What Circuits, Masks and Filters Should Be Used in Home Non-Invasive Mechanical Ventilation
Abstract
1. Introduction
2. Circuits and Expiratory Valves
2.1. Double Limb System
2.1.1. Implications for Monitoring
2.1.2. Implications for Ventilator Performance
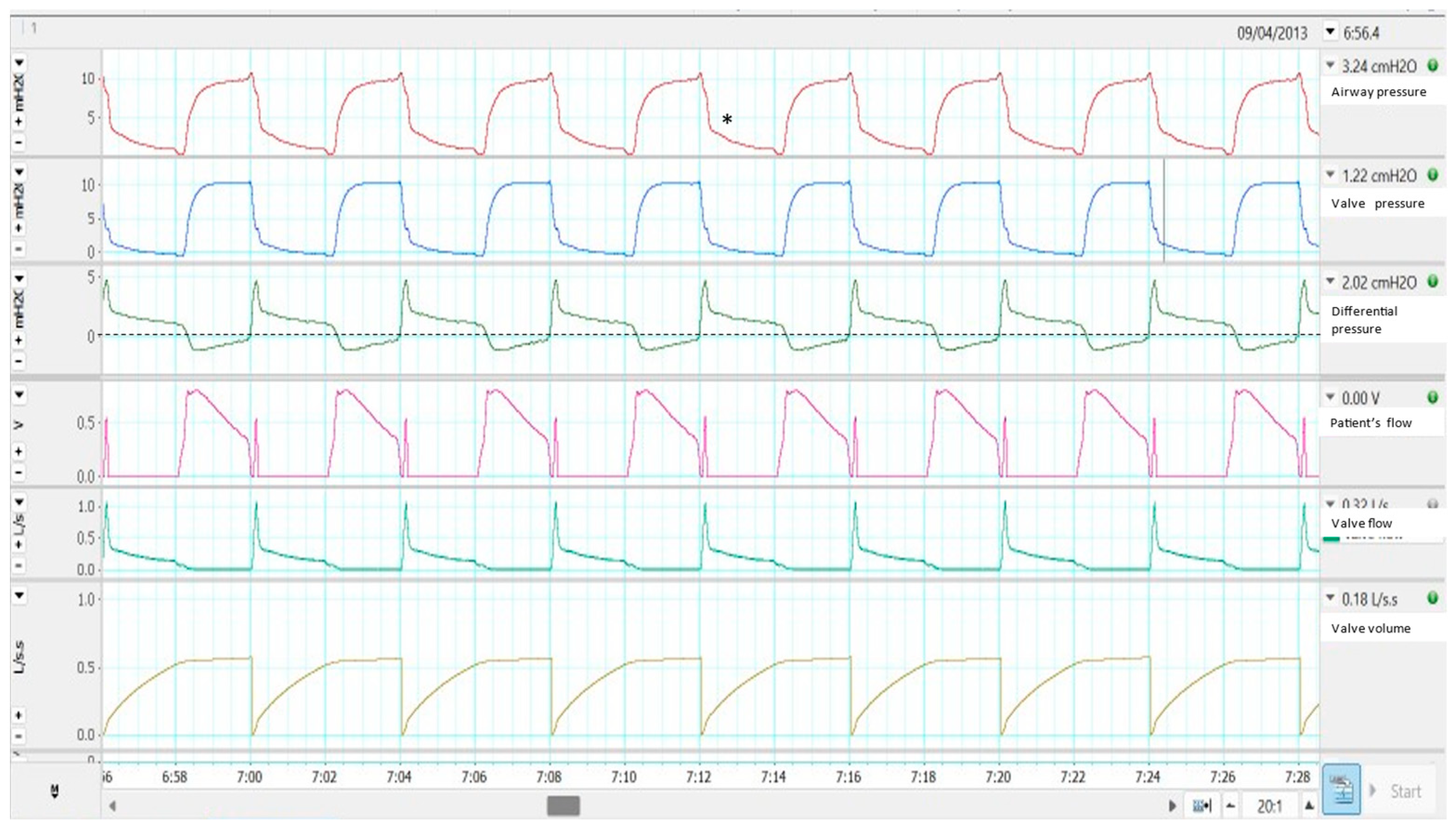
2.2. Single Limb with Active Valve
Effects on Monitoring
