Updated Immunotherapy for Gastric Cancer
Abstract
1. Introduction
2. Genetic and Molecular Profiles and Immunotherapy in Gastric Cancer
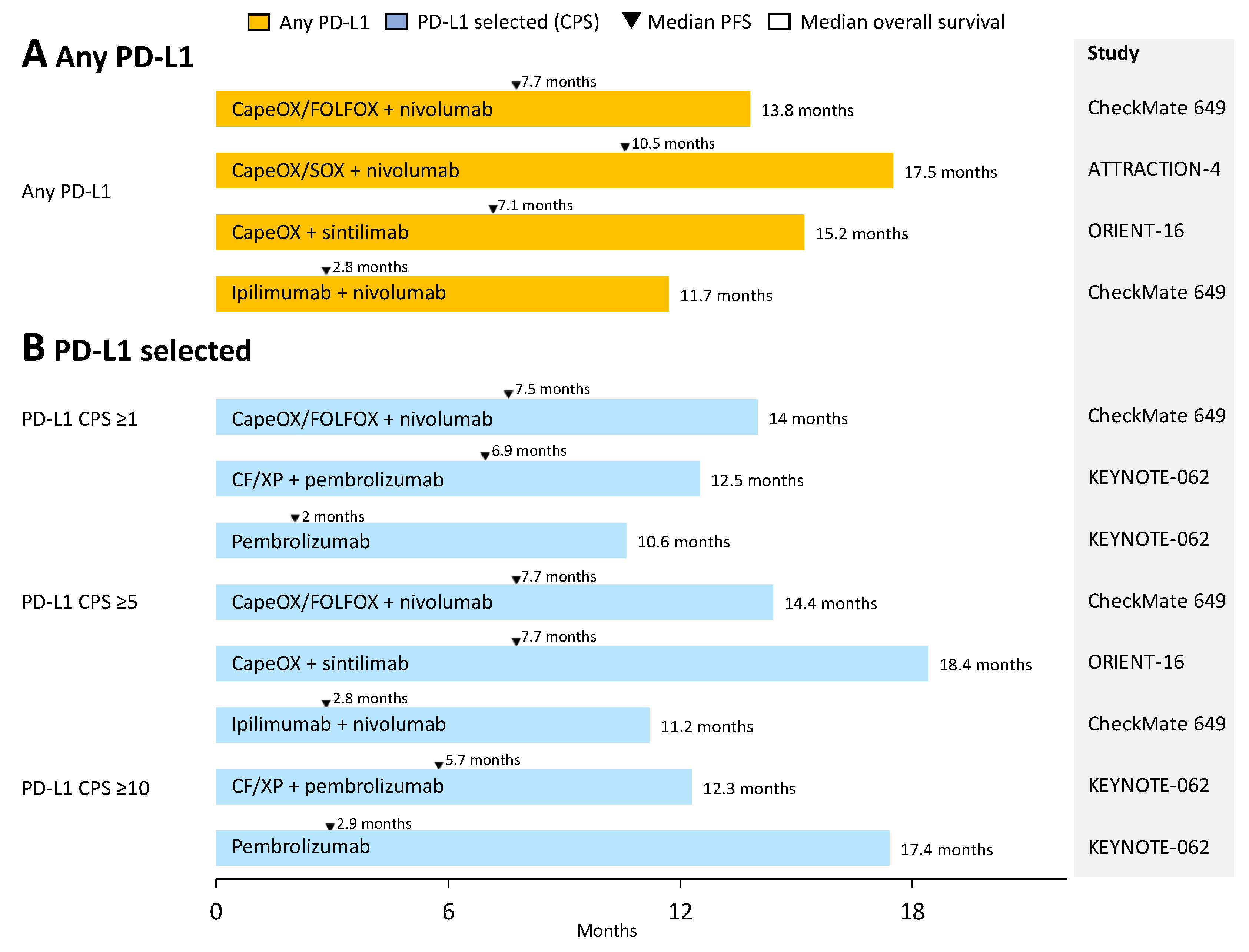
3. Anti-PD-1/PD-L1 Antibodies Plus Chemotherapy as First-Line Therapy
4. Anti-PD-1/PD-L1 Antibody Monotherapy
4.1. First-Line Therapy
4.2. Second or Later-Line Therapy
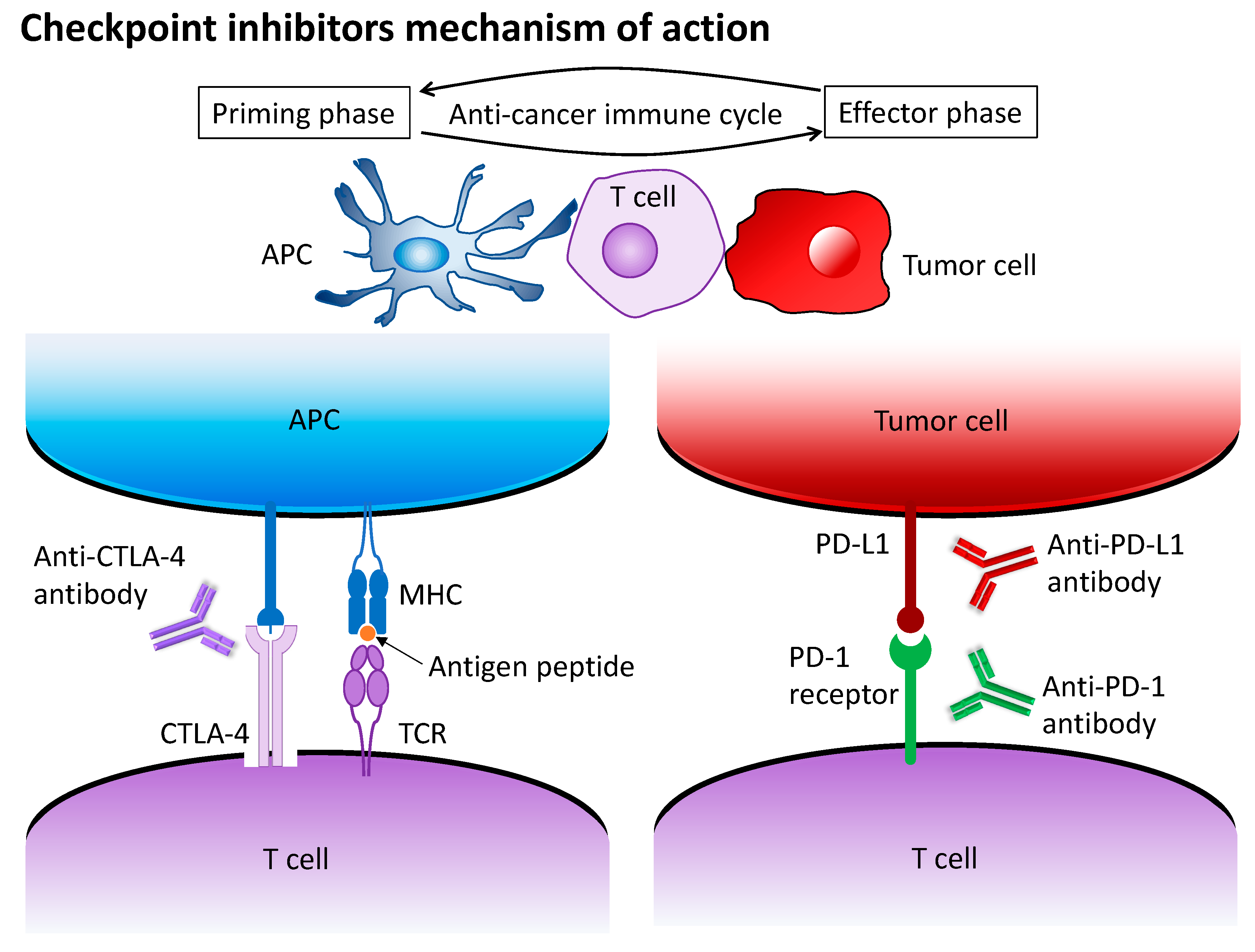
5. Anti-PD-1/PD-L1 Antibodies Plus Anti-CTLA-4 Antibodies
6. Anti-PD-1/PD-L1 Antibodies Plus Anti-HER2 Therapy
7. Perioperative/Curative Immunotherapy in Locally Advanced Gastric Cancer
8. Toxicity Profile of Immune Checkpoint Inhibitors
9. Molecular and Genetic Biomarkers in Gastric Cancer
9.1. PD-L1 CPS
9.2. MSI-H
9.3. Tumor Mutational Burden
9.4. EBV
9.5. Investigational Biomarkers
10. Regimen Selection Strategies for HER2-Negative Gastric Cancer in a First-Line Setting: Immunochemotherapy Combination versus Chemotherapy Alone
11. Potential Regimens and Future Directions
11.1. Anti-PD-1/PD-L1 Antibodies Plus Multikinase or Vascular Endothelial Growth Factor Inhibitors
11.2. CAR-T
12. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pyrhönen, S.; Kuitunen, T.; Nyandoto, P.; Kouri, M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br. J. Cancer 1995, 71, 587–591. [Google Scholar] [CrossRef]
- Murad, A.M.; Santiago, F.F.; Petroianu, A.; Rocha, P.R.S.; Rodrigues, M.A.G.; Rausch, M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993, 72, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B.; Ekström, K.; Hoffman, K.; Graf, W.; Sjödén, P.-O.; Haglund, U.; Svensson, C.; Enander, L.-K.; Linné, T.; Sellsröm, H.; et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann. Oncol. 1997, 8, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, W.; Narahara, H.; Hara, T.; Takagane, A.; Akiya, T.; Takagi, M.; Miyashita, K.; Nishizaki, T.; Kobayashi, O.; Takiyama, W.; et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008, 9, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Higuchi, K.; Nishikawa, K.; Gotoh, M.; Fuse, N.; Sugimoto, N.; Nishina, T.; Amagai, K.; Chin, K.; Niwa, Y.; et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemo-therapy-naive patients with advanced gastric cancer. Ann. Oncol. 2015, 26, 141–148. [Google Scholar] [CrossRef]
- Cunningham, D.; Starling, N.; Rao, S.; Iveson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R. Capecitabine and Oxaliplatin for Advanced Esophagogastric Cancer. N. Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, A.; Yoshida, S.; Saijo, N. Disparities in Gastric Cancer Chemotherapy Between the East and West. J. Clin. Oncol. 2006, 24, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 3, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-K.; Boku, N.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Bendell, J.C.