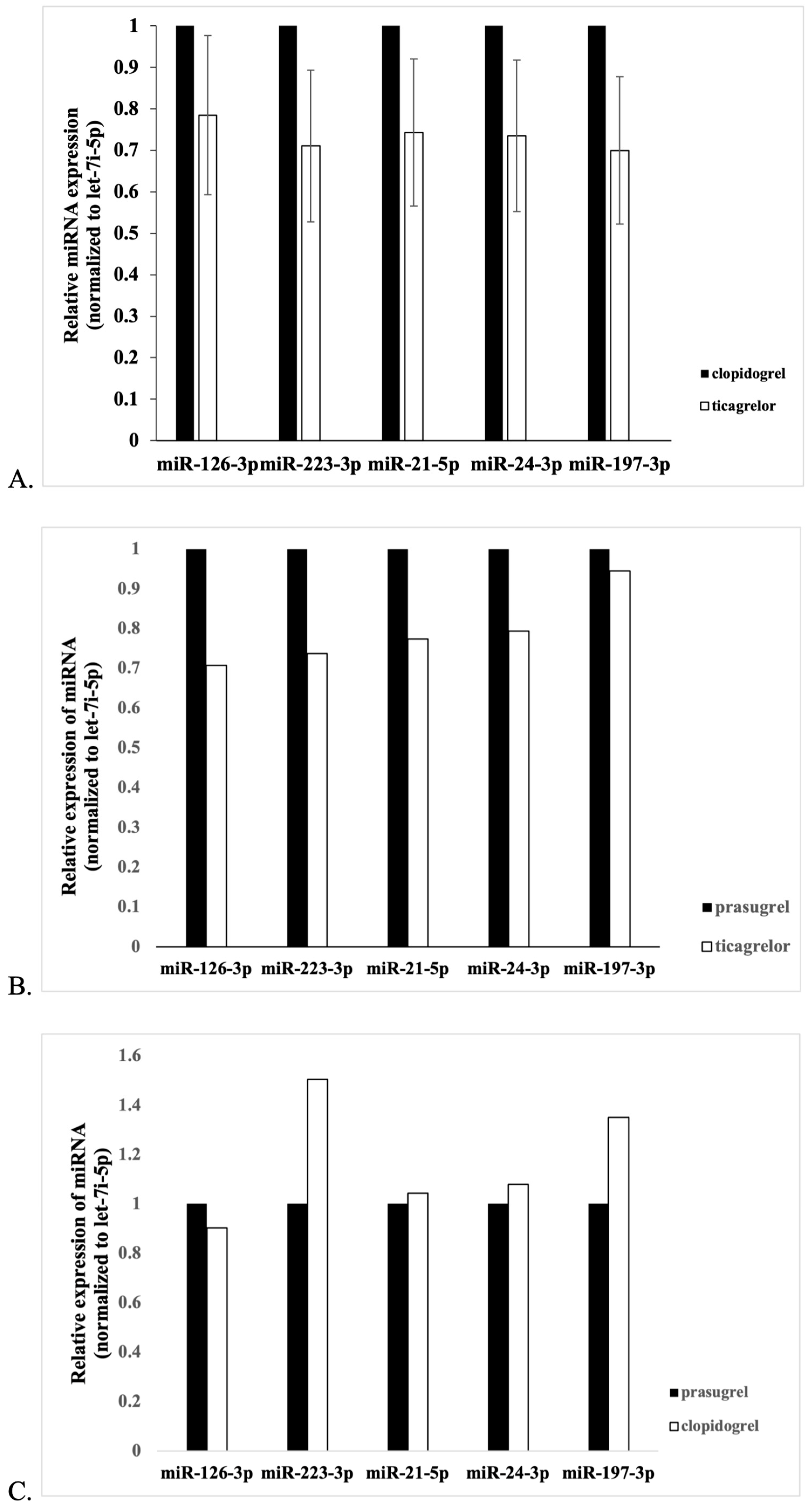
3.2. Comparison of miR Expression between Groups
3.3. Expression of miR vs. Platelet Function and Platelet Turnover
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef] [PubMed]
- Aradi, D.; Komocsi, A.; Vorobcsuk, A.; Rideg, O.; Tokes-Fuzesi, M.; Magyarlaki, T.; Horvath, I.G.; Serebruany, V.L. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: Systematic review and meta-analysis. Am. Heart J. 2010, 160, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Aradi, D.; Storey, R.F.; Komocsi, A.; Trenk, D.; Gulba, D.; Kiss, R.G.; Husted, S.; Bonello, L.; Sibbing, D.; Collet, J.P.; et al. Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur. Heart J. 2014, 35, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Breet, N.J.; van Werkum, J.W.; Bouman, H.J.; Kelder, J.C.; Harmsze, A.M.; Hackeng, C.M.; ten Berg, J.M. High on-treatment platelet reactivity to both aspirin and clopidogrel is associated with the highest risk of adverse events following percutaneous coronary intervention. Heart 2011, 97, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, D.; Xanthopoulou, I.; Gkizas, V.; Kassimis, G.; Theodoropoulos, K.C.; Makris, G.; Koutsogiannis, N.; Damelou, A.; Tsigkas, G.; Davlouros, P.; et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Interv. 2012, 5, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Gurbel, P.A.; Erlinge, D.; Ohman, E.M.; Neely, B.; Neely, M.; Goodman, S.G.; Huber, K.; Chan, M.Y.; Cornel, J.H.; Brown, E.; et al. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: The TRILOGY ACS platelet function substudy. JAMA 2012, 308, 1785–1794. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Roule, V.; Ardouin, P.; Repesse, Y.; Le Querrec, A.; Blanchart, K.; Lemaitre, A.; Sabatier, R.; Borel-Derlon, A.; Beygui, F. Point of Care Tests VerifyNow P2Y12 and INNOVANCE PFA P2Y Compared to Light Transmittance Aggregometry after Fibrinolysis. Clin. Appl. Thromb. Hemost. 2018, 24, 1109–1116. [Google Scholar] [CrossRef]
- Procyk, G.; Klimczak-Tomaniak, D.; Sygitowicz, G.; Tomaniak, M. Circulating and Platelet MicroRNAs in Cardiovascular Risk Assessment and Antiplatelet Therapy Monitoring. J. Clin. Med. 2022, 11, 1763. [Google Scholar] [CrossRef]
