Clinical Remission in Patients Affected by Severe Eosinophilic Asthma on Dupilumab Therapy: A Long-Term Real-Life Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Asthma Control Test
2.2. Pulmonary Function Tests
2.3. Inflammatory Characterization
2.4. Definition of Clinical Remission
- lack of need to use OCS;
- lack of exacerbations;
- achievement of ACT score ≥ 20;
- achievement of a percentage of pre-bd FEV1 ≥ 80% of the predicted.
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Population at Baseline (T0)
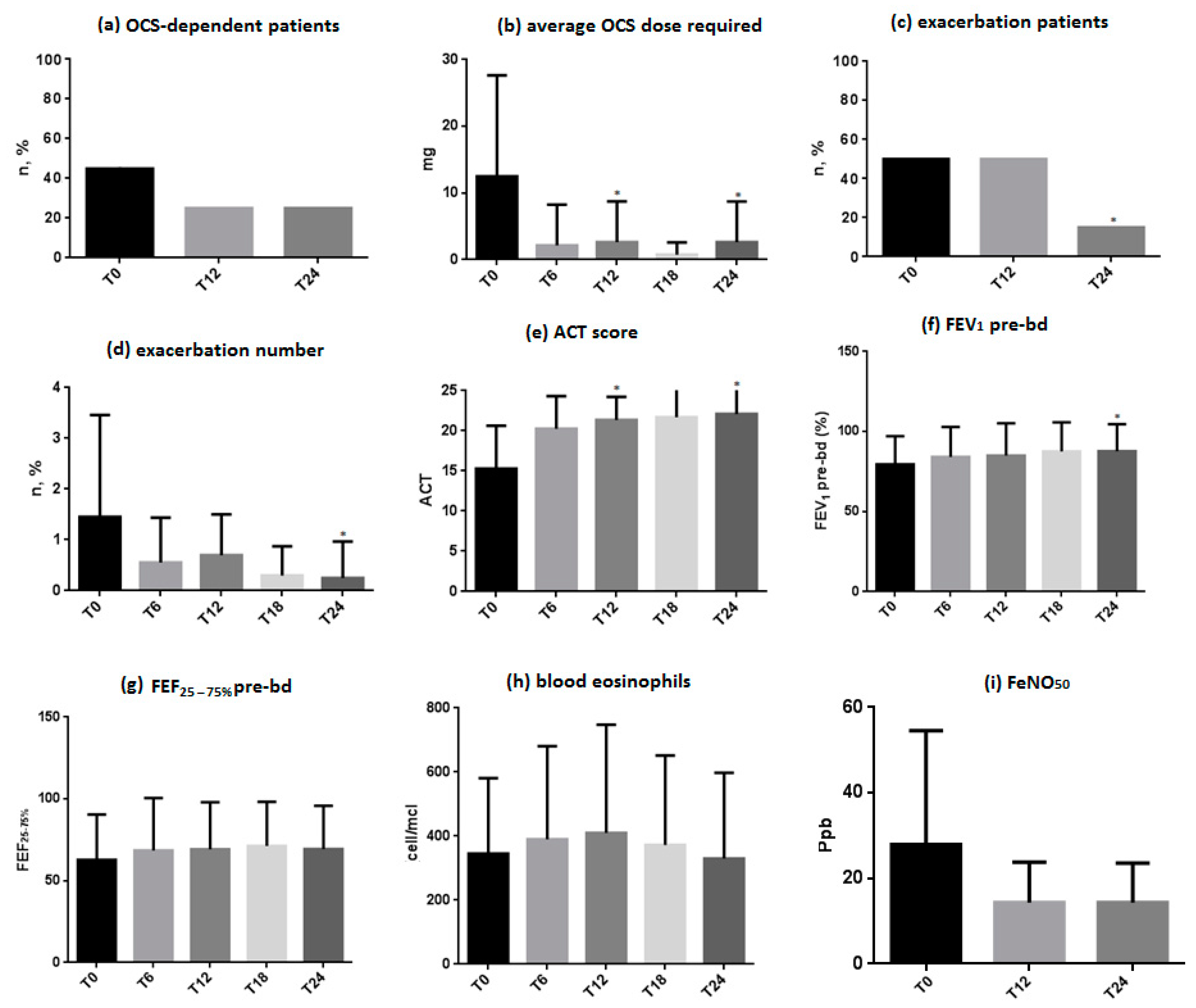
3.2. OCS-Dependent Patients and Average OCS Dose Required
3.3. Exacerbation Patients and Number of Exacerbations
3.4. ACT Score
3.5. Respiratory Function
3.6. Blood Eosinophils
3.7. FeNO50 Levels
3.8. Clinical Remission
3.9. Analysis of the Characteristics Shown at Baseline by Patients in Clinical Remission (Partial + Complete) at T24
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Asthma Network The Global Asthma Report. Int. J. Tuberc. Lung Dis. 2022, 26, 1–104. [CrossRef]
- Rogliani, P.; Calzetta, L.; Matera, M.G.; Laitano, R.; Ritondo, B.L.; Hanania, N.A.; Cazzola, M. Severe Asthma and Biological Therapy: When, Which, and for Whom. Pulm. Ther. 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- 2023 GINA Main Report—Global Initiative for Asthma—GINA. Available online: https://ginasthma.org/2023-gina-main-report/ (accessed on 18 October 2023).