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Bono, P.; Jaeger, D.; Evans, T.J.; et al. CheckMate-032: Phase I/II, open-label study of safety and activity of nivolumab (nivo) alone or with ipilimumab (ipi) in advanced and metastatic (A/M) gastric cancer (GC). J. Clin. Oncol. 2016, 34, 4010. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.-J.; Fuchs, C.; Wyrwicz, L.; Lee, K.-W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients with First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.-P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.-H.; Fornaro, L.; Olesiński, T.; Caglevic, C.; Chung, H.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gas-tro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Di Bartolomeo, M.; Morano, F.; Raimondi, A.; Miceli, R.; Corallo, S.; Tamborini, E.; Perrone, F.; Antista, M.; Niger, M.; Pellegrinelli, A.; et al. Prognostic and Predictive Value of Microsatellite Instability, Inflammatory Reaction and PD-L1 in Gastric Cancer Patients Treated with Either Adjuvant 5-FU/LV or Sequential FOLFIRI Followed by Cisplatin and Docetaxel: A Translational Analysis from the ITACA-S Trial. Oncologist 2020, 25, e460–e468. [Google Scholar]
- Nie, R.C.; Chen, G.M.; Yuan, S.Q.; Kim, J.W.; Zhou, J.; Nie, M.; Feng, C.Y.; Chen, Y.B.; Chen, S.; Zhou, Z.W.; et al. Adjuvant Chemotherapy for Gastric Cancer Patients with Mismatch Repair Deficiency or Microsatellite Instability: Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2021, 29, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Miceli, R.; An, J.; Di Bartolomeo, M.; Morano, F.; Kim, S.T.; Park, S.H.; Choi, M.G.; Lee, J.H.; Raimondi, A.; Fucà, G.; et al. Prognostic Impact of Microsatellite Instability in Asian Gastric Cancer Patients Enrolled in the ARTIST Trial. Oncology 2019, 97, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Explor-atory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol 2017, 3, 1197–1203. [Google Scholar] [CrossRef]
- Yun, S.; Koh, J.; Nam, S.K.; Kwak, Y.; Ahn, S.-H.; Park, J.D.; Kim, H.-H.; Kim, W.H.; Lee, H.S. Immunoscore is a strong predictor of survival in the prognosis of stage II/III gastric cancer patients following 5-FU-based adjuvant chemotherapy. Cancer Immunol. Immunother. 2020, 70, 431–441. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, H.; Shin, S.J.; Kim, H.Y.; Lee, J.; Yang, H.-K.; Kim, W.H.; Kim, Y.-W.; Kook, M.-C.; Park, Y.K.; et al. Microsatellite Instability and Programmed Cell Death-Ligand 1 Expression in Stage II/III Gastric Cancer: Post Hoc Analysis of the CLASSIC Randomized Controlled study. Ann. Surg. 2019, 270, 309–316. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.-M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology GC, Version 2. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 25 October 2022).