- Kirschner, M.B.; Kao, S.C.; Edelman, J.J.; Armstrong, N.J.; Vallely, M.P.; van Zandwijk, N.; Reid, G. Haemolysis during sample preparation alters microRNA content of plasma. PLoS ONE 2011, 6, e24145. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.P.; Schneeweiss, T.; Cremer, R.; Biesinger, B.; Hengstenberg, C.; Pruller, F.; Wallner, M.; Kolesnik, E.; von Lewinski, D.; Lang, I.M.; et al. Platelet reactivity patterns in patients treated with dual antiplatelet therapy. Eur. J. Clin. Investig. 2019, 49, e13102. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Zampetaki, A.; Dudek, K.; Kaudewitz, D.; King, A.; Kirkby, N.S.; Crosby-Nwaobi, R.; Prokopi, M.; Drozdov, I.; Langley, S.R.; et al. Circulating microRNAs as novel biomarkers for platelet activation. Circ. Res. 2013, 112, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Landry, P.; Plante, I.; Ouellet, D.L.; Perron, M.P.; Rousseau, G.; Provost, P. Existence of a microRNA pathway in anucleate platelets. Nat. Struct. Mol. Biol. 2009, 16, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhou, X.; Ji, W.J.; Zhang, Y.Y.; Ma, Y.Q.; Zhang, J.Q.; Li, Y.M. The Emerging Role of miR-223 in Platelet Reactivity: Implications in Antiplatelet Therapy. Biomed. Res. Int. 2015, 2015, 981841. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhou, X.; Ji, W.J.; Shi, R.; Lu, R.Y.; Li, J.L.; Yang, G.H.; Luo, T.; Zhang, J.Q.; Zhao, J.H.; et al. Decreased circulating microRNA-223 level predicts high on-treatment platelet reactivity in patients with troponin-negative non-ST elevation acute coronary syndrome. J. Thromb. Thrombolysis 2014, 38, 65–72. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, C.W.; Simmons, R.D.; Jo, H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: Mechanosensitive athero-miRs. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2206–2216. [Google Scholar] [CrossRef]
- Xu, X.; Kriegel, A.J.; Jiao, X.; Liu, H.; Bai, X.; Olson, J.; Liang, M.; Ding, X. miR-21 in ischemia/reperfusion injury: A double-edged sword? Physiol. Genom. 2014, 46, 789–797. [Google Scholar] [CrossRef]
- Wang, X.; Sundquist, K.; Svensson, P.J.; Rastkhani, H.; Palmer, K.; Memon, A.A.; Sundquist, J.; Zoller, B. Association of recurrent venous thromboembolism and circulating microRNAs. Clin. Epigenet. 2019, 11, 28. [Google Scholar] [CrossRef]
- Barwari, T.; Eminaga, S.; Mayr, U.; Lu, R.; Armstrong, P.C.; Chan, M.V.; Sahraei, M.; Fernandez-Fuertes, M.; Moreau, T.; Barallobre-Barreiro, J.; et al. Inhibition of profibrotic microRNA-21 affects platelets and their releasate. JCI Insight 2018, 3, 123335. [Google Scholar] [CrossRef]
