Determination of Peak Oxygen Uptake in Patients with Acute Myocardial Infarction: The Role of Arterial Stiffness in Cardio–Vascular–Skeletal Muscle Coupling
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics
2.3. Measured Parameters
2.3.1. CAVI
2.3.2. CPX
2.3.3. Skeletal Muscle Mass and Handgrip Strength
2.3.4. Echocardiography
2.3.5. Hematology and Biochemistry Data
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Physical Function and CPX
3.3. Comparison of VO2 Peak according to CAVI Classification
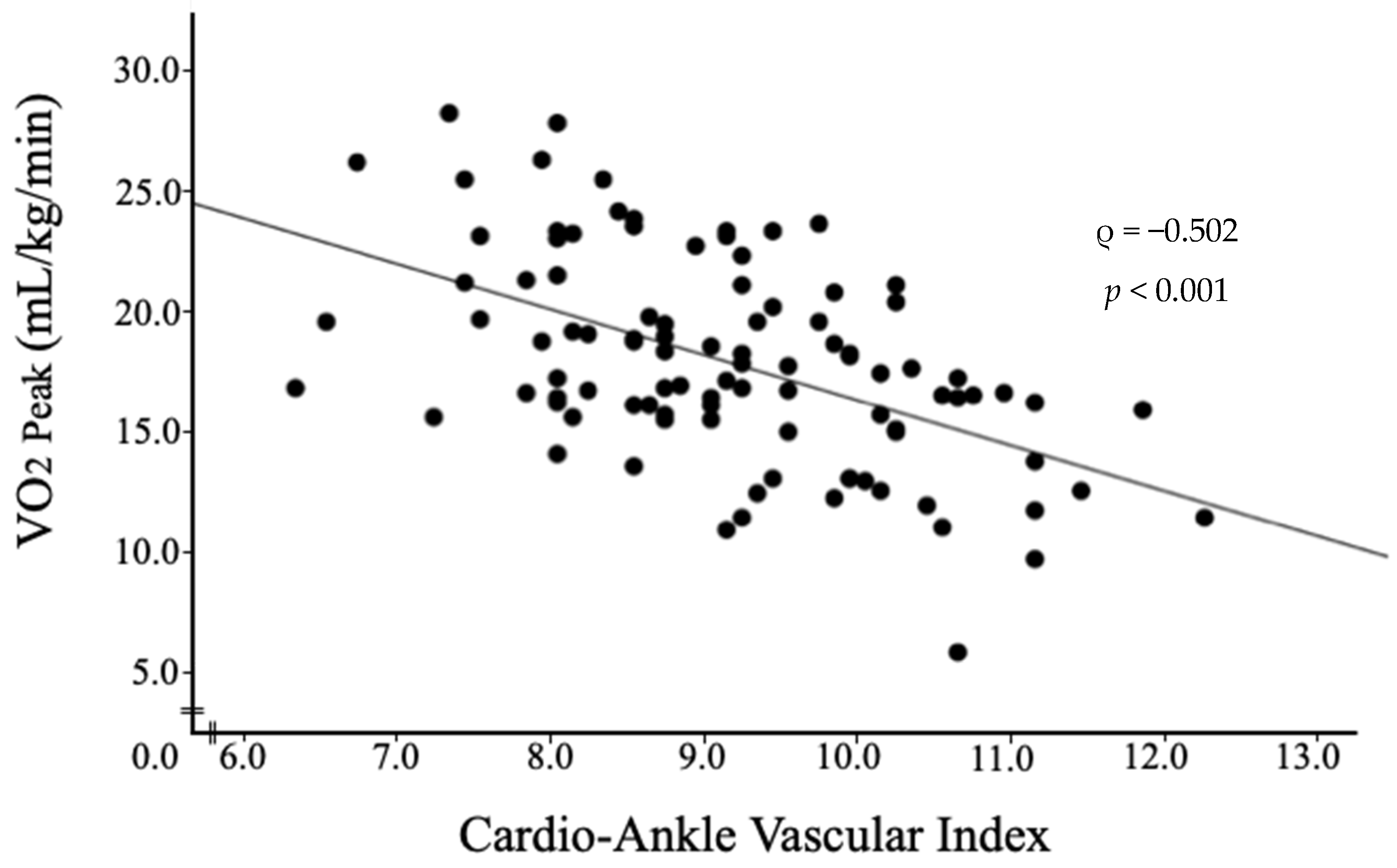
3.4. Association between VO2 Peak and Clinical Parameters
3.5. Factors Contributing to VO2 Peak
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimokawa, H.; Miura, M.; Nochioka, K.; Sakata, Y. Heart failure as a general pandemic in Asia. Eur. J. Heart Fail. 2015, 17, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Mori, M.; Komoto, S. Japanese national plan for promotion of measures against cerebrovascular and cardiovascular disease. Circulation 2021, 143, 1929–1931. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sakata, Y.; Sato, K.; Nochioka, K.; Miura, M.; Abe, R.; Oikawa, T.; Kasahara, S.; Aoyanagi, H.; Yamanaka, S.; et al. Clinical characteristics and prognostic factors in elderly patients with chronic heart failure-a report from the CHART-2 study. Int. J. Cardiol. Heart Vasc. 2020, 27, 100497. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- McNallan, S.M.; Singh, M.; Chamberlain, A.M.; Kane, R.L.; Dunlay, S.M.; Redfield, M.M.; Weston, S.A.; Roger, V.L. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013, 1, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Fülster, S.; Tacke, M.; Sandek, A.; Ebner, N.; Tschöpe, C.; Doehner, W.; Anker, S.D.; von Haehling, S. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SI-CA-HF). Eur. Heart J. 2013, 34, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Al-Mallah, M.H.; Keteyian, S.J.; Brawner, C.A.; Whelton, S.; Blaha, M.J. Rationale and design of the Henry Ford Exercise Testing Project (the FIT project). Clin. Cardiol. 2014, 37, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Ezzatvar, Y.; Izquierdo, M.; Núñez, J.; Calatayud, J.; Ramírez-Vélez, R.; García-Hermoso, A. Cardiorespiratory fitness measured with cardiopulmonary exercise testing and mortality in patients with cardiovascular disease: A systematic review and meta-analysis. J. Sport Health Sci. 2021, 10, 609–619. [Google Scholar] [CrossRef]