- Shitara, K.; Ajani, J.A.; Moehler, M.; Garrido, M.; Gallardo, C.; Shen, L.; Yamaguchi, K.; Wyrwicz, L.; Skoczylas, T.; Bragagnoli, A.C.; et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022, 603, 942–948. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Shu, Y.; Liu, L.; Kong, L.; Yang, L.; Wang, B.; Sun, G.; Ji, Y.; Cao, G.; et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): Multicentre, randomised, double blind, phase 3 trial. BMJ 2022, 377, e068714. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Cutsem, E.V.; Bang, Y.-J.; Fuchs, C.; Wyrwicz, L.; Lee, K.; Kudaba, I.; Garrido, M.; Chung, H.; Salguero, H.C.; et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. J. Clin. Oncol. 2019, 37, LBA4007. [Google Scholar] [CrossRef]
- Lei, M.; Siemers, N.O.; Pandya, D.; Chang, H.; Sanchez, T.K.; Harbison, C.T.; Szabo, P.M.; Janjigian, Y.Y.; Ott, P.A.; Sharma, P.; et al. Analyses of PD-L1 and Inflammatory Gene Expression Association with Efficacy of Nivolumab ± Ipilimumab in Gastric Cancer/Gastroesophageal Junction Cancer. Clin. Cancer Res. 2021, 27, 3926–3935. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.A.; Fuchs, C.S.; Tabernero, J.; Shitara, K.; Muro, K.; Van Cutsem, E.; Bang, Y.-J.; Chung, H.C.; Yamaguchi, K.; Varga, E.; et al. Efficacy of Pembrolizumab Monotherapy for Advanced Gastric/Gastroesophageal Junction Cancer with Programmed Death Ligand 1 Combined Positive Score ≥10. Clin. Cancer Res. 2021, 27, 1923–1931. [Google Scholar] [CrossRef]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.-M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef]
- Kang, Y.K.; Chen, L.T.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRAC-TION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar]
- Xu, J.; Jiang, H.; Pan, Y.; Gu, K.; Cang, S.; Han, L.; Shu, Y.; Li, J.; Zhao, J.; Pan, H.; et al. LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): First results of a randomized, double-blind, phase III study. Ann. Oncol. 2021, 32, S1331. [Google Scholar] [CrossRef]
- Emens, L.A.; Middleton, G. The Interplay of Immunotherapy and Chemotherapy: Harnessing Potential Synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2009, 29, 482–491. [Google Scholar] [CrossRef]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Sawaki, A.; Yamada, Y.; Yamaguchi, K.; Nishina, T.; Doi, T.; Satoh, T.; Chin, K.; Boku, N.; Omuro, Y.; Komatsu, Y.; et al. Regional differences in advanced gastric cancer: Exploratory analyses of the AV-AGAST placebo arm. Gastric Cancer 2018, 21, 429–438. [Google Scholar] [CrossRef]
- Kubota, Y.; Aoki, Y.; Kawazoe, A.; Shitara, K. Role of Nivolumab in the Management of First-Line Unresectable Advanced or Recurrent Gastric Cancer in Combination with Chemotherapy: Lessons from the Japanese Experience. Cancer Manag. Res. 2022, 14, 3083–3094. [Google Scholar] [CrossRef]
- Su, X.; Zhan, P.; Gavine, P.R.; Morgan, S.; Womack, C.; Ni, X.; Shen, D.; Bang, Y.-J.; Im, S.-A.; Kim, H.; et al. FGFR2 amplification has prognostic significance in gastric cancer: Results from a large inter-national multicentre study. Br. J. Cancer 2014, 110, 967–975. [Google Scholar] [CrossRef]
- Dulak, A.M.; Schumacher, S.E.; van Lieshout, J.; Imamura, Y.; Fox, C.; Shim, B.; Ramos, A.H.; Saksena, G.; Baca, S.C.; Baselga, J.; et al. Gastrointestinal Adenocarcinomas of the Esophagus, Stomach, and Colon Exhibit Distinct Patterns of Genome Instability and Oncogenesis. Cancer Res 2012, 72, 4383–4393. [Google Scholar] [CrossRef]
- van Grieken, N.C.T.; Aoyama, T.; Chambers, P.A.; Bottomley, D.; Ward, L.C.; Inam, I.; Buffart, T.E.; Das, K.; Lim, T.; Pang, B.; et al. KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: Results from a large international multicentre study. Br. J. Cancer 2013, 108, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Gagnon-Bartsch, J.A.; Tan, I.B.; Earle, S.; Ruff, L.; Pettinger, K.; Ylstra, B.; van Grieken, N.; Rha, S.Y.; Chung, H.C.; et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocar-cinomas. Gut 2015, 64, 1721–1731. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Shitara, K.; Van Cutsem, E.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.C.; Lee, J.; Castro-Salguero, H.R.; et al. Pembrolizumab with or without chemotherapy versus chemotherapy alone for patients with PD-L1–positive advanced gastric or gastroesophageal junction adenocarcinoma: Update from the phase 3 KEYNOTE-062 trial. J. Clin. Oncol. 2022, 40, 243. [Google Scholar] [CrossRef]
- Moehler, M.; Ryu, M.-H.; Dvorkin, M.; Lee, K.-W.; Coşkun, H.; Wong, R.; Chung, H.; Poltoratsky, A.; Tsuji, A.; Yen, C.J.; et al. Maintenance avelumab versus continuation of first-line chemotherapy in gastric cancer: JAVELIN Gastric 100 study design. Futur. Oncol. 2019, 15, 567–577. [Google Scholar] [CrossRef]
