Acute Kidney Injury and Hepatorenal Syndrome in Patients with Cirrhosis
Abstract
:1. Introduction
2. Assessment of Kidney Function in Patients with Cirrhosis
3. Definition and Diagnosis of Hepatorenal Syndrome
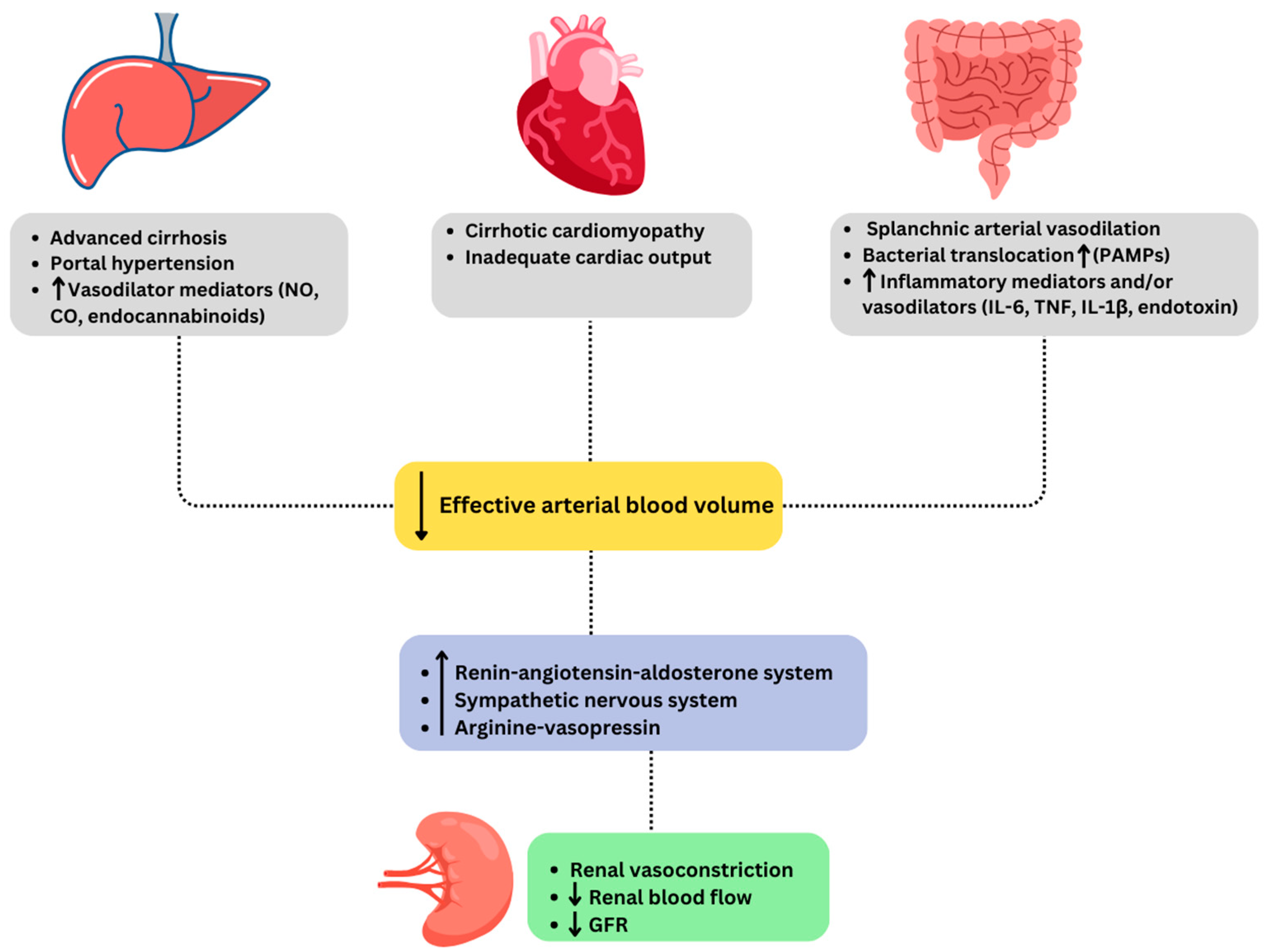
4. Pathophysiology of Hepatorenal Syndrome
5. Diagnostic Workup of Acute Kidney Injury in Patients with Cirrhosis
Conventional and Emerging Biomarkers
6. Acute Kidney Injury in Patients with Acute-on-Chronic Liver Failure
7. Prevention of Acute Kidney Injury and Hepatorenal Syndrome-Acute Kidney Injury
8. Management of Acute Kidney Injury and Hepatorenal Syndrome-Acute Kidney Injury
8.1. Terlipressin
8.2. Norepinephrine
8.3. Midodrine and Octreotide
9. Transjugular Intrahepatic Portosystemic Shunt
10. Renal Replacement Therapy
11. Liver Transplantation
12. Future Directions
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nadim, M.K.; Garcia-Tsao, G. Acute Kidney Injury in Patients with Cirrhosis. N. Engl. J. Med. 2023, 388, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Kiani, C.; Zori, A.G. Recent advances in pathophysiology, diagnosis and management of hepatorenal syndrome: A review. World J. Hepatol. 2023, 15, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Biggins, S.W.; Angeli, P.; Garcia-Tsao, G.; Ginès, P.; Ling, S.C.; Nadim, M.K.; Wong, F.; Kim, W.R. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1014–1048. [Google Scholar] [CrossRef] [PubMed]
- Cholongitas, E.; Senzolo, M.; Patch, D.; Shaw, S.; O’Beirne, J.; Burroughs, A.K. Cirrhotics admitted to intensive care unit: The impact of acute renal failure on mortality. Eur. J. Gastroenterol. Hepatol. 2009, 21, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.O.; Francoz, C. Global strategy for the diagnosis and management of acute kidney injury in patients with liver cirrhosis. United Eur. Gastroenterol. J. 2021, 9, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Parikh, N.D. Diagnosis and Management of Cirrhosis and Its Complications: A Review. JAMA 2023, 329, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, J.; Bartels, C.; Berssenbrügge, C.; Schmidt, H.; Meister, T. Hepatorenal Syndrome: Outcome of Response to Therapy and Predictors of Survival. Gastroenterol. Res. Pract. 2015, 2015, 457613. [Google Scholar] [CrossRef]
- Angeli, P.; Ginès, P.; Wong, F.; Bernardi, M.; Boyer, T.D.; Gerbes, A.; Moreau, R.; Jalan, R.; Sarin, S.K.; Piano, S.; et al. Diagnosis and Management of Acute Kidney Injury in Patients with Cirrhosis: Revised Consensus Recommendations of the International Club of Ascites. J. Hepatol. 2015, 62, 968–974. [Google Scholar] [CrossRef]
- Francoz, C.; Prié, D.; Abdelrazek, W.; Moreau, R.; Mandot, A.; Belghiti, J.; Valla, D.; Durand, F. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: Impact on the model for end-stage liver disease score. Liver Transpl. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transpl. Soc. 2010, 16, 1169–1177. [Google Scholar] [CrossRef]