- Pierce, G.L. Mechanisms and subclinical consequences of aortic stiffness. Hypertension 2017, 70, 848–853. [Google Scholar] [CrossRef]
- Lin, C.J.; Cocciolone, A.J.; Wagenseil, J.E. Elastin, arterial mechanics, and stenosis. Am. J. Physiol. Cell Physiol. 2022, 322, C875–C886. [Google Scholar] [CrossRef]
- Dipla, K.; Triantafyllou, A.; Koletsos, N.; Papadopoulos, S.; Sachpekidis, V.; Vrabas, I.S.; Gkaliagkousi, E.; Zafeiridis, A.; Douma, S. Impaired muscle oxygenation and elevated exercise blood pressure in hypertensive patients: Links with vascular stiffness. Hypertension 2017, 70, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Rhim, H.C.; Park, S.H.; Park, S.; Lee, J.Y. Relationship between physical fitness and arterial stiffness in Korean older adults. Medicine 2022, 101, e30617. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J. Atheroscler. Thromb. 2006, 13, 101–107. [Google Scholar] [CrossRef]
- Okamoto, Y.; Miyoshi, T.; Ichikawa, K.; Takaya, Y.; Nakamura, K.; Ito, H. Cardio-ankle vascular index as an arterial stiffness marker improves the prediction of cardiovascular events in patients without cardiovascular diseases. J. Cardiovasc. Dev. Dis. 2022, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ohira, M.; Iizuka, T.; Mikamo, H.; Nakagami, T.; Suzuki, M.; Hirano, K.; Takahashi, M.; Shimizu, K.; Sugiyama, Y.; et al. Cardio-ankle vascular index relates to left ventricular ejection fraction in patients with heart failure. A retrospective study. Int. Heart J. 2013, 54, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamamoto, T.; Takahara, A.; Shirai, K. Clinical assessment of arterial stiffness with cardio-ankle vascular index: Theory and applications. J. Hypertens. 2015, 33, 1742–1757, discussion 1757. [Google Scholar] [CrossRef]
- Shimizu, K.; Takahashi, M.; Sato, S.; Saiki, A.; Nagayama, D.; Hitsumoto, T.; Takahara, A.; Shirai, K. Rapid rise in cardio-ankle vascular index as a predictor of impending cardiovascular events -smooth muscle cell contraction hypothesis for plaque rupture. Vasc. Health Risk Manag. 2022, 18, 879–886. [Google Scholar] [CrossRef]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef]
- Saiki, A.; Ohira, M.; Yamaguchi, T.; Nagayama, D.; Shimizu, N.; Shirai, K.; Tatsuno, I. New horizons of arterial stiffness developed using cardio-ankle vascular index (CAVI). J. Atheroscler. Thromb. 2020, 27, 732–748. [Google Scholar] [CrossRef]