- Moehler, M.; Dvorkin, M.; Boku, N.; Özgüroğlu, M.; Ryu, M.-H.; Muntean, A.S.; Lonardi, S.; Nechaeva, M.; Bragagnoli, A.C.; Coşkun, H.S.; et al. Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J. Clin. Oncol. 2021, 39, 966–977. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Ruiz, E.; Van Cutsem, E.; Lee, K.-W.; Wyrwicz, L.; Schenker, M.; Alsina, M.; Ryu, M.-H.; Chung, H.-C.; Evesque, L.; et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN Gastric 300. Ann. Oncol. 2018, 29, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.-J.; Liu, Z.; Cheng, Q. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J. Exp. Clin. Cancer Res. 2021, 40, 184. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair–Deficient/Microsatellite Instability–High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Pauligk, C.; Götze, T.; Thuss-Patience, P.; Riera-Knorrenschild, J.; Goekkurt, E.; Ettrich, T.; Pink, D.; Lindig, U.; Luley, K.; Dechow, T.; et al. 1443P Modified FOLFOX versus modified FOLFOX plus nivolumab and ipilimumab in patients with previously untreated advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction–Safety Results from AIO-STO-0417: A randomized phase II trial of the German Gastric Group of the AIO. Ann. Oncol. 2020, 31, S908. [Google Scholar] [CrossRef]
- Lorenzen, S.; Thuss-Patience, P.C.; Riera Knorrenschild, J.; Goekkurt, E.; Dechow, T.N.; Hofheinz, R.D.; Luley, K.; Ettrich, T.; Pink, D.; Lindig, U.; et al. FOLFOX versus FOLFOX plus nivolumab and ipilimumab adminis-tered in parallel or sequentially versus FLOT plus nivolumab administered in parallel in patients with previously untreated advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction: A randomized phase 2 trial of the AIO. J. Clin. Oncol. 2022, 40, 4043. [Google Scholar]
- Lorenzen, S.; Thuss-Patience, P.; Folprecht, G.; Knorrenschild, J.R.; Heinemann, V.; Goekkurt, E.; Dechow, T.; Ettrich, T.; Luley, K.; Moulin, J.-C.; et al. 1203O FOLFOX plus nivolumab and ipilimumab versus FOLFOX induction followed by nivolumab and ipilimumab in patients with previously untreated advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction: Results from the randomized phase II Moonlight trial of the AIO. Ann. Oncol. 2022, 33, S1099. [Google Scholar] [CrossRef]
- Tougeron, D.; Dahan, L.; El Hajbi, F.; Le Malicot, K.; Evesque, L.; Aparicio, T.; Bouché, O.; Bonichon-Lamichhane, N.; Chibaudel, B.; Angelergues, A.; et al. The PRODIGE 59-DURIGAST trial: A randomized phase II study evaluating FOLFIRI plus durvalumab and FOLFIRI plus durvalumab plus tremelimumab in second-line treatment of patients with advanced gastric or gastro-esophageal junction adenocarcinoma. J. Clin. Oncol. 2022, 40, 4036. [Google Scholar] [CrossRef]
- Tougeron, D.; Dahan, L.; El Hajbi, F.; Le Malicot, K.; Evesque, L.; Aparicio, T.; Bouche, O.; Lamichhane, N.B.; Chibaudel, B.; Angelergues, A.; et al. 1204MO PRODIGE 59–DURIGAST trial: A randomised phase II study evaluating FOLFIRI plus durvalumab and FOLFIRI plus durvalumab plus tremelimumab in second-line treatment of patients with advanced gastric or gastro-oesophageal junction adenocarcinoma. Ann. Oncol. 2022, 33, S1099–S1100. [Google Scholar] [CrossRef]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Muller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O.; et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, 315ra188. [Google Scholar] [CrossRef]
- Chaganty, B.K.R.; Qiu, S.; Gest, A.; Lu, Y.; Ivan, C.; Calin, G.; Weiner, L.M.; Fan, Z. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion. Cancer Lett. 2018, 430, 47–56. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Maron, S.B.; Chatila, W.K.; Millang, B.; Chavan, S.S.; Alterman, C.; Chou, J.F.; Segal, M.F.; Simmons, M.Z.; Momtaz, P.; et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: An open-label, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Kang, Y.K.; Park, H.; Uronis, H.; Lee, K.-W.; Ng, M.; Enzinger, P.; Park, S.H.; Gold, P.; Lacy, J.; et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): A single-arm, phase 1b-2 trial. Lancet Oncol. 2020, 21, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.; Park, H.; Shim, B.Y.; Kim, S.T.; Oh, D.-Y.; Spira, A.; Ulahannan, S.; Avery, E.J.; Boland, P.M.; Chao, J.; et al. 1379P Margetuximab (M) with retifanlimab (R) in HER2+, PD-L1+ 1st-line unresec-table/metastatic gastroesophageal adenocarcinoma (GEA): MAHOGANY cohort A. Ann. Oncol. 2021, 32, S1043–S1044. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Iwata, T.N.; Ishii, C.; Ishida, S.; Ogitani, Y.; Wada, T.; Agatsuma, T. A HER2-Targeting Antibody–Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol. Cancer Ther. 2018, 17, 1494–1503. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Oh, D.-Y.; Rha, S.Y.; Lee, K.-W.; Steeghs, N.; Chao, Y.; Di Bartolomeo, M.; García, M.D.; Mohammad, N.H.; Stein, A.; et al. Dose-escalation and dose-expansion study of trastuzumab deruxtecan (T-DXd) mono-therapy and combinations in patients (pts) with advanced/metastatic HER2+ gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): DESTINY-Gastric03. J. Clin. Oncol. 2022, 40, 295. [Google Scholar] [CrossRef]