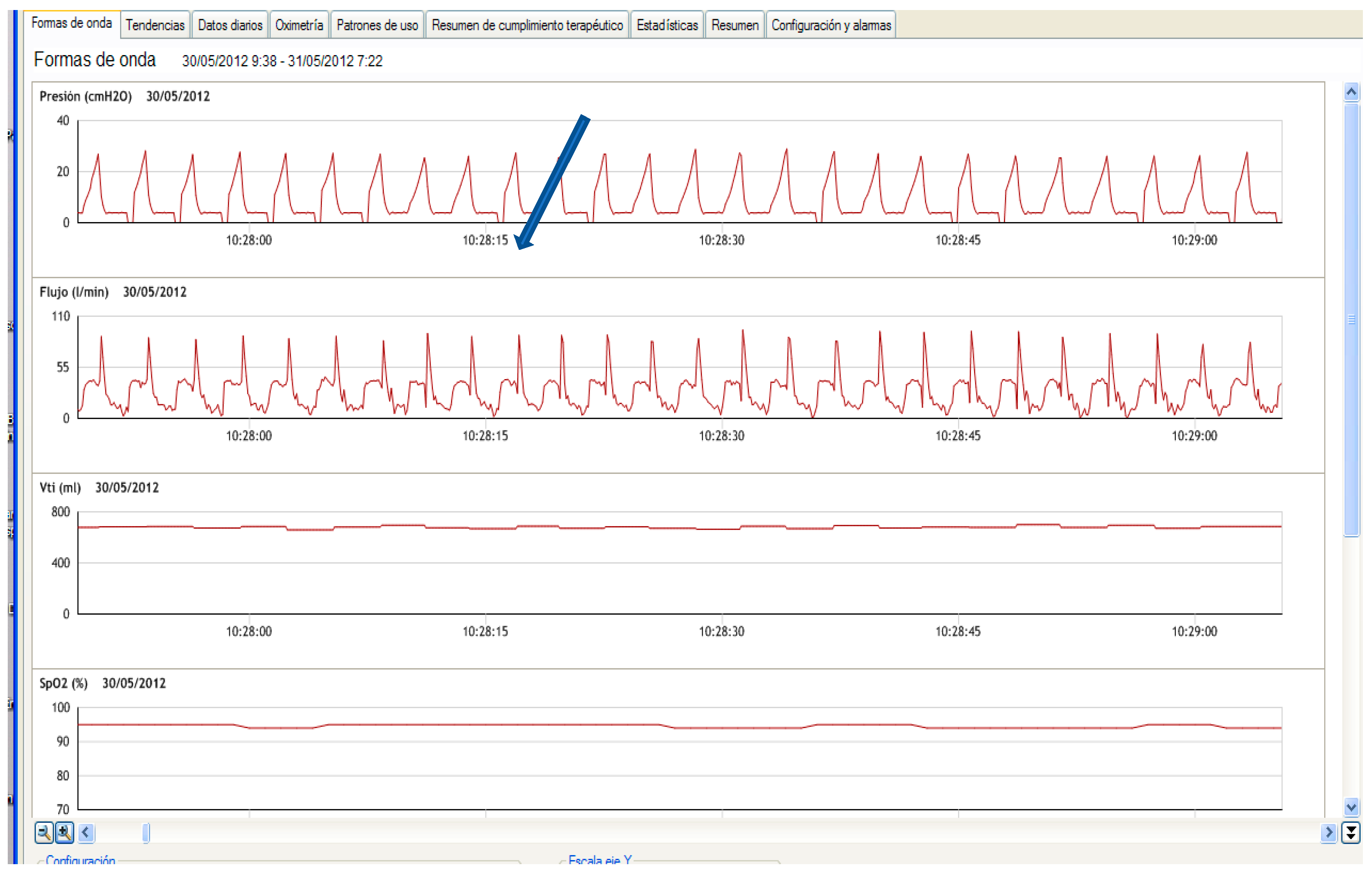
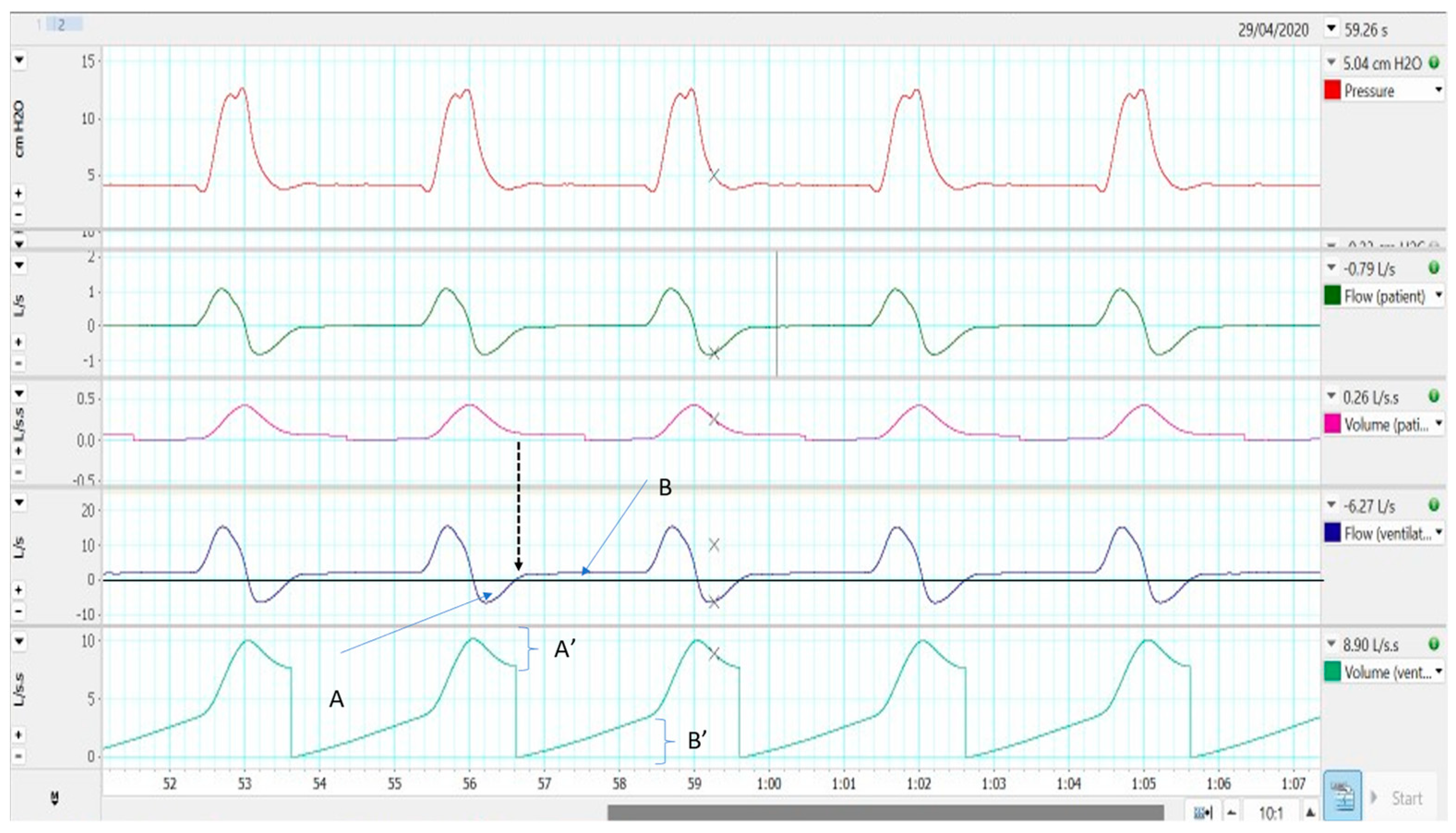
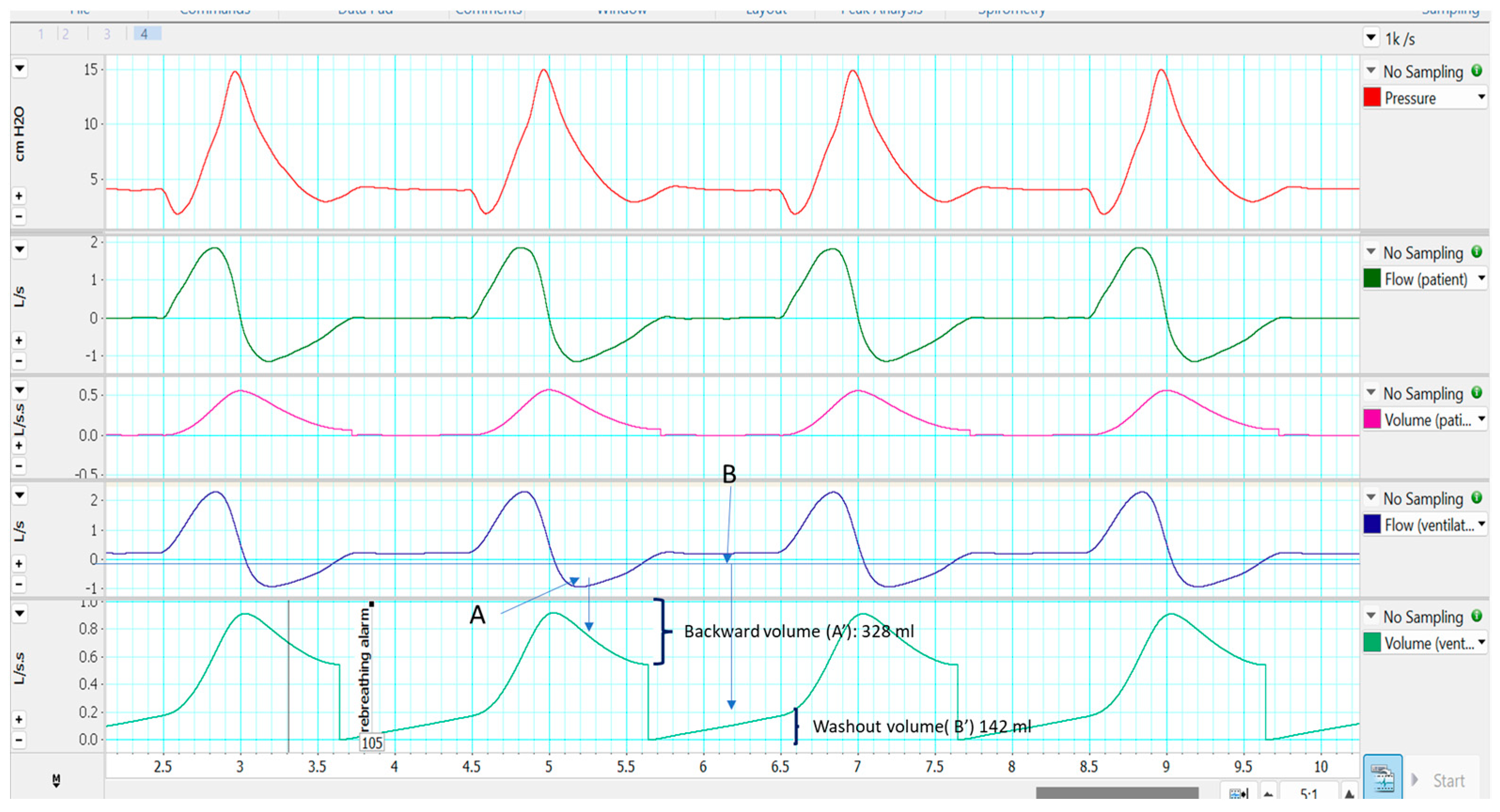
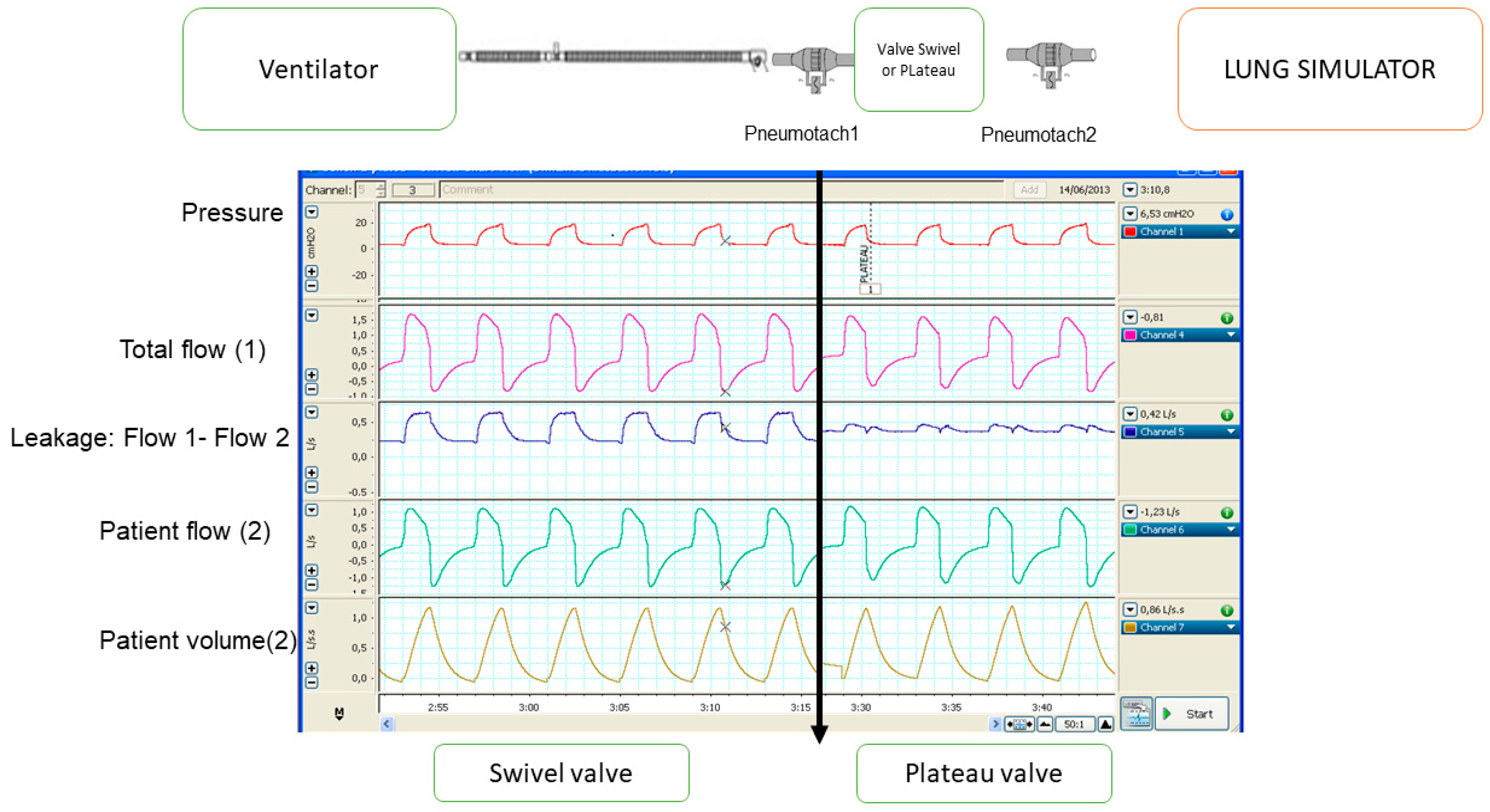
2.3. Single Limb with Intentional Leakage
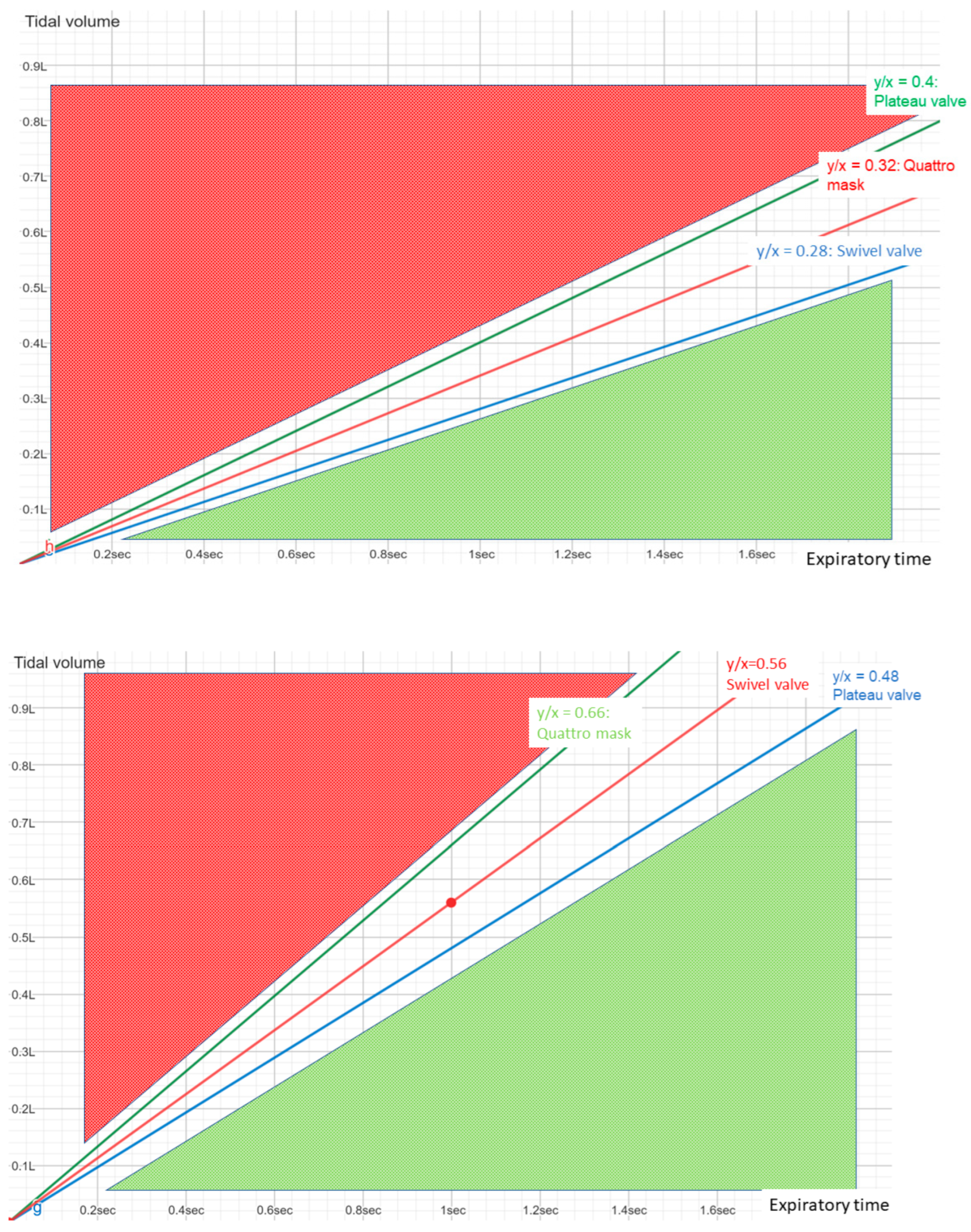
2.3.1. Effectiveness in Preventing Rebreathing
2.3.2. Influence on Monitoring
- Both parameters (tidal volume and leakage) are closely related, as the total flow is the sum of tidal volume plus the leakage. Therefore, if one is underestimated, the other will also be overestimated.
- The reference point commonly used to determine leakage (and indirectly tidal volume) is usually the transition between expiration and inspiration, because at that point, the patient’s flow is zero and therefore all circulating flow will correspond to leakage. In fact, it is analogous to a single equation with two unknowns, in which the total amount of flow is known for the entire cycle, but their components (patient’s flow and leaks) are not known. Only in two points of the cycle (transitions from inspiration to expiration and vice versa) one of the values (leakage) is known since the patient’s flow is zero. The choice of the expiratory–inspiratory transition as a reference is related to the flatter slope in the flow waveform at this point. Then, small deviations in the curve would not represent a big error in the estimations.