- Zampetaki, A.; Willeit, P.; Tilling, L.; Drozdov, I.; Prokopi, M.; Renard, J.M.; Mayr, A.; Weger, S.; Schett, G.; Shah, A.; et al. Prospective study on circulating MicroRNAs and risk of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Hromadka, M.; Motovska, Z.; Hlinomaz, O.; Kala, P.; Tousek, F.; Jarkovsky, J.; Beranova, M.; Jansky, P.; Svoboda, M.; Krepelkova, I.; et al. MiR-126-3p and MiR-223-3p as Biomarkers for Prediction of Thrombotic Risk in Patients with Acute Myocardial Infarction and Primary Angioplasty. J. Pers. Med. 2021, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Chyrchel, B.; Toton-Zuranska, J.; Kruszelnicka, O.; Chyrchel, M.; Mielecki, W.; Kolton-Wroz, M.; Wolkow, P.; Surdacki, A. Association of plasma miR-223 and platelet reactivity in patients with coronary artery disease on dual antiplatelet therapy: A preliminary report. Platelets 2015, 26, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.M.; Edelstein, L.C.; Nagalla, S.; Woodley, A.B.; Chen, E.S.; Kong, X.; Ma, L.; Fortina, P.; Kunapuli, S.; Holinstat, M.; et al. Human platelet microRNA-mRNA networks associated with age and gender revealed by integrated plateletomics. Blood 2014, 123, e37–e45. [Google Scholar] [CrossRef]
| All (n = 79) | Clopidogrel (n = 40) | Ticagrelor (n = 21) | Prasugrel (n = 18) | p-Value | |
|---|---|---|---|---|---|
| Mean age, y (SD) | 61.3 (11.7) | 66.1 (9.2) | 60.3 (12.2) | 56.1 (12.4) | 0.002 |
| Male sex, n (%) | 61 (77.2) | 30 (75.0) | 15 (71.4) | 16 (88.9) | 0.386 |
| HT, n (%) | 58 (73.4) | 29 (72.5) | 14 (66.7) | 15 (83.3) | 0.493 |
| DM, n (%) | 21 (26.6) | 11 (27.5) | 3 (14.3) | 7 (38.9) | 0.219 |
| HL, n (%) | 60 (75.9) | 34 (85.0) | 16 (76.2) | 10 (55.5) | 0.053 |
| Past smokers, n (%) | 16 (20.3) | 11 (27.5) | 2 (9.5) | 3 (16.7) | 0.230 |
| Current smokers, n (%) | 27 (34.2) | 10 (25.0) | 10 (47.6) | 7 (38.9) | 0.186 |
| Previous MI, n (%) | 17 (21.5) | 9 (22.5) | 5 (23.8) | 3 (16.7) | 0.844 |
| Previous PCI, n (%) | 19 (24.1) | 11 (27.5) | 6 (28.6) | 2 (11.1) | 0.342 |
| Previous CABG, n (%) | 3 (3.8) | 1 (2.5) | 2 (9.5) | 0 (0.0) | 0.249 |
| Mean LVEF, % (SD) | 49.1 (10.2) | 49.0 (11.7) | 47.9 (7.9) | 50.3 (9.4) | 0.818 |
| STEMI | 44 (55.7) | 22 (55.0) | 10 (47.6) | 12 (66.7) | 0.487 |
| Statin, n (%) | 79 (100) | 40 (100) | 21 (100) | 18 (100) | 1.0 |
| BB, n (%) | 69 (87.3) | 37 (92.5) | 18 (85.7) | 14 (77.8) | 0.286 |
| ACEI/ARB, n (%) | 74 (93.7) | 38 (95.0) | 20 (95.2) | 16 (88.9) | 0.637 |
| CCB, n (%) | 12 (15.2) | 8 (20.0) | 2 (9.5) | 2 (11.1) | 0.479 |
| PPI, n (%) | 74 (93.7) | 37 (92.5) | 20 (95.2) | 17 (94.4) | 0.906 |
| Creatinine [mg/dL] median (25; 75 percentile) | 0.94 (0.82; 1.13) | 0.97 (0.84; 1.15) | 0.91 (0.81; 1.12) | 0.94 (0.80; 1.1) | 0.831 |
| Hgb [g/dL] median (25; 75 percentile) | 13.8 (12.4; 14.6) | 13.6 (12.2; 14.7) | 13.9 (12.2; 14.4) | 14.3 (12.4; 15.5) | 0.662 |