- Stein, A.; Paschold, L.; Tintelnot, J.; Goekkurt, E.; Henkes, S.-S.; Simnica, D.; Schultheiss, C.; Willscher, E.; Bauer, M.; Wickenhauser, C.; et al. Efficacy of Ipilimumab vs FOLFOX in Combination With Nivolumab and Trastuzumab in Patients With Previously Untreated ERBB2-Positive Esophagogastric Adenocarcinoma: The AIO INTEGA Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1150–1158. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Lorenzen, S.; Thuss-Patience, P.C.; Homann, N.; Schenk, M.; Lindig, U.; Heuer, V.; Kretzschmar, A.; Goekkurt, E.; Haag, G.M.; et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esoph-agogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J. Clin. Oncol. 2022, 40, 4003. [Google Scholar]
- Becker, K.; Mueller, J.D.; Schulmacher, C.; Ott, K.; Fink, U.; Busch, R.; Bottcher, K.; Siewert, J.R.; Hofler, H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003, 98, 1521–1530. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recom-mendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Sasaki, E.; Masuishi, T.; Taniguchi, H.; Kadowaki, S.; Ito, S.; Yatabe, Y.; Muro, K. PD-L1 immunohistochemistry comparison of 22C3 and 28-8 assays for gastric cancer. J. Gastrointest. Oncol. 2021, 12, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Hagi, T.; Kurokawa, Y.; Kawabata, R.; Omori, T.; Matsuyama, J.; Fujitani, K.; Hirao, M.; Akamaru, Y.; Takahashi, T.; Yamasaki, M.; et al. Multicentre biomarker cohort study on the efficacy of nivolumab treatment for gastric cancer. Br. J. Cancer 2020, 123, 965–972. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, K.-M. PD-L1 expression in gastric cancer: Interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod. Pathol. 2021, 34, 1719–1727. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Treatment Guidelines 2022 (6th edition). 2021. Available online: https://link.springer.com/article/10.1007/s10120-022-01331-8 (accessed on 28 March 2023).
- Fuchs, C.S.; Özgüroğlu, M.; Bang, Y.-J.; Di Bartolomeo, M.; Mandala, M.; Ryu, M.-H.; Fornaro, L.; Olesinski, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer 2021, 25, 197–206. [Google Scholar] [CrossRef]
- Yamashita, K.; Iwatsuki, M.; Harada, K.; Koga, Y.; Kiyozumi, Y.; Eto, K.; Hiyoshi, Y.; Ishimoto, T.; Iwagami, S.; Baba, Y.; et al. Can PD-L1 expression evaluated by biopsy sample accurately reflect its expression in the whole tumour in gastric cancer? Br. J. Cancer 2019, 121, 278–280. [Google Scholar] [CrossRef]
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor Microsatellite-Instability Status as a Predictor of Benefit from Fluorouracil-Based Adjuvant Chemotherapy for Colon Cancer. N. Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Insta-bility/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Chao, J.; Fuchs, C.S.; Shitara, K.; Tabernero, J.; Muro, K.; Van Cutsem, E.; Bang, Y.-J.; De Vita, F.; Landers, G.; Yen, C.-J.; et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol 2021, 7, 895–902. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Lorenzen, S.; Homann, N.; Thuss-Patience, P.; Schenk, M.; Lindig, U.; Kretzschmar, A.; Heuer, V.; Goekkurt, E.; Haag, G.; et al. 1429P Pathological regression in patients with microsatellite instability (MSI) receiving perioperative atezolizumab in combination with FLOT vs. FLOT alone for resectable esophagogastric adenocarcinoma: Results from the DANTE trial of the German Gastric Group at the AIO and SAKK. Ann. Oncol. 2021, 32, S1069. [Google Scholar] [CrossRef]
- André, T.; Tougeron, D.; Piessen, G.; de la Fouchardière, C.; Louvet, C.; Adenis, A.; Jary, M.; Tournigand, C.; Aparicio, T.; Desrame, J.; et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability–High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 2023, 41, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Gilbert, L.; Tinker, A.V.; Brown, J.; Mathews, C.; Press, J.; Sabatier, R.; O’Malley, D.; Samouelian, V.; Boni, V.; et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: Interim results from GARNET-a phase I, single-arm study. J. Immunother. Cancer 2022, 10, e003777. [Google Scholar] [PubMed]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Puliga, E.; Corso, S.; Pietrantonio, F.; Giordano, S. Microsatellite instability in Gastric Cancer: Between lights and shadows. Cancer Treat. Rev. 2021, 95, 102175. [Google Scholar] [CrossRef]