- Simonetto, D.A.; Gines, P.; Kamath, P.S. Hepatorenal syndrome: Pathophysiology, diagnosis, and management. BMJ 2020, 370, m2687. [Google Scholar] [CrossRef]
- Francoz, C.; Nadim, M.K.; Durand, F. Kidney biomarkers in cirrhosis. J. Hepatol. 2016, 65, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Mindikoglu, A.L.; Dowling, T.C.; Weir, M.R.; Seliger, S.L.; Christenson, R.H.; Magder, L.S. Performance of chronic kidney disease epidemiology collaboration creatinine-cystatin C equation for estimating kidney function in cirrhosis. Hepatology 2014, 59, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Angeli, P.; Garcia-Tsao, G.; Nadim, M.K.; Parikh, C.R. News in Pathophysiology, Definition and Classification of Hepatorenal Syndrome: A Step beyond the International Club of Ascites (ICA) Consensus Document. J. Hepatol. 2019, 71, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Solé, C.; Solà, E.; Huelin, P.; Carol, M.; Moreira, R.; Cereijo, U.; Mas, J.; Graupera, I.; Pose, E.; Napoleone, L.; et al. Characterization of inflammatory response in hepatorenal syndrome: Relationship with kidney outcome and survival. Liver Int. 2019, 39, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.Y.; Ginès, P.; Schrier, R.W. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N. Engl. J. Med. 1998, 339, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Krag, A.; Bendtsen, F.; Henriksen, J.H.; Møller, S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut 2010, 59, 105–110. [Google Scholar] [CrossRef]
- Møller, S.; Henriksen, J.H. Cirrhotic cardiomyopathy. J. Hepatol. 2010, 53, 179–190. [Google Scholar] [CrossRef]
- Chayanupatkul, M.; Liangpunsakul, S. Cirrhotic cardiomyopathy: Review of pathophysiology and treatment. Hepatol. Int. 2014, 8, 308–315. [Google Scholar] [CrossRef]
- Liu, S.; Meng, Q.; Xu, Y.; Zhou, J. Hepatorenal syndrome in acute-on-chronic liver failure with acute kidney injury: More questions requiring discussion. Gastroenterol. Rep. 2021, 9, 505–520. [Google Scholar] [CrossRef]
- Mindikoglu, A.L.; Dowling, T.C.; Wong-You-Cheong, J.J.; Christenson, R.H.; Magder, L.S.; Hutson, W.R.; Seliger, S.L.; Weir, M.R. A pilot study to evaluate renal hemodynamics in cirrhosis by simultaneous glomerular filtration rate, renal plasma flow, renal resistive indices and biomarkers measurements. Am. J. Nephrol. 2014, 39, 543–552. [Google Scholar] [CrossRef]