- Adachi, H. Cardiopulmonary exercise test. Int. Heart J. 2017, 58, 654–665. [Google Scholar] [CrossRef]
- ATS/ACCP. American Thoracic Society/American College of Chest Physicians Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.D.; Nepal, P.; Akinlonu, A.; Nekkalapudi, D.; Kim, K.; Cativo, E.H.; Visco, F.; Mushiyev, S.; Pekler, G. Low skeletal muscle mass independently predicts mortality in patients with chronic heart failure after an acute hospitalization. Cardiology 2019, 142, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Yamashita, M.; Uchida, S.; Ueno, K.; Maekawa, E.; Terada, T.; et al. SARC-F predicts poor motor function, quality of life, and prognosis in older patients with cardiovascular disease and cognitive impairment. Exp. Gerontol. 2023, 171, 112021. [Google Scholar] [CrossRef]
- Pietrobelli, A.; Morini, P.; Battistini, N.; Chiumello, G.; Nuñez, C.; Heymsfield, S.B. Appendicular skeletal muscle mass: Prediction from multiple frequency segmental bioimpedance analysis. Eur. J. Clin. Nutr. 1998, 52, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiog-raphy’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- London, G.M.; Pannier, B. Arterial functions: How to interpret the complex physiology. Nephrol. Dial. Transplant. 2010, 25, 3815–3823. [Google Scholar] [CrossRef]
- Briet, M.; Boutouyrie, P.; Laurent, S.; London, G.M. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012, 82, 388–400. [Google Scholar] [CrossRef]
- Shiba, T.; Takahashi, M.; Matsumoto, T.; Shirai, K.; Hori, Y. Arterial stiffness shown by the cardio-ankle vascular index is an important contributor to optic nerve head microcirculation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 99–105. [Google Scholar] [CrossRef]
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul. Pharmacol. 2016, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Tanisawa, K.; Ito, T.; Sun, X.; Kawakami, R.; Oshima, S.; Gando, Y.; Cao, Z.B.; Sakamoto, S.; Higuchi, M. Cardiorespiratory fitness is a strong predictor of the cardio-ankle vascular index in hypertensive middle-aged and elderly Japanese men. J. Atheroscler. Thromb. 2015, 22, 379–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Karpman, V.L.; Fick, A. The theoretical analysis of Fick’s equation. On the centennial of the use of Fick’s principle in physiology. Z. Kardiol. 