- If the leak flow and the pressure (EPAP/PEEP) are known, the resistance of the system during the expiratory phase can be estimated by means of the Poiseuille’s law for fluids. Usually, the leakage during inspiration is calculated by applying the resistance value to the inspiratory phase. In other words, a linear or near-linear relationship is assumed. However, there are some situations in clinical practice that do not fulfil this linearity (asymmetric leaks). For example, a poorly attached interface can produce leakage in the system only during inspiratory phase. In this case, the leakage (and the tidal volume) can be wrongly estimated [17].
- Based mainly on this single datum (resistance during expiratory phase), an attempt is made to determine what happens for the whole respiratory cycle. Therefore, if this point is taken as a reference (at the end of the cycle), the analysis must be applied to the next cycle, not to the same cycle that generated this datum. This can be misleading in very heterogeneous cycles.
- The estimation of leakage (and therefore tidal volume) must consider that the actual data (pressure and flow) are obtained inside the ventilator, and that there is a pressure loss through the system dependent on the outlet flow and the properties of the tubing (resistance and compliance, by Poiseuille’s law). Some manufacturers have incorporated tests for the correction of the pressure values (pre-use tests) for greater reliability in the estimation of the tidal volume [18]. Another option would be the placement of a proximal pressure sensor, such as in some acute care ventilators.
2.3.3. Influences on Ventilator Performance
3. Interfaces
- Nasal masks: cover the nasal structure, with support on the nasal bridge and upper jaw area, especially.
- Nasal pillows: cover only the nasal orifices.
- Oronasal masks, whey can be classified into two subtypes: the conventional oronasal mask, with support on the nasal bridge and lower jaw, and the subnasal (“hybrid”) mask, with support on the lower jaw but respecting the nasal bridge by means of support on the cartilaginous area of the nose.
- Full-face masks: nose and mouth coverage and forehead support.
- Oral-only interfaces: mouthpiece and lip-seal masks.
4. Filters and Other Items
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mokhlesi, B.; Masa, J.F.; Brozek, J.L.; Gurubhagavatula, I.; Murphy, P.B.; Piper, A.J.; Tulaimat, A.; Afshar, M.; Balachandran, J.S.; Dweik, R.A.; et al. Evaluation and Management of Obesity Hypoventilation Syndrome. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019, 200, e6–e24. [Google Scholar] [CrossRef]
- Ergan, B.; Oczkowski, S.; Rochwerg, B.; Carlucci, A.; Chatwin, M.; Clini, E.; Elliott, M.; Gonzalez-Bermejo, J.; Hart, N.; Lujan, M.; et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur. Respir. J. 2019, 54, 1901003. [Google Scholar] [CrossRef]
- O’Brien, D.; Stavroulakis, T.; Baxter, S.; Norman, P.; Bianchi, S.; Elliott, M.; Johnson, M.; Clowes, M.; Garcia-Sánchez, A.; Hobson, E.; et al. The optimisation of noninvasive ventilation in amyotrophic lateral sclerosis: A systematic review. Eur. Respir. J. 2019, 54, 1900261. [Google Scholar] [CrossRef]
- Rabec, C.; Rodenstein, D.; Leger, P.; Rouault, S.; Perrin, C.; Gonzalez-Bermejo, J. SomnoNIV group Ventilator modes and settings during non-invasive ventilation: Effects on respiratory events and implications for their identification. Thorax 2011, 66, 170–178. [Google Scholar] [CrossRef]
- Masselli, G.M.P.; Silvestri, S.; Sciuto, S.A.; Cappa, P. Circuit compliance compensation in lung protective ventilation. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; Volume 2006, pp. 5603–5606. [Google Scholar] [CrossRef]
- Lalmolda, C.; Prados, H.; Mateu, G.; Noray, M.; Pomares, X.; Luján, M. Titration of Mechanical Insufflation-Exsufflation Optimal Pressure Combinations in Neuromuscular Diseases by Flow/Pressure Waveform Analysis. Arch. Bronconeumol. 2019, 55, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Leger, P. Noninvasive positive pressure ventilation at home. Respir. Care 1994, 39, 501–510, discussion 511–514. [Google Scholar] [PubMed]