- Tiwari, N.; Wong, F. Hepatorenal syndrome: Updates. Clin. Liver Dis. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Patidar, K.R.; Kang, L.; Bajaj, J.S.; Carl, D.; Sanyal, A.J. Fractional Excretion of Urea: A Simple Tool for the Differential Diagnosis of Acute Kidney Injury in Cirrhosis. Hepatology 2018, 68, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.; Sheikh, M.F.; Lamb, E.; Agarwal, B.; Jalan, R. Acute kidney injury in acute-on-chronic liver failure: Where does hepatorenal syndrome fit? Kidney Int. 2017, 92, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.M. Hepatorenal Syndrome: Pathophysiology, Diagnosis, and Treatment. Med. Clin. N. Am. 2023, 107, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Brown, R.S.; Farrand, E.; Pichardo, E.M.; Forster, C.S.; Valle, D.A.S.-D.; Adkins, S.H.; Sise, M.E.; Oliver, J.A.; Radhakrishnan, J.; et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig. Dis. Sci. 2012, 57, 2362–2370. [Google Scholar] [CrossRef]
- Fagundes, C.; Pépin, M.N.; Guevara, M.; Barreto, R.; Casals, G.; Solà, E.; Pereira, G.; Rodríguez, E.; Garcia, E.; Prado, V.; et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J. Hepatol. 2012, 57, 267–273. [Google Scholar] [CrossRef]
- Belcher, J.M.; Sanyal, A.J.; Peixoto, A.J.; Perazella, M.A.; Lim, J.; Thiessen-Philbrook, H.; Ansari, N.; Coca, S.G.; Garcia-Tsao, G.; Parikh, C.R.; et al. Kidney Biomarkers and Differential Diagnosis of Patients with Cirrhosis and Acute Kidney Injury. Hepatology 2014, 60, 622–632. [Google Scholar] [CrossRef]
- Mårtensson, J.; Bellomo, R. The rise and fall of NGAL in acute kidney injury. Blood Purif. 2014, 37, 304–310. [Google Scholar] [CrossRef]
- Macdonald, S.P.J.; Stone, S.F.; Neil, C.L.; van Eeden, P.E.; Fatovich, D.M.; Arendts, G.; Brown, S.G.A. Sustained elevation of resistin, NGAL and IL-8 are associated with severe sepsis/septic shock in the emergency department. PLoS ONE 2014, 9, e110678. [Google Scholar] [CrossRef]
- Ariza, X.; Solà, E.; Elia, C.; Barreto, R.; Moreira, R.; Morales-Ruiz, M.; Graupera, I.; Rodríguez, E.; Huelin, P.; Solé, C.; et al. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS ONE 2015, 10, e0128145. [Google Scholar] [CrossRef]
- Belcher, J.M.; Garcia-Tsao, G.; Sanyal, A.J.; Thiessen-Philbrook, H.; Peixoto, A.J.; Perazella, M.A.; Ansari, N.; Lim, J.; Coca, S.G.; Parikh, C.R. Urinary Biomarkers and Progression of AKI in Patients with Cirrhosis. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ge, B.; Liu, Y.; Feng, J. The efficacy of biomarkers in the diagnosis of acute kidney injury secondary to liver cirrhosis. Medicine 2021, 100, e25411. [Google Scholar] [CrossRef] [PubMed]
- Yewale, R.V.; Ramakrishna, B.S.; Venugopal, G.; Doraiswami, B.V.; Rajini, K. Urine neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury and prognosis in decompensated chronic liver disease: A prospective study. Indian J. Gastroenterol. 2023, 42, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013, 144, 1426–1437.e9. [Google Scholar] [CrossRef] [PubMed]
- Huelin, P.; Piano, S.; Solà, E.; Stanco, M.; Solé, C.; Moreira, R.; Pose, E.; Fasolato, S.; Fabrellas, N.; de Prada, G.; et al. Validation of a Staging System for Acute Kidney Injury in Patients with Cirrhosis and Association with Acute-on-Chronic Liver Failure. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2017, 15, 438–445.e5. [Google Scholar] [CrossRef]