- Tsiavia, T.; Henny, J.; Goldberg, M.; Zins, M.; Roche, N.; Orsi, L.; Nadif, R. Blood inflammatory phenotypes were associated with distinct clinical expressions of asthma in adults from a large population-based cohort. eBioMedicine 2022, 76, 103875. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, J.R.; Lloyd, C.M. Chronic inflammation and asthma. Mutat. Res. 2010, 690, 24. [Google Scholar] [CrossRef] [PubMed]
- Canonica, G.W.; Blasi, F.; Carpagnano, G.E.; Guida, G.; Heffler, E.; Paggiaro, P.; Allegrini, C.; Antonelli, A.; Aruanno, A.; Bacci, E.; et al. Severe Asthma Network Italy Definition of Clinical Remission in Severe Asthma: A Delphi Consensus. J. Allergy Clin. Immunol. Pract. 2023, 11, 3629–3637. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Bafadhel, M.; Busse, W.W.; Casale, T.B.; Kocks, J.W.H.; Pavord, I.D.; Szefler, S.J.; Woodruff, P.G.; de Giorgio-Miller, A.; Trudo, F.; et al. An expert consensus framework for asthma remission as a treatment goal. J. Allergy Clin. Immunol. 2020, 145, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Ul-Haq, Z.; Naz, S.; Mesaik, M.A. Interleukin-4 receptor signaling and its binding mechanism: A therapeutic insight from inhibitors tool box. Cytokine Growth Factor Rev. 2016, 32, 3–15. [Google Scholar] [CrossRef]
- LaPorte, S.L.; Juo, Z.S.; Vaclavikova, J.; Colf, L.A.; Qi, X.; Heller, N.M.; Keegan, A.D.; Garcia, K.C. Molecular and Structural Basis of Cytokine Receptor Pleiotropy in the Interleukin-4/13 System. Cell 2008, 132, 259–272. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the Asthma Control Test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Stocks, J.; Quanjer, P. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur. Respir. J. 1995, 8, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; An, J.; Xie, J.; Huang, R.; Xie, Y.; He, L.; Xv, H.; Qian, G.; Li, J. FEF25–75% Is a More Sensitive Measure Reflecting Airway Dysfunction in Patients with Asthma: A Comparison Study Using FEF25–75% and FEV1%. J. Allergy Clin. Immunol. Pract. 2021, 9, 3649–3659.e6. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Merman, E.; Stanbrook, M.B.; Anand, A. Liberty Asthma Venture Trial: Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. Can. J. Respir. Crit. Care Sleep Med. 2019, 3, 121–122. [Google Scholar] [CrossRef]
- Dupin, C.; Belhadi, D.; Guilleminault, L.; Gamez, A.S.; Berger, P.; De Blay, F.; Bonniaud, P.; Leroyer, C.; Mahay, G.; Girodet, P.O.; et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin. Exp. Allergy 2020, 50, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Campisi, R.; Crimi, C.; Nolasco, S.; Beghè, B.; Antonicelli, L.; Guarnieri, G.; Scichilone, N.; Porto, M.; Macchia, L.; Scioscia, G.; et al. Real-World Experience with Dupilumab in Severe Asthma: One-Year Data from an Italian Named Patient Program. J. Asthma Allergy 2021, 14, 575. [Google Scholar] [CrossRef]
- Le Floch-ramondou, A.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Murphy, A.; Sleeman, M.; Orengo, J. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. J. Allergy Clin. Immunol. 2020, 145, AB158. [Google Scholar] [CrossRef]
- Eger, K.; Pet, L.; Weersink, E.J.M.; Bel, E.H. Complications of switching from anti–IL-5 or anti–IL-5R to dupilumab in corticosteroid-dependent severe asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 2913–2915. [Google Scholar] [CrossRef]
- Pavord, I.D.; Israel, E.; Szefler, S.J.; Brusselle, G.; Rabe, K.F.; Chen, Z.; Altincatal, A.; Radwan, A.; Pandit-Abid, N.; Amin, N.; et al. Dupilumab Induces Clinical Remission in Patients with Uncontrolled, Moderate-to-Severe, Type 2 Inflammatory Asthma. Available online: www.atsjournals.org (accessed on 19 October 2023).