- Carlucci, A.; Schreiber, A.; Mattei, A.; Malovini, A.; Bellinati, J.; Ceriana, P.; Gregoretti, C. The configuration of bi-level ventilator circuits may affect compensation for non-intentional leaks during volume-targeted ventilation. Intensive Care Med. 2013, 39, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Owen, S.J.; Donaldson, G.C.; Ambrosino, N.; Escarabill, J.; Farre, R.; Fauroux, B.; Robert, D.; Schoenhofer, B.; Simonds, A.K.; Wedzicha, J.A. Patterns of home mechanical ventilation use in Europe: Results from the Eurovent survey. Eur. Respir. J. 2005, 25, 1025–1031. [Google Scholar] [CrossRef]
- Pierucci, P.; Crimi, C.; Carlucci, A.; Carpagnano, G.E.; Janssens, J.-P.; Lujan, M.; Noto, A.; Wijkstra, P.J.; Windisch, W.; Scala, R. REINVENT: ERS International survey on REstrictive thoracic diseases IN long term home noninvasive VENTilation. ERJ Open Res. 2021, 7, 00911-2020. [Google Scholar] [CrossRef] [PubMed]
- Luján, M.; Lalmolda, C.; Ergan, B. Basic Concepts for Tidal Volume and Leakage Estimation in Non-Invasive Ventilation. Turk. Thorac. J. 2019, 20, 140–146. [Google Scholar] [CrossRef]
- Ou, Y.-E.; Lin, Z.-M.; Hua, D.-M.; Jiang, Y.; Huo, Y.-T.; Luo, Q.; Chen, R.-C. Evaluation of carbon dioxide rebreathing during exercise assisted by noninvasive ventilation with plateau exhalation valve. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 291–298. [Google Scholar] [CrossRef]
- Schettino, G.P.P.; Chatmongkolchart, S.; Hess, D.R.; Kacmarek, R.M. Position of exhalation port and mask design affect CO2 rebreathing during noninvasive positive pressure ventilation. Crit. Care Med. 2003, 31, 2178–2182. [Google Scholar] [CrossRef]
- Szkulmowski, Z.; Belkhouja, K.; Le, Q.-H.; Robert, D.; Argaud, L. Bilevel positive airway pressure ventilation: Factors influencing carbon dioxide rebreathing. Intensive Care Med. 2010, 36, 688–691. [Google Scholar] [CrossRef]
- Rezoagli, E.; Signori, D.; Grassi, A.; Rabboni, F.; Lucchini, A.; Bellani, G.; Foti, G. A Novel Mask with Selective Ports for Inflow and Outflow Reduces CO2 Rebreathing during Non-Invasive Ventilation: A Physiological Study in Healthy Volunteers. Respir. Int. Rev. Thorac. Dis. 2023, 102, 1–11. [Google Scholar] [CrossRef]
- Signori, D.; Bellani, G.; Calcinati, S.; Grassi, A.; Patroniti, N.; Foti, G. Effect of Face Mask Design and Bias Flow on Rebreathing during Noninvasive Ventilation. Respir. Care 2019, 64, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Sogo, A.; Montanyà, J.; Monsó, E.; Blanch, L.; Pomares, X.; Lujàn, M. Effect of dynamic random leaks on the monitoring accuracy of home mechanical ventilators: A bench study. BMC Pulm. Med. 2013, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Luján, M.; Sogo, A.; Pomares, X.; Monsó, E.; Sales, B.; Blanch, L. Effect of leak and breathing pattern on the accuracy of tidal volume estimation by commercial home ventilators: A bench study. Respir. Care 2013, 58, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Luján, M.; Sogo, A.; Grimau, C.; Pomares, X.; Blanch, L.; Monsó, E. Influence of dynamic leaks in volume-targeted pressure support noninvasive ventilation: A bench study. Respir. Care 2015, 60, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, P.; Portacci, A.; Carpagnano, G.E.; Banfi, P.; Crimi, C.; Misseri, G.; Gregoretti, C. The right interface for the right patient in noninvasive ventilation: A systematic review. Expert Rev. Respir. Med. 2022, 16, 931–944. [Google Scholar] [CrossRef]
- Lebret, M.; Léotard, A.; Pépin, J.L.; Windisch, W.; Ekkernkamp, E.; Pallero, M.; Sánchez-Quiroga, M.-Á.; Hart, N.; Kelly, J.L.; Patout, M.; et al. Nasal versus oronasal masks for home non-invasive ventilation in patients with chronic hypercapnia: A systematic review and individual participant data meta-analysis. Thorax 2021, 76, 1108–1116. [Google Scholar] [CrossRef]
- Schorr, F.; Genta, P.R.; Gregório, M.G.; Danzi-Soares, N.J.; Lorenzi-Filho, G. Continuous positive airway pressure delivered by oronasal mask may not be effective for obstructive sleep apnoea. Eur. Respir. J. 2012, 40, 503–505. [Google Scholar] [CrossRef]
- Ebben, M.R.; Milrad, S.; Dyke, J.P.; Phillips, C.D.; Krieger, A.C. Comparison of the upper airway dynamics of oronasal and nasal masks with positive airway pressure treatment using cine magnetic resonance imaging. Sleep Breath. Schlaf Atm. 2016, 20, 79–85. [Google Scholar] [CrossRef]
- Toussaint, M.; Chatwin, M.; Gonçalves, M.R.; Gonzalez-Bermejo, J.; Benditt, J.O.; McKim, D.; Sancho, J.; Hov, B.; Sansone, V.; Prigent, H.; et al. Mouthpiece ventilation in neuromuscular disorders: Narrative review of technical issues important for clinical success. Respir. Med. 2021, 180, 106373. [Google Scholar] [CrossRef]