- Clària, J.; Stauber, R.E.; Coenraad, M.J.; Moreau, R.; Jalan, R.; Pavesi, M.; Amorós, À.; Titos, E.; Alcaraz-Quiles, J.; Oettl, K.; et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology 2016, 64, 1249–1264. [Google Scholar] [CrossRef]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.P.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- Umgelter, A.; Reindl, W.; Franzen, M.; Lenhardt, C.; Huber, W.; Schmid, R.M. Renal resistive index and renal function before and after paracentesis in patients with hepatorenal syndrome and tense ascites. Intensive Care Med. 2009, 35, 152–156. [Google Scholar] [CrossRef]
- Sort, P.; Navasa, M.; Arroyo, V.; Aldeguer, X.; Planas, R.; Ruiz-Del-Arbol, L.; Castells, L.; Vargas, V.; Soriano, G.; Guevara, M.; et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N. Engl. J. Med. 1999, 341, 403–409. [Google Scholar] [CrossRef]
- Thévenot, T.; Bureau, C.; Oberti, F.; Anty, R.; Louvet, A.; Plessier, A.; Rudler, M.; Heurgué-Berlot, A.; Rosa, I.; Talbodec, N.; et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J. Hepatol. 2015, 62, 822–830. [Google Scholar] [CrossRef]
- Kanduri, S.R.; Velez, J.C.Q. Kidney Dysfunction in the Setting of Liver Failure: Core Curriculum 2024. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2023. [Google Scholar] [CrossRef] [PubMed]
- Amathieu, R.; Al-Khafaji, A.; Sileanu, F.E.; Foldes, E.; DeSensi, R.; Hilmi, I.; Kellum, J.A. Significance of oliguria in critically ill patients with chronic liver disease. Hepatology 2017, 66, 1592–1600. [Google Scholar] [CrossRef]
- Wong, F.; Kwo, P. Practical Management of HRS-AKI in the Era of Terlipressin: What the Gastroenterologist Needs to Know. Am. J. Gastroenterol. 2023, 118, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Velez, J.C.Q.; Nietert, P.J. Therapeutic response to vasoconstrictors in hepatorenal syndrome parallels increase in mean arterial pressure: A pooled analysis of clinical trials. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2011, 58, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Cavallin, M.; Piano, S.; Romano, A.; Fasolato, S.; Frigo, A.C.; Benetti, G.; Gola, E.; Morando, F.; Stanco, M.; Rosi, S.; et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology 2016, 63, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Arab, J.P.; Premkumar, M.; Benítez, C.; Ravikumar, S.T.; Kumar, P.; Sharma, M.; Reddy, D.N.; Simonetto, D.A.; Rao, P.N. Terlipressin has stood the test of time: Clinical overview in 2020 and future perspectives. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 2888–2905. [Google Scholar] [CrossRef]