1975, 64, 801–808. [Google Scholar] [PubMed]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Park, S.H.; Kim, W.S.; Jang, W.Y.; Park, E.J.; Kang, D.O.; Park, Y.; Na, J.O.; Kim, J.W.; Kim, E.J.; et al. Handgrip strength as a predictor of exercise capacity in coronary heart disease. J. Cardiopulm. Rehabil. Prev. 2020, 40, E10–E13. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, S.; Takada, S.; Matsushima, S.; Okita, K.; Tsutsui, H. Skeletal muscle abnormalities in heart failure. Int. Heart J. 2015, 56, 475–484. [Google Scholar] [CrossRef]
- Okita, K.; Kinugawa, S.; Tsutsui, H. Exercise intolerance in chronic heart failure--skeletal muscle dysfunction and potential therapies. Circ. J. 2013, 77, 293–300. [Google Scholar] [CrossRef]
- Ogawa, A.; Shimizu, K.; Nakagami, T.; Maruoka, H.; Shirai, K. Physical function and cardio-ankle vascular index in elderly heart failure patients. Int. Heart J. 2020, 61, 769–775. [Google Scholar] [CrossRef]
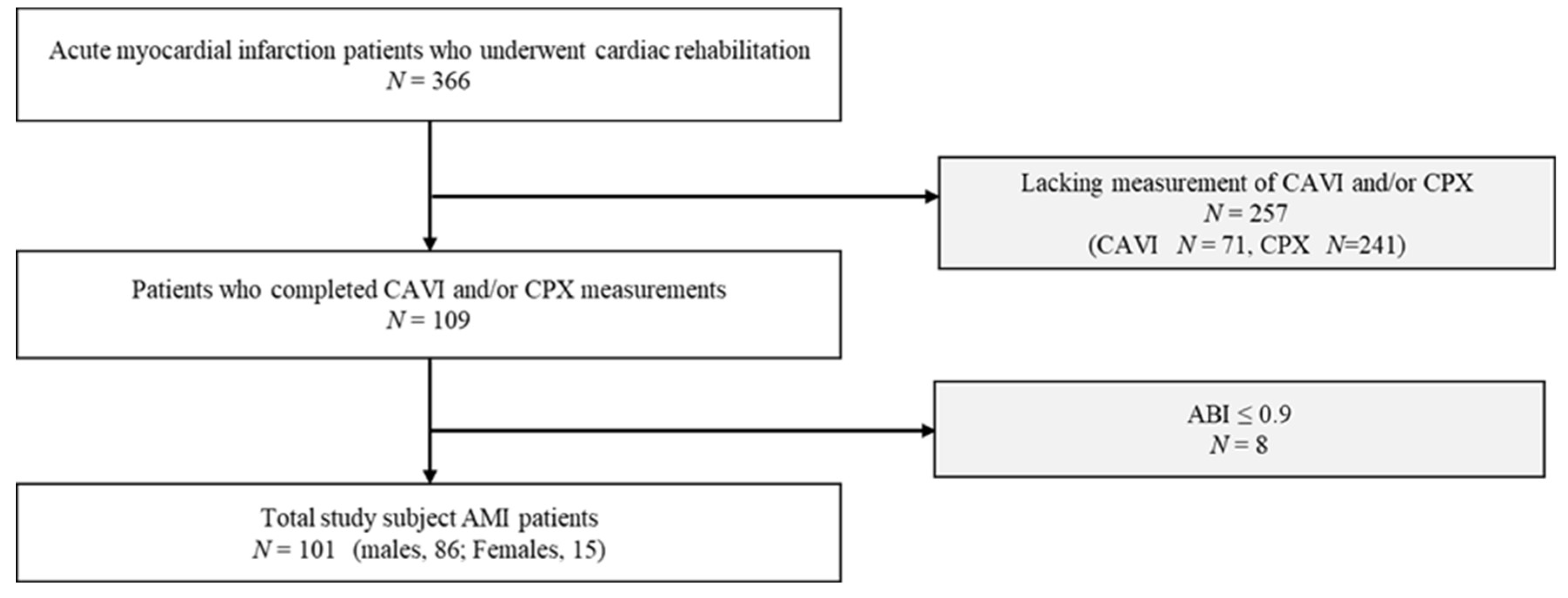

| Variable | Patients with AMI n = 101 |
|---|---|
| Male, n (%) | 86 (85.1) |
| Age, years | 67.5 (56.0, 74.0) |
| BMI, kg/m2 | 22.3 (20.9, 25.2) |
| sBP, mmHg | 115.0 (106.5, 126.5) |
| dBP, mmHg | 71.0 (63.5, 80.5) |
| HR, bpm | 68.0 (59.3, 77.8) |
| Alb, g/dL | 3.8 (3.6, 4.2) |
| Cre, mg/dL | 0.92 (0.82, 1.06) |
| Hb, mg/dL | 13.4 (11.9, 14.2) |
| CPK Peak | 1162.0 (374.5, 2240.0) |
| BNP, pg/mL | 86.9 (52.0, 163.2) |
| EF, % | 61.0 (53.5, 67.0) |
| E/e′ | 11.2 (9.0, 15.5) |
| CAVI | 9.1 (8.2, 9.9) |
| Smoking, n (%) | 34 (33.7) |
| Complications, n (%) | |
| AF | 5 (4.9) |
| HT | 42 (41.6) |
| DM | 31 (31.7) |
| DL | 63 (62.4) |
| Use of medications, n (%) | |
| Ca-antagonists | 9 (8.9) |
| RAS-inhibitors | 53 (52.5) |
| β-blockers | 72 (71.3) |
| Diuretics | 28 (27.7) |
| Statins | 76 (75.2) |
| Nitrate drug | 42 (41.6) |
| Variable | Patients with AMI n = 101 |
|---|---|
| SMI, kg/m2 | 6.37 (5.68, 7.38) |
| Handgrip strength, kg | 21.7 (16.2, 28.2) |
| VO2 AT, mL/kg/min | 13.7 (11.4, 15.7) |
| VO2 Peak, mL/kg/min | 17.7 (15.6, 20.7) |
| HR AT, beat/min | 104.5 (94.8, 111.0) |
| HR Peak, beat/min | 124.5 (110.3, 134.0) |
| VO2/HR Peak, mL/beat | 9.5 (7.9, 10.9) |
| VE vs. VCO2 slope | 32.0 (29.1, 37.0) |
| ΔVO2/ΔWR, mL/min/watt | 8.1 (7.0, 9.5) |
| Variable | Correlation Coefficient (ρ) | 95% CI | p-Value |
|---|---|---|---|
| Age | −0.487 | −0.628–−0.315 | <0.001 |
| BMI | 0.136 | −0.075–0.335 | 0.191 |
| sBP | −0.026 | −0.230–0.180 | 0.798 |
| dBP | 0.024 | −0.182–0.228 | 0.812 |