- Wang, F.H.; Wei, X.; Xu, N.; Shen, L.; Dai, G.; Yuan, X.; Chen, Y.; Yang, S.; Shi, J.; Hu, X.; et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann. Oncol. 2019, 30, 1479–1486. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.-J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.-H.; Vivaldi, C.; Olesinski, T.; Chung, H.C.; Muro, K.; et al. The association of tissue tumor mutational burden (tTMB) using the Foundation Medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061. J. Clin. Oncol. 2020, 38, 4537. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Sanchez-Vega, F.; Jonsson, P.; Chatila, W.K.; Hechtman, J.F.; Ku, G.Y.; Riches, J.C.; Tuvy, Y.; Kundra, R.; Bouvier, N.; et al. Genetic Predictors of Response to Systemic Therapy in Esophagogastric Cancer. Cancer Discov. 2018, 8, 49–58. [Google Scholar] [CrossRef]
- Greally, M.; Chou, J.F.; Chatila, W.K.; Margolis, M.; Capanu, M.; Hechtman, J.F.; Tuvy, Y.; Kundra, R.; Daian, F.; Ladanyi, M.; et al. Clinical and Molecular Predictors of Response to Immune Checkpoint Inhibitors in Patients with Advanced Esophagogastric Cancer. Clin. Cancer Res. 2019, 25, 6160–6169. [Google Scholar] [CrossRef]
- Sasaki, S.; Nishikawa, J.; Sakai, K.; Iizasa, H.; Yoshiyama, H.; Yanagihara, M.; Shuto, T.; Shimokuri, K.; Kanda, T.; Suehiro, Y.; et al. EBV-associated gastric cancer evades T-cell immunity by PD-1/PD-L1 interactions. Gastric Cancer 2019, 22, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Kawazoe, A.; Shitara, K.; Kuboki, Y.; Bando, H.; Kojima, T.; Yoshino, T.; Ohtsu, A.; Ochiai, A.; Togashi, Y.; Nishikawa, H.; et al. Clinicopathological features of 22C3 PD-L1 expression with mismatch repair, Epstein–Barr virus status, and cancer genome alterations in metastatic gastric cancer. Gastric Cancer 2019, 22, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Saito, M.; Nakajima, S.; Saito, K.; Nakayama, Y.; Kase, K.; Yamada, L.; Kanke, Y.; Hanayama, H.; Onozawa, H.; et al. PD-L1 overexpression in EBV-positive gastric cancer is caused by unique genomic or epigenomic mechanisms. Sci. Rep. 2021, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Garmezy, B.; Gheeya, J.; Lin, H.Y.; Huang, Y.; Kim, T.; Jiang, X.; Thein, K.; Pilié, P.; Zeineddine, F.; Wang, W.; et al. Clinical and Molecular Characterization of POLE Mutations as Predictive Biomarkers of Response to Immune Checkpoint Inhibitors in Advanced Cancers. JCO Precis. Oncol. 2022, 6, e2100267. [Google Scholar] [CrossRef]
- Zhu, M.; Cui, H.; Zhang, L.; Zhao, K.; Jia, X.; Jin, H. Assessment of POLE and POLD1 mutations as prognosis and immunotherapy biomarkers for stomach adenocarcinoma. Transl. Cancer Res. 2022, 11, 193–205. [Google Scholar] [CrossRef]
- Sundar, R.; Huang, K.; Qamra, A.; Kim, K.-M.; Kim, S.; Kang, W.; Tan, A.; Lee, J.; Tan, P. Epigenomic promoter alterations predict for benefit from immune checkpoint inhibition in metastatic gastric cancer. Ann. Oncol. 2019, 30, 424–430. [Google Scholar] [CrossRef]
- Sundar, R.; Huang, K.-K.; Kumar, V.; Ramnarayanan, K.; Demircioglu, D.; Her, Z.; Ong, X.; Isa, Z.F.B.A.; Xing, M.; Tan, A.L.-K.; et al. Epigenetic promoter alterations in GI tumour immune-editing and resistance to immune checkpoint inhibition. Gut 2021, 71, 1277–1288. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Piao, Y.; Makiyama, A. Achieving sequential therapy in advanced gastric cancer: The importance of appropriate patient management for the elderly and/or those with ascites. Gastric Cancer 2020, 23, 363–372. [Google Scholar] [CrossRef]
- Iizumi, S.; Takashima, A.; Sakamaki, K.; Morita, S.; Boku, N. Survival impact of post-progression chemotherapy in advanced gastric cancer: Systematic review and meta-analysis. Cancer Chemother. Pharmacol. 2018, 81, 981–989. [Google Scholar] [CrossRef]
- Iwasa, S.; Kudo, T.; Takahari, D.; Hara, H.; Kato, K.; Satoh, T. Practical guidance for the evaluation of disease progression and the decision to change treatment in patients with advanced gastric cancer receiving chemotherapy. Int. J. Clin. Oncol. 2020, 25, 1223–1232. [Google Scholar] [CrossRef]