- Boyer, T.D.; Sanyal, A.J.; Garcia-Tsao, G.; Blei, A.; Carl, D.; Bexon, A.S.; Teuber, P. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: Relationship of serum creatinine to hemodynamics. J. Hepatol. 2011, 55, 315–321. [Google Scholar] [CrossRef]
- Wong, F.; Pappas, S.C.; Curry, M.P.; Reddy, K.R.; Rubin, R.A.; Porayko, M.K.; Gonzalez, S.A.; Mumtaz, K.; Lim, N.; Simonetto, D.A.; et al. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N. Engl. J. Med. 2021, 384, 818–828. [Google Scholar] [CrossRef]
- Arora, V.; Maiwall, R.; Rajan, V.; Jindal, A.; Shasthry, S.M.; Kumar, G.; Jain, P.; Sarin, S.K. Terlipressin Is Superior to Noradrenaline in the Management of Acute Kidney Injury in Acute on Chronic Liver Failure. Hepatology 2020, 71, 600. [Google Scholar] [CrossRef]
- Wong, F. Latest Treatment of Acute Kidney Injury in Cirrhosis. Curr. Treat. Options Gastroenterol. 2020, 18, 281–294. [Google Scholar] [CrossRef]
- Bernardi, M.; Angeli, P.; Claria, J.; Moreau, R.; Gines, P.; Jalan, R.; Caraceni, P.; Fernandez, J.; Gerbes, A.L.; O’Brien, A.J.; et al. Albumin in decompensated cirrhosis: New concepts and perspectives. Gut 2020, 69, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- China, L.; Freemantle, N.; Forrest, E.; Kallis, Y.; Ryder, S.D.; Wright, G.; Portal, A.J.; Salles, N.B.; Gilroy, D.W.; O’brien, A. A Randomized Trial of Albumin Infusions in Hospitalized Patients with Cirrhosis. N. Engl. J. Med. 2021, 384, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; Ginès, P.; Uriz, J.; Cárdenas, A.; Calahorra, B.; Heras, D.D.L.; Guevara, M.; Bataller, R.; Jiménez, W.; Arroyo, V.; et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: Results of a prospective, nonrandomized study. Hepatology 2002, 36, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Piano, S.; Schmidt, H.H.; Ariza, X.; Amoros, A.; Romano, A.; Hüsing-Kabar, A.; Solà, E.; Gerbes, A.; Bernardi, M.; Alessandria, C.; et al. Association Between Grade of Acute on Chronic Liver Failure and Response to Terlipressin and Albumin in Patients with Hepatorenal Syndrome. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2018, 16, 1792–1800.e3. [Google Scholar] [CrossRef] [PubMed]
- Nazar, A.; Pereira, G.H.; Guevara, M.; Martín-Llahi, M.; Pepin, M.-N.; Marinelli, M.; Solá, E.; Baccaro, M.E.; Terra, C.; Arroyo, V.; et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology 2010, 51, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Pappas, S.C.; Reddy, K.R.; Vargas, H.; Curry, M.P.; Sanyal, A.; Jamil, K. Terlipressin use and respiratory failure in patients with hepatorenal syndrome type 1 and severe acute-on-chronic liver failure. Aliment. Pharmacol. Ther. 2022, 56, 1284–1293. [Google Scholar] [CrossRef]
- Singh, V.; Ghosh, S.; Singh, B.; Kumar, P.; Sharma, N.; Bhalla, A.; Sharma, A.; Choudhary, N.; Chawla, Y.; Nain, C. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: A randomized study. J. Hepatol. 2012, 56, 1293–1298. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Shrama, B.C.; Sarin, S.K. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am. J. Gastroenterol. 2008, 103, 1689–1697. [Google Scholar] [CrossRef]