- Ambrose, C.S.; Chipps, B.E.; Moore, W.C.; Soong, W.; Trevor, J.; Ledford, D.K.; Carr, W.W.; Lugogo, N.; Trudo, F.; Tran, T.N.; et al. The CHRONICLE Study of US Adults with Subspecialist-Treated Severe Asthma: Objectives, Design, and Initial Results. Pragmatic Obs. Res. 2020, 11, 77. [Google Scholar] [CrossRef]
- Prato, R.; Martinelli, D.; Calabria, S.; Quarato, C.M.I.; Piccinni, C.; Carpagnano, G.E.; Rossi, A.; Blasi, F.; Fortunato, F.; Foschino Barbaro, M.P. Inclusion of vaccination into clinical pathways for COPD and asthma: Current challenges and future perspectives in Italy. Epidemiol. Prev. 2023, 47, 47–56. [Google Scholar]
- de Boer, G.M.; Braunstahl, G.J.; van der Ploeg, E.K.; van Zelst, C.M.; van Bruggen, A.; Epping, G.; van Nimwegen, M.; Verhoeven, G.; Birnie, E.; Boxma-de Klerk, B.M.; et al. Bacterial lysate add-on therapy to reduce exacerbations in severe asthma: A double-blind placebo-controlled trial. Clin. Exp. Allergy 2021, 51, 1172. [Google Scholar] [CrossRef] [PubMed]
- Canonica, G.W.; Bourdin, A.; Peters, A.T.; Desrosiers, M.; Bachert, C.; Weidinger, S.; Simpson, E.L.; Daizadeh, N.; Chen, Z.; Kamat, S.; et al. Dupilumab Demonstrates Rapid Onset of Response Across Three Type 2 Inflammatory Diseases. J. Allergy Clin. Immunol. Pract. 2022, 10, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Castro, M.; O’Riordan, T.; Hanania, N.A.; Pavord, I.D.; Quirce, S.; Chipps, B.E.; Wenzel, S.E.; Thangavelu, K.; Rice, M.S.; et al. Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Lombardo, N.; Busceti, M.T.; Piazzetta, G.; Crimi, C.; Calabrese, C.; Vatrella, A.; Pelaia, G. Short-Term Evaluation of Dupilumab Effects in Patients with Severe Asthma and Nasal Polyposis. J. Asthma Allergy 2021, 14, 1165. [Google Scholar] [CrossRef] [PubMed]
- Fomina, D.S.; Fedosenko, S.V.; Bobrikova, E.N.; Chernov, A.A.; Mukhina, O.A.; Lebedkina, M.S.; Karaulov, A.V.; Nurtazina, A.Y.; Lysenko, M.A. Efficacy of dupilumab in real practice in the treatment of severe forms of asthma and atopic dermatitis (comparative retrospective study). Ter. Arkh. 2023, 95, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Lipworth, B. Real-life effects of dupilumab on airway oscillometry in severe uncontrolled asthma. Ann. Allergy, Asthma Immunol. 2023, 131, 664–666. [Google Scholar] [CrossRef]
- Malinovschi, A.; Fonseca, J.A.; Jacinto, T.; Alving, K.; Janson, C. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J. Allergy Clin. Immunol. 2013, 132, 821–827.e5. [Google Scholar] [CrossRef]
- Tay, T.R.; Radhakrishna, N.; Hore-Lacy, F.; Smith, C.; Hoy, R.; Dabscheck, E.; Hew, M. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology 2016, 21, 1384–1390. [Google Scholar] [CrossRef]
- Jones, R.L.; Nzekwu, M.M.U. The effects of body mass index on lung volumes. Chest 2006, 130, 827–833. [Google Scholar] [CrossRef]