- Leger, P.; Bedicam, J.M.; Cornette, A.; Reybet-Degat, O.; Langevin, B.; Polu, J.M.; Jeannin, L.; Robert, D. Nasal intermittent positive pressure ventilation. Long-term follow-up in patients with severe chronic respiratory insufficiency. Chest 1994, 105, 100–105. [Google Scholar] [CrossRef]
- Willox, M.; Metherall, P.; Jeays-Ward, K.; McCarthy, A.D.; Barker, N.; Reed, H.; Elphick, H.E. Custom-made 3D printed masks for children using non-invasive ventilation: A feasibility study of production method and testing of outcomes in adult volunteers. J. Med. Eng. Technol. 2020, 44, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Shikama, M.; Nakagami, G.; Noguchi, H.; Mori, T.; Sanada, H. Development of Personalized Fitting Device with 3-Dimensional Solution for Prevention of NIV Oronasal Mask-Related Pressure Ulcers. Respir. Care 2018, 63, 1024–1032. [Google Scholar] [CrossRef]
- Patout, M.; Fresnel, E.; Lujan, M.; Rabec, C.; Carlucci, A.; Razakamanantsoa, L.; Kerfourn, A.; Nunes, H.; Tandjaoui-Lambiotte, Y.; Cuvelier, A.; et al. Recommended approaches to minimize aerosol dispersion of SARS-CoV2 during noninvasive ventilatory support can deteriorate ventilator performances: A benchmark comparative study. Chest 2021, 160, 175–186. [Google Scholar] [CrossRef]
- Rabec, C.; Fresnel, E.; Rétory, Y.; Zhu, K.; Joly, K.; Kerfourn, A.; Dudoignon, B.; Mendoza, A.; Cuvelier, A.; Muir, J.-F.; et al. Addition of bacterial filter alters positive airway pressure and non-invasive ventilation performances. Eur. Respir. J. 2022, 59, 2102636. [Google Scholar] [CrossRef] [PubMed]
- Storre, J.H.; Huttmann, S.E.; Ekkernkamp, E.; Walterspacher, S.; Schmoor, C.; Dreher, M.; Windisch, W. Oxygen supplementation in noninvasive home mechanical ventilation: The crucial roles of CO2 exhalation systems and leakages. Respir. Care 2014, 59, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.-J.; Schroeder, M.; Domanski, U.; Dewald, B.; Nilius, G. Non-invasive Ventilation: Effect of Vented and Non-vented Exhalation Systems on Inspiratory CO2 and O2 Concentrations, Ventilation, and Breathing Pattern. Lung 2022, 200, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lalmolda, C.; Flórez, P.; Grimau, C.; Larrosa, R.; Corral, M.; Sayas, J.; Luján, M. A bench-to-bedside study about trigger asynchronies induced by the introduction of external gas into the non-invasive mechanical ventilation circuit. Sci. Rep. 2021, 11, 23814. [Google Scholar] [CrossRef] [PubMed]
- Gregoretti, C.; Navalesi, P.; Ghannadian, S.; Carlucci, A.; Pelosi, P. Choosing a ventilator for home mechanical ventilation. Breathe 2013, 9, 394–409. [Google Scholar] [CrossRef]
| Item | Types | Advantages | Disadvantages |
|---|---|---|---|
| Limb systems | Double limb system | Accuracy in monitoring. only compressible volume needs correction | Bulky system |
| Single limb with active valve | PEEP not strictly necessary | Flow expiratory waveform not displayed. Semiology may be confusing. | |
| Single limb with intentional leakage | Simple system | Rebreathing under certain conditions should be considered. Leakage and tidal volume estimation are challenging. | |
| Masks | Nasal | Less instrumental dead space | Oral leakage. |
| Oronasal | No oral leakage | Can worsen upper airway obstructions. | |
| Filters | Protective effect | Interferences in trigger function and pressurization. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luján, M.; Flórez, P.; Pomares, X. What Circuits, Masks and Filters Should Be Used in Home Non-Invasive Mechanical Ventilation. J. Clin. Med. 2023, 12, 2692. https://doi.org/10.3390/jcm12072692
Luján M, Flórez P, Pomares X. What Circuits, Masks and Filters Should Be Used in Home Non-Invasive Mechanical Ventilation. Journal of Clinical Medicine. 2023; 12(7):2692. https://doi.org/10.3390/jcm12072692
Chicago/Turabian StyleLuján, Manel, Pablo Flórez, and Xavier Pomares. 2023. "What Circuits, Masks and Filters Should Be Used in Home Non-Invasive Mechanical Ventilation" Journal of Clinical Medicine 12, no. 7: 2692. https://doi.org/10.3390/jcm12072692
APA StyleLuján, M., Flórez, P., & Pomares, X. (2023). What Circuits, Masks and Filters Should Be Used in Home Non-Invasive Mechanical Ventilation. Journal of Clinical Medicine, 12(7), 2692. https://doi.org/10.3390/jcm12072692