- Thomson, M.J.; Taylor, A.; Sharma, P.; Lok, A.S.; Tapper, E.B. Limited Progress in Hepatorenal Syndrome (HRS) Reversal and Survival 2002-2018: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2020, 65, 1539–1548. [Google Scholar] [CrossRef]
- Kwong, A.; Kim, W.R.; Kwo, P.Y.; Wang, U.; Cheng, X. Feasibility and Effectiveness of Norepinephrine Outside the Intensive Care Setting for Treatment of Hepatorenal Syndrome. Liver Transpl. 2021, 27, 1095. [Google Scholar] [CrossRef]
- Singal, A.K.; Palmer, G.; Melick, L.; Abdallah, M.; Kwo, P. Vasoconstrictor Therapy for Acute Kidney Injury Hepatorenal Syndrome: A Meta-Analysis of Randomized Studies. Gastro Hep Adv. 2023, 2, 455–464. [Google Scholar] [CrossRef]
- Brensing, K.A.; Textor, J.; Perz, J.; Schiedermaier, P.; Raab, P.; Strunk, H.; Klehr, H.; Kramer, H.; Spengler, U.; Schild, H.; et al. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: A phase II study. Gut 2000, 47, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; ter Borg, P.C.J. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Maddukuri, G.; Jaipaul, N.; Cai, C.X. Role of renal replacement therapy in patients with type 1 hepatorenal syndrome receiving combination treatment of vasoconstrictor plus albumin. J. Crit. Care 2015, 30, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Bera, C.; Wong, F. Management of hepatorenal syndrome in liver cirrhosis: A recent update. Ther. Adv. Gastroenterol. 2022, 15, 17562848221102679. [Google Scholar] [CrossRef] [PubMed]
- Nadim, M.K.; Kellum, J.A.; Davenport, A.; Wong, F.; Davis, C.; Pannu, N.; Tolwani, A.; Bellomo, R.; Genyk, Y.S.; ADQI Workgroup. Hepatorenal syndrome: The 8th International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2012, 16, R23. [Google Scholar] [CrossRef]
- Wong, F.; Leung, W.; Al Beshir, M.; Marquez, M.; Renner, E.L. Outcomes of patients with cirrhosis and hepatorenal syndrome type 1 treated with liver transplantation. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2015, 21, 300–307. [Google Scholar] [CrossRef]
- Israni, A.K.; Xiong, H.; Liu, J.; Salkowski, N.; Trotter, J.F.; Snyder, J.J.; Kasiske, B.L. Predicting End-Stage Renal Disease After Liver Transplant. Am. J. Transplant. 2013, 13, 1782–1792. [Google Scholar] [CrossRef]
- Allegretti, A.S.; Israelsen, M.; Krag, A.; Jovani, M.; Goldin, A.H.; Schulman, A.R.; Winter, R.W.; Gluud, L.L. Terlipressin versus placebo or no intervention for people with cirrhosis and hepatorenal syndrome. Cochrane Database Syst. Rev. 2017, 6, CD005162. [Google Scholar] [CrossRef]
- Adebayo, D.; Wong, F. Pathophysiology of Hepatorenal Syndrome—Cute Kidney Injury. Clin. Gastroenterol. Hepatol. 2023, 21 (Suppl. S10), S1–S10. [Google Scholar] [CrossRef]