| CPKPeak | −0.09 | −0.410–0.251 | 0.598 |
| Alb | 0.297 | 0.094–0.476 | 0.004 |
| Cre | −0.121 | −0.319–0.087 | 0.240 |
| Hb | 0.454 | 0.270–0.603 | <0.001 |
| BNP | −0.326 | −0.509–−0.115 | 0.002 |
| EF | 0.049 | −0.204–0.296 | 0.698 |
| E/e′ | −0.212 | −0.403–−0.004 | 0.04 |
| CAVI | −0.502 | −0.640–−0.333 | <0.001 |
| SMI | 0.479 | 0.302–0.624 | <0.001 |
| Handgrip strength | 0.501 | 0.325–0.643 | <0.001 |
| Variable | r | r2 | β | 95% CI | p-Value |
|---|---|---|---|---|---|
| Age | 0.657 | 0.432 | 0.098 | –4.494–35.936 | 0.125 |
| Sex | −0.103 | –4.132–1.917 | 0.467 | ||
| Alb | −0.011 | –2.243–2.042 | 0.926 | ||
| Hb | 0.106 | –0.296–0.789 | 0.368 | ||
| BNP | −0.046 | –0.008–0.005 | 0.678 | ||
| E/e′ | −0.043 | –0.225–0.149 | 0.684 | ||
| CAVI | −0.258 | –1.856–−0.070 | 0.035 | ||
| SMI | −0.014 | –1.170–1.075 | 0.933 | ||
| Handgrip strength | 0.541 | 0.074–0.402 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, A.; Kanzaki, S.; Ikeda, Y.; Iwakawa, M.; Nakagami, T.; Sato, S.; Mikamo, H.; Kido, S.; Nakajima, A.; Shimizu, K. Determination of Peak Oxygen Uptake in Patients with Acute Myocardial Infarction: The Role of Arterial Stiffness in Cardio–Vascular–Skeletal Muscle Coupling. J. Clin. Med. 2024, 13, 42. https://doi.org/10.3390/jcm13010042
Ogawa A, Kanzaki S, Ikeda Y, Iwakawa M, Nakagami T, Sato S, Mikamo H, Kido S, Nakajima A, Shimizu K. Determination of Peak Oxygen Uptake in Patients with Acute Myocardial Infarction: The Role of Arterial Stiffness in Cardio–Vascular–Skeletal Muscle Coupling. Journal of Clinical Medicine. 2024; 13(1):42. https://doi.org/10.3390/jcm13010042
Chicago/Turabian StyleOgawa, Akihiro, Shinya Kanzaki, Yuki Ikeda, Masahiro Iwakawa, Takahiro Nakagami, Shuji Sato, Hiroshi Mikamo, Satoshi Kido, Arata Nakajima, and Kazuhiro Shimizu. 2024. "Determination of Peak Oxygen Uptake in Patients with Acute Myocardial Infarction: The Role of Arterial Stiffness in Cardio–Vascular–Skeletal Muscle Coupling" Journal of Clinical Medicine 13, no. 1: 42. https://doi.org/10.3390/jcm13010042
APA StyleOgawa, A., Kanzaki, S., Ikeda, Y., Iwakawa, M., Nakagami, T., Sato, S., Mikamo, H., Kido, S., Nakajima, A., & Shimizu, K. (2024). Determination of Peak Oxygen Uptake in Patients with Acute Myocardial Infarction: The Role of Arterial Stiffness in Cardio–Vascular–Skeletal Muscle Coupling. Journal of Clinical Medicine, 13(1), 42. https://doi.org/10.3390/jcm13010042