| PLT 103/μL median (25; 75 percentile) | 220 (194; 252) | 215 (201; 250) | 223 (192; 265) | 227 (176; 271) | 0.336 |
| miR-126-3p | miR-223-3p | miR-21-5p | miR-24-3p | miR-197-3p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | |
| Aggregometry (n = 63) | 0.600 | 0.067 | 0.119 | 0.199 | 0.128 | 0.194 | 0.363 | 0.117 | 0.324 | 0.126 |
| Aggregometry, clopidogrel (n = 29) | 0.468 | −0.14 | 0.971 | −0.007 | 0.491 | −0.133 | 0.909 | −0.022 | 0.984 | −0.004 |
| Aggregometry, potent P2Y12 inhibitors (n = 34) | 0.271 | 0.194 | 0.019 * | 0.400 | 0.013 * | 0.423 | 0.196 | 0.227 | 0.212 | 0.220 |
| Aggregometry, ticagrelor (n = 19) | 0.661 | 0.125 | 0.180 | 0.321 | 0.068 | 0.427 | 0.811 | 0.059 | 0.668 | 0.105 |
| Aggregometry, prasugrel (n = 14) | 0.850 | 0.054 | 0.930 | −0.025 | 0.732 | 0.096 | 0.685 | 0.114 | 0.732 | −0.096 |
| IPF (n = 37) | 0.295 | 0.177 | 0.256 | 0.126 | 0.391 | 0.145 | 0.012 * | 0.411 | 0.044 * | 0.333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumiężna, K.; Bednarek, A.; Sygitowicz, G.; Maciejak-Jastrzębska, A.; Baruś, P.; Hunia, J.; Klimczak-Tomaniak, D.; Kochman, J.; Grabowski, M.; Tomaniak, M. Platelet microRNAs as Potential Novel Biomarkers for Antiplatelet Therapy with P2Y12 Inhibitors and Their Association with Platelet Function. J. Clin. Med. 2024, 13, 63. https://doi.org/10.3390/jcm13010063
Gumiężna K, Bednarek A, Sygitowicz G, Maciejak-Jastrzębska A, Baruś P, Hunia J, Klimczak-Tomaniak D, Kochman J, Grabowski M, Tomaniak M. Platelet microRNAs as Potential Novel Biomarkers for Antiplatelet Therapy with P2Y12 Inhibitors and Their Association with Platelet Function. Journal of Clinical Medicine. 2024; 13(1):63. https://doi.org/10.3390/jcm13010063
Chicago/Turabian StyleGumiężna, Karolina, Adrian Bednarek, Grażyna Sygitowicz, Agata Maciejak-Jastrzębska, Piotr Baruś, Jaromir Hunia, Dominika Klimczak-Tomaniak, Janusz Kochman, Marcin Grabowski, and Mariusz Tomaniak. 2024. "Platelet microRNAs as Potential Novel Biomarkers for Antiplatelet Therapy with P2Y12 Inhibitors and Their Association with Platelet Function" Journal of Clinical Medicine 13, no. 1: 63. https://doi.org/10.3390/jcm13010063
APA StyleGumiężna, K., Bednarek, A., Sygitowicz, G., Maciejak-Jastrzębska, A., Baruś, P., Hunia, J., Klimczak-Tomaniak, D., Kochman, J., Grabowski, M., & Tomaniak, M. (2024). Platelet microRNAs as Potential Novel Biomarkers for Antiplatelet Therapy with P2Y12 Inhibitors and Their Association with Platelet Function. Journal of Clinical Medicine, 13(1), 63. https://doi.org/10.3390/jcm13010063