| Subject | Definition | ||
|---|---|---|---|
| Baseline sCr | A value of sCr obtained in the previous three months, when available, can be used as baseline sCr. In patients with more than one value within the previous three months, the value closest to the admission time to the hospital should be used. In patients without a previous sCr value, the sCr on admission should be used as baseline. | ||
| Definition of AKI |
| ||
| Staging of AKI |
| ||
| Progression of AKI | Progression | Regression | |
| Progression of AKI to a higher stage and/or need for RRT | Regression of AKI to a lower stage | ||
| Response to treatment | No response | Partial response | Full response |
| No regression of AKI | Regression of AKI stage with a reduction in sCr ≥ 0.3 mg/dL (26.5 μmol/L) above the baseline value | Return of sCr to a value within 0.3 mg/dL (26.5 μmol/L) of the baseline value | |
| Diagnosis of cirrhosis and ascites |
| Diagnosis of AKI according to ICA-AKI criteria |
| No response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin 1 g per kg of body weight |
| Absence of shock |
| No current or recent use of nephrotoxic drugs (NSAIDs, aminoglycosides, iodinated contrast media, etc.) |
No macroscopic signs of structural kidney injury a defined as:
|
| Old Terminology | New Terminology | Definition |
|---|---|---|
| HRS-1 a | HRS-AKI | (a) Absolute increase in sCr ≥ 0.3 mg/dL within 48 h and/or (b) Urinary output ≤ 0.5 mL/kg B.W. ≥6 h b or (c) Percent increase in sCr ≥ 50% using the last available value of outpatient sCr within 3 months as the baseline value |
| HRS-2 a | HRS-AKD | (a) eGFR < 60 mL/min per 1.73 m2 for <3 months in the absence of other (structural) causes |
| HRS-NAKI | (b) Percent increase in sCr < 50% using the last available value of outpatient sCr within 3 months as the baseline value | |
| HRS-CKD | (a) eGFR < 60 mL/min per 1.73 m2 for ≥3 months in the absence of other (structural) causes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozturk, N.B.; Dinc, E.J.; Swami, A.; Gurakar, A. Acute Kidney Injury and Hepatorenal Syndrome in Patients with Cirrhosis. J. Clin. Med. 2024, 13, 199. https://doi.org/10.3390/jcm13010199
Ozturk NB, Dinc EJ, Swami A, Gurakar A. Acute Kidney Injury and Hepatorenal Syndrome in Patients with Cirrhosis. Journal of Clinical Medicine. 2024; 13(1):199. https://doi.org/10.3390/jcm13010199
Chicago/Turabian StyleOzturk, Nazli Begum, Ece Janet Dinc, Abhishek Swami, and Ahmet Gurakar. 2024. "Acute Kidney Injury and Hepatorenal Syndrome in Patients with Cirrhosis" Journal of Clinical Medicine 13, no. 1: 199. https://doi.org/10.3390/jcm13010199
APA StyleOzturk, N. B., Dinc, E. J., Swami, A., & Gurakar, A. (2024). Acute Kidney Injury and Hepatorenal Syndrome in Patients with Cirrhosis. Journal of Clinical Medicine, 13(1), 199. https://doi.org/10.3390/jcm13010199