- Komatsu, Y.; Hironaka, S.; Tanizawa, Y.; Cai, Z.; Piao, Y.; Boku, N. Treatment Pattern for Advanced Gastric Cancer in Japan and Factors Associated with Sequential Treatment: A Retrospective Administrative Claims Database Study. Adv. Ther. 2021, 39, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Jeurnink, S.M.; Steyerberg, E.W.; van Hooft, J.E.; van Eijck, C.H.; Schwartz, M.P.; Vleggaar, F.P.; Kuipers, E.J.; Siersema, P.D. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): A multicenter randomized trial. Gastrointest. Endosc. 2010, 71, 490–499. [Google Scholar] [CrossRef]
- Ogata, T.; Narita, Y.; Kumanishi, R.; Nakazawa, T.; Matsubara, Y.; Kato, K.; Nozawa, K.; Honda, K.; Masuishi, T.; Bando, H. Clinical Impact of Oral Intake in Second-line or Third-line Chemotherapy for 589 Patients With Advanced Gastric Cancer: A Retrospective Cohort Study. Am. J. Clin. Oncol. 2021, 44, 388–394. [Google Scholar] [CrossRef]
- No, J.H.; Kim, S.W.; Lim, C.-H.; Kim, J.S.; Cho, Y.K.; Park, J.M.; Lee, I.S.; Choi, M.-G.; Choi, K.Y. Long-term outcome of palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer in patients with good performance status: Endoscopic stenting versus surgery. Gastrointest. Endosc. 2013, 78, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.B.; Shen, W.S.; Xi, H.Q.; Wei, B.; Chen, L. Palliative Therapy for Gastric Outlet Obstruction Caused by Unresectable Gastric Cancer: A Meta-analysis Comparison of Gastrojejunostomy with Endoscopic Stenting. Chin. Med. J. (Engl.) 2016, 129, 1113–1121. [Google Scholar] [CrossRef]
- Xu, R.-H.; Zhang, Y.; Pan, H.; Feng, J.; Zhang, T.; Liu, T.; Qin, Y.; Qin, S.; Yin, X.; Liu, B.; et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): A randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Sho, M.; Yamato, I.; Yoshiji, H.; Wakatsuki, K.; Nishiwada, S.; Yagita, H.; Nakajima, Y. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin. Exp. Immunol. 2013, 172, 500–506. [Google Scholar] [CrossRef]
- Nakajima, T.E.; Kadowaki, S.; Minashi, K.; Nishina, T.; Yamanaka, T.; Hayashi, Y.; Izawa, N.; Muro, K.; Hironaka, S.; Kajiwara, T.; et al. Multicenter Phase I/II Study of Nivolumab Combined with Paclitaxel Plus Ramucirumab as Second-line Treatment in Patients with Advanced Gastric Cancer. Clin. Cancer Res. 2021, 27, 1029–1036. [Google Scholar] [CrossRef]
- Kato, K.; Satoh, T.; Muro, K.; Yoshikawa, T.; Tamura, T.; Hamamoto, Y.; Chin, K.; Minashi, K.; Tsuda, M.; Yamaguchi, K.; et al. A subanalysis of Japanese patients in a randomized, double-blind, placebo-controlled, phase 3 trial of nivolumab for patients with advanced gastric or gastro-esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2). Gastric Cancer 2018, 22, 344–354. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Högner, A.; Goekkurt, E.; Stahl, M.; Kretzschmar, A.; Schädlich, B.; Goetze, T.O.; Stocker, G.; Reichardt, P.; Kullmann, F.; et al. Ramucirumab, avelumab, and paclitaxel (RAP) as second-line treatment in gastro-esophageal adenocarcinoma, a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie (AIO). J. Clin. Oncol. 2022, 40, 4051. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients with Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Kawazoe, A.; Fukuoka, S.; Nakamura, Y.; Kuboki, Y.; Wakabayashi, M.; Nomura, S.; Mikamoto, Y.; Shima, H.; Fujishiro, N.; Higuchi, T.; et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): An open-label, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial interim results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef] [PubMed]

| East Asia | West | |
|---|---|---|
| Prevalence | High | Low |
| Proportion of gastroesophageal junction cancer | Low | High |
| Early cancer | Common | Rare |
| Helicobacter pylori-induced gastric cancer | Common | Rare |
| Standard surgery | D2 dissection | D1-D2 dissection |
| 5-year survival by surgery | 70% | 30–40% |
| Standard adjuvant chemotherapy | Post-operative chemotherapy in Japan (S-1/CapeOX/SOX/DS) | Perioperative chemotherapy (Neo and post adjuvant FLOT) |
| Standard chemotherapy regimen for advanced gastric cancer | Doublet | Doublet or triplet |
| Line | Agent | Target | Patient Selection | Trial | Phase | Experimental Arm | Control Arm | Key Results | Approval (USA/EU/JPN/KOR/CHN/TWN) |
|---|---|---|---|---|---|---|---|---|---|
| Locally advanced | Atezolizumab | PD-L1 | Perioperative | DANTE | II | Atezo + FLOT → op → atezo + FLOT | FLOT → op → FLOT | Awaited: High pathological response in higher PD-L1 CPS and MSI-high | |
| Nivolumab | PD-1 | pStage III | ATTRACTION-5 | III | Adj nivo + S-1/CapeOX | Adj S-1/CapeOX | Ongoing | ||
| Ipilimumab+ nivolumab | CTLA-4 + PD-1 | Comp preop CTx | VESTIGE | II | Adj ipi+ nivo | Adj CTx | Ongoing | ||
| Pembrolizumab | PD-1 | Perioperative | KEYNOTE-585 | III | Pembro + XP/CF/FLOT → op → pembro + XP/CF/FLOT | Placebo | Ongoing | ||
| Durvalumab | PD-L1 | Perioperative | MATTERHORN | III | Durva + FLOT→ op → durva + FLOT | Placebo | Ongoing | ||