- Al-Alwan, A.; Bates, J.H.T.; Chapman, D.G.; Kaminsky, D.A.; DeSarno, M.J.; Irvin, C.G.; Dixon, A.E. The Nonallergic asthma of obesity: A matter of distal lung compliance. Am. J. Respir. Crit. Care Med. 2014, 189, 1494–1502. [Google Scholar] [CrossRef]
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, R113–R127. [Google Scholar] [CrossRef]
- Holguin, F.; Comhair, S.A.A.; Hazen, S.L.; Powers, R.W.; Khatri, S.S.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Fitzpatrick, A.M.; et al. An Association between l-Arginine/Asymmetric Dimethyl Arginine Balance, Obesity, and the Age of Asthma Onset Phenotype. Am. J. Respir. Crit. Care Med. 2013, 187, 153. [Google Scholar] [CrossRef]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Pretto, J.J.; Morgan, P.J.; Callister, R.; Wood, L.G. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: A randomized trial. Clin. Exp. Allergy 2013, 43, 36–49. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Goleva, E.; Strand, M.; Beuther, D.A.; Leung, D.Y.M. Body Mass and Glucocorticoid Response in Asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 682. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Li, M.; Xu, C.; Davis, J.D.; Kanamaluru, V.; Lu, Q. Population pharmacokinetic analysis of dupilumab in adult and adolescent patients with asthma. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 941. [Google Scholar] [CrossRef]
- Chen, Z.; Salam, M.T.; Alderete, T.L.; Habre, R.; Bastain, T.M.; Berhane, K.; Gilliland, F.D. Effects of childhood asthma on the development of obesity among school-aged children. Am. J. Respir. Crit. Care Med. 2017, 195, 1181–1188. [Google Scholar] [CrossRef]
- Sweeney, J.; Patterson, C.C.; Menzies-Gow, A.; Niven, R.M.; Mansur, A.H.; Bucknall, C.; Chaudhuri, R.; Price, D.; Brightling, C.E.; Heaney, L.G. Comorbidity in severe asthma requiring systemic corticosteroid therapy: Cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016, 71, 339–346. [Google Scholar] [CrossRef]


| Characteristics | Results (n = 20) |
|---|---|
| Age, years (mean ± SD) | 52.25 ± 10.35 |
| Females, n (%) | 16 (80%) |
| Males, n (%) | 4 (20%) |
| Current smokers, n (%) | 2 (10%) |
| Past smokers, n (%) | 8 (40%) |
| BMI, kg/m2 (mean ± SD) | 30.04 ± 7.60 |
| Age of asthma onset, years (mean ± SD) | 40.68 ± 11.52 |
| Childhood-onset asthma, n (%) | 3 (15%) |
| Adult-onset asthma, n (%) | 17 (85%) |
| Atopy, n (%) | 16 (80%) |
| Gastroesophageal reflux, n (%) | 8 (40%) |
| Nasal polyposis, n (%) | 5 (25%) |
| OSAS, n (%) | 3 (15%) |
| Anxiety and depression, n (%) | 3 (15%) |
| Atopic dermatitis, n (%) | 2 (10%) |
| Osteoporosis, n (%) | 2 (10%) |
| Exacerbation patients, n (%) | 10 (50%) |
| Exacerbation/year, n (mean ± SD) | 1.45 ± 1.58 |
| OCS-dependent patients, n (%) | 10 (50%) |
| OCS dosage, mg (mean ± SD) | 12.5 ± 13.75 |
| ACT score (mean ± DS) | 15.30 ± 4.16 |
| FEV1/FVC (mean ± DS) | 68.35 ± 9.09 |
| FEV1% pre bd, (mean ± DS) | 79.50 ± 14.40 |
| FEV1, Liters, (mean ± DS) | 91.15 ± 14.32 |
| FEF25–75%, (mean ± DS) | 62.75 ± 23.82 |
| Eosinophils, cells/µL (mean ± DS) | 345.50 ± 194.15 |
| Eosinophils > 300 cells/µL, n (%) | 8 (40%) |
| FeNO50, ppb (mean ± DS) | 27.94 ± 17.72 |
| FeNO50 > 25 ppb, n (%) | 7 (35%) |
| Total IgE, kU/L (mean ± DS) | 825.40 ± 661.94 |
| Characteristics at Baseline (T0) | Clinical Remission at T24 (n = 12) | No Remission at T24 (n = 8) | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Sex female | 9 (56.3%) | 7 (43.7%) | 0.43 | 0.04 | 5.06 | 0.62 |
| Smoking (past or current) | 5 (50.0%) | 5 (50.0%) | 0.43 | 0.07 | 2.68 | 0.65 |
| BMI > 30 kg/m2 | 1 (14.3%) | 6 (85.7%) | 0.03 | 0.002 | 0.41 | 0.004 * |
| Adult-onset asthma | 9 (52.9%) | 8 (47.1%) | 0.16 | 0.007 | 3.56 | 0.24 |
| Atopy | 10 (62.5%) | 6 (37.5%) | 1.67 | 0.18 | 15.14 | 1.00 |
| Gastroesophageal reflux | 4 (50.0%) | 4 (50.0%) | 0.50 | 0.08 | 3.13 | 0.65 |
| Nasal polyposis | 4 (66.7%) | 2 (33.3%) | 1.50 | 0.20 | 11.09 | 1.00 |
| OSAS | 2 (66.7%) | 1 (33.3%) | 1.40 | 0.11 | 18.63 | 1.00 |
| Anxiety and depression | 1 (33.3%) | 2 (66.7%) | 0.27 | 0.02 | 3.67 | 0.54 |
| Atopic dermatitis | 2 (100.0%) | 0 (0.0%) | 4.05 | 0.17 | 96.26 | 0.49 |
| Osteoporosis | 2 (100.0%) | 0 (0.0%) | 4.05 | 0.17 | 96.26 | 0.49 |
| FEF25–75% < 65% | 7 (63.6%) | 4 (36.4%) | 1.40 | 0.23 | 8.46 | 1.00 |
| Eosinophils > 300 cell/mcL | 4 (50.0%) | 4 (50.0%) | 0.50 | 0.08 | 3.13 | 0.65 |
| FeNO50 > 25 ppb | 5 (71.4%) | 2 (28.6%) | 2.14 | 0.30 | 15.36 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarato, C.M.I.; Tondo, P.; Lacedonia, D.; Soccio, P.; Fuso, P.; Sabato, E.; Hoxhallari, A.; Foschino Barbaro, M.P.; Scioscia, G. Clinical Remission in Patients Affected by Severe Eosinophilic Asthma on Dupilumab Therapy: A Long-Term Real-Life Study. J. Clin. Med. 2024, 13, 291. https://doi.org/10.3390/jcm13010291
Quarato CMI, Tondo P, Lacedonia D, Soccio P, Fuso P, Sabato E, Hoxhallari A, Foschino Barbaro MP, Scioscia G. Clinical Remission in Patients Affected by Severe Eosinophilic Asthma on Dupilumab Therapy: A Long-Term Real-Life Study. Journal of Clinical Medicine. 2024; 13(1):291. https://doi.org/10.3390/jcm13010291
Chicago/Turabian StyleQuarato, Carla Maria Irene, Pasquale Tondo, Donato Lacedonia, Piera Soccio, Paolo Fuso, Eugenio Sabato, Anela Hoxhallari, Maria Pia Foschino Barbaro, and Giulia Scioscia. 2024. "Clinical Remission in Patients Affected by Severe Eosinophilic Asthma on Dupilumab Therapy: A Long-Term Real-Life Study" Journal of Clinical Medicine 13, no. 1: 291. https://doi.org/10.3390/jcm13010291
APA StyleQuarato, C. M. I., Tondo, P., Lacedonia, D., Soccio, P., Fuso, P., Sabato, E., Hoxhallari, A., Foschino Barbaro, M. P., & Scioscia, G. (2024). Clinical Remission in Patients Affected by Severe Eosinophilic Asthma on Dupilumab Therapy: A Long-Term Real-Life Study. Journal of Clinical Medicine, 13(1), 291. https://doi.org/10.3390/jcm13010291