| Ipilimumab + nivolumab | CTLA-4 + PD-1 | MSI-H or dMMR | NEONIPIGA | II | Ipi + nivo → op → nivo | – | High CR rate (58.6%), no patient relapse | ||
| First-line | Nivolumab | PD-1 | HER2- | CheckMate 649 | III | Nivo + CapeOX/FOLFOX | CapeOX/FOLFOX | Positive: OS Δ3.3 months (CPS ≥ 5), OS Δ2.2 months (all randomized) | USA, EU (CPS ≥ 5), JPN, KOR, CHN, TWN |
| Ipilimumab + nivolumab | CTLA-4 + PD-1 | III | Ipi + nivo | CapeOX/FOLFOX | Negative | ||||
| Nivolumab | PD-1 | HER2- | ATTRACTION-4 | III | Nivo + SOX/CapeOX | SOX/CapeOX | Positive for PFS/negative for OS | ||
| Pembrolizumab | PD-1 | HER2- | KEYNOTE-062 | III | Pembro + XP/CF | XP/CF | Negative | ||
| III | Pembro | Noninferior for OS (CPS ≥ 10) | USA (CPS ≥ 10) | ||||||
| Pembrolizumab | PD-1 | HER2- | KEYNOTE-859 | III | Pembro + CapeOX/CF | Placebo | Positive: Press release only | ||
| Avelumab | PD-L1 | HER2- | JAVELIN Gastric 100 | III | Avel maintenance | CapeOX/FOLFOX cont, | Negative | ||
| Sintilimab | PD-1 | HER2- | ORIENT-16 | III | Sinti + CapeOX | Placebo | Positive: OS Δ5.5/Δ2.9 months (CPS ≥ 5/any PD-L1) | CHN | |
| Tislelizumab | PD-1 | HER2- | RATIONALE 305 | III | Tisle + CapeOX/CF | Placebo | Positive: Press release only | ||
| Ipilimumab + nivolumab | CTLA-4 + PD-1 | HER2- | MOONLIGHT | II | Ipi + Nivo + FOLFOX/ Nivo + FLOT | FOLFOX → Ipi + nivo | Median OS 16.46 months in Ipi + Nivo + FOLFOX (CPS ≥ 1) | ||
| Pembrolizumab + lenvatinib | PD-1 + multikinase | HER2- | LEAP-015 | III | Pembro + lenva + CapeOX/FOLFOX | CapeOX/FOLFOX | Ongoing | ||
| Pembrolizumab | PD-1 + HER2 | HER2+ | KEYNOTE-811 | III | Pembro + trastuzumab + CF/CapeOX/SOX | Placebo | OS awaited; ORR, 74.4% vs. 51.9% | USA | |
| Retifanlimab/tebotelimab + margetuximab | PD-1 (+LAG-3) + HER2 | HER2+ and CPS ≥ 1 | MAHOGANY | III | Reti/tebote + marge +/− CTx | Trastuzumab + CTx | On going: ORR, 64.8% in Reti + marge arm | ||
| Durvalumab + T-DXd | PD-L1 + HER2 | HER2+ | DESTINY-Gastric03 | Ib/II | T-DXd ± durva ± CTx | – | Ongoing: ORR, 42.9~50% | ||
| Ipilimumab + nivoluamb | CTLA-4 + PD-1 + HER2 | HER2+ | INTEGA | II | Ipi + nivo + trastuzumab | Nivo + trastuzumab + FOLFOX | 12 mo OS rate, 57% vs. 70% | ||
| First or second-line | Pembrolizumab + lenvatinib | PD-1 + multikinase | all | EPOC1706 | II | Pembro + Lenva | – | ORR, 69% | |
| Second-line | Pembrolizumab | PD-1 | CPS ≥ 1 | KEYNOTE-061 | III | Pembro | Paclitaxel | Negative | |
| Durvalumab ± tremelimumab | PD-L1± CTLA-4 | all | DURIGAST | II | Durva + treme + FOLFIRI | Durva + FOLFIRI | Negative: PFS at 4 mo, 57.8% vs. 44.7% | ||
| Nivolumab + paclitaxel + ramucirumab | PD-1 + VEGF | all | - | II | Nivo + paclitaxel + ramucirumab | – | 6-month OS rate, 46.5% | ||
| Avelumab + paclitaxel + ramucirumab | PD-L1 + VEGF | all | RAP | II | Ave + paclitaxel + ramucirumab | – | 6-month OS rate, 71.2% | ||
| Second or later line | Nivolumab ± ipilimumab | PD-1 ± CTLA-4 | all | CheckMate 032 | II | Nivo ± ipi | – | ORR, 8~24% | |
| Pembrolizumab | PD-1 | MSI-H or dMMR | KEYNOTE-158 | II | Pembro | – | ORR, 45.8% (gastric cancer) | USA, JPN | |
| Pembrolizumab | PD-1 | TMB-H | II | ORR, 28% (not MSI-H/dMMR) | USA, JPN | ||||
| Dostarlimab | PD-1 | dMMR or POLE mut | GARNET | II | Dostar | – | ORR, 38.7% | USA | |
| Nivolumab + regorafenib | PD-1 + multikinase | all | INTEGRATEIIB | III | Nivo + rego | Chemotherapy | Ongoing | ||
| Third-line | Avelumab | PD-L1 | all | JAVELIN Gastric 300 | III | Ave | Irinotecan or taxane | Negative | |
| Third or later-line | Nivolumab | PD-1 | all | ATTRACTION-2 | III | Nivo | Placebo | Positive | JPN, KOR, CHN, TWN |
| Pembrolizumab | PD-1 | all | KEYNOTE-059 | II | Pembro | – | ORR, 15.5% (CPS ≥ 1) | Withdraw Indication in USA (CPS ≥ 1) | |
| Nivolumab + regorafenib | PD-1 + multikinase | all | REGONIVO | II | Nivo + rego | – | ORR, 44% | ||
| Pembrolizumab + lenvatinib | PD-1 + multikinase | all | LEAP-005 | II | Pembro + lenva | – | ORR, 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narita, Y.; Muro, K. Updated Immunotherapy for Gastric Cancer. J. Clin. Med. 2023, 12, 2636. https://doi.org/10.3390/jcm12072636
Narita Y, Muro K. Updated Immunotherapy for Gastric Cancer. Journal of Clinical Medicine. 2023; 12(7):2636. https://doi.org/10.3390/jcm12072636
Chicago/Turabian StyleNarita, Yukiya, and Kei Muro. 2023. "Updated Immunotherapy for Gastric Cancer" Journal of Clinical Medicine 12, no. 7: 2636. https://doi.org/10.3390/jcm12072636
APA StyleNarita, Y., & Muro, K. (2023). Updated Immunotherapy for Gastric Cancer. Journal of Clinical Medicine, 12(7), 2636. https://doi.org/10.3390/jcm12072636





