Nutrients Associated with Sleep Bruxism
Abstract
1. Introduction
2. Materials and Methods
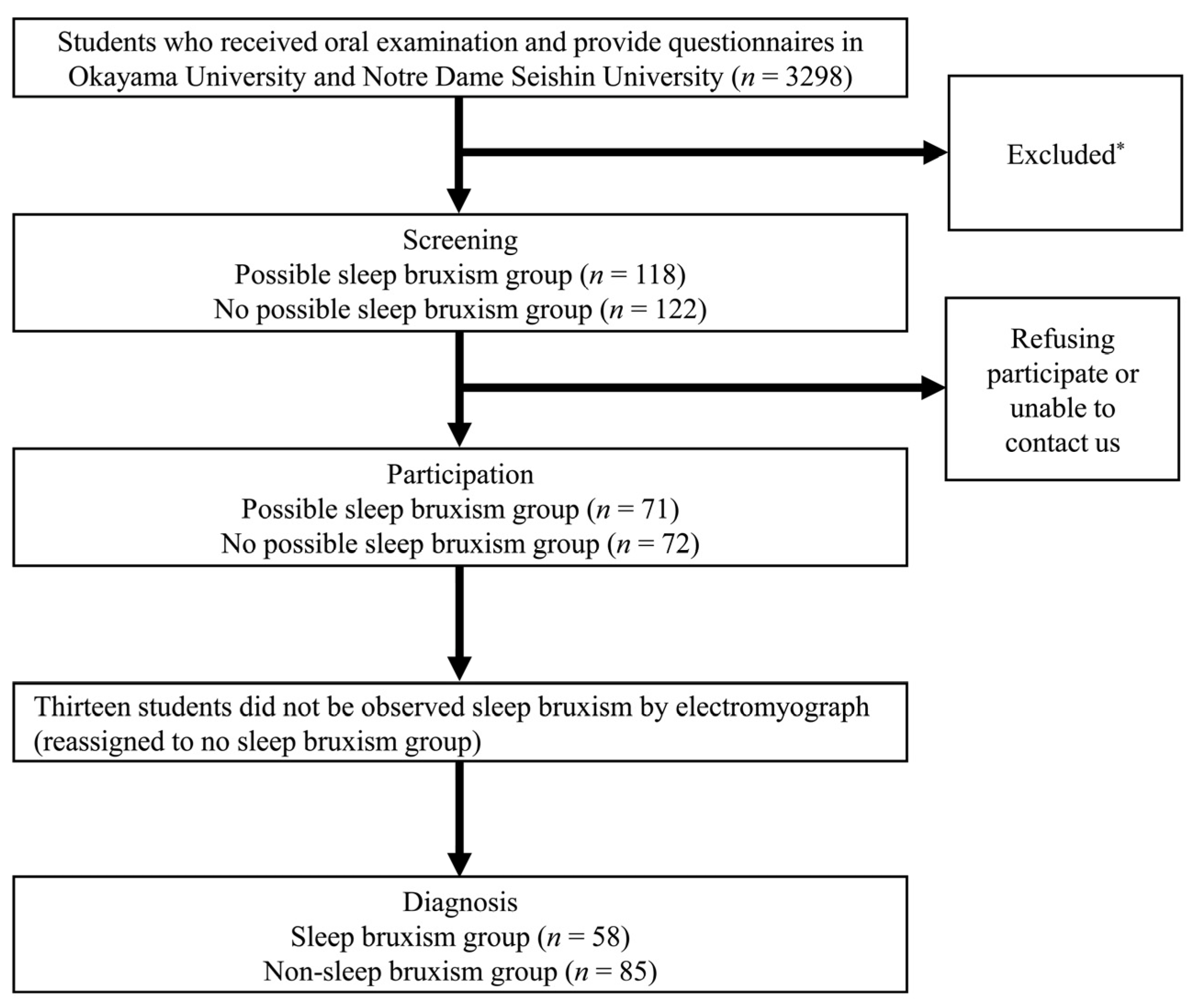
2.1. Study Population
2.2. Ethical Procedures and Informed Consent
2.3. Assessment of Risk Factors for SB and of Subjective Sleep Quality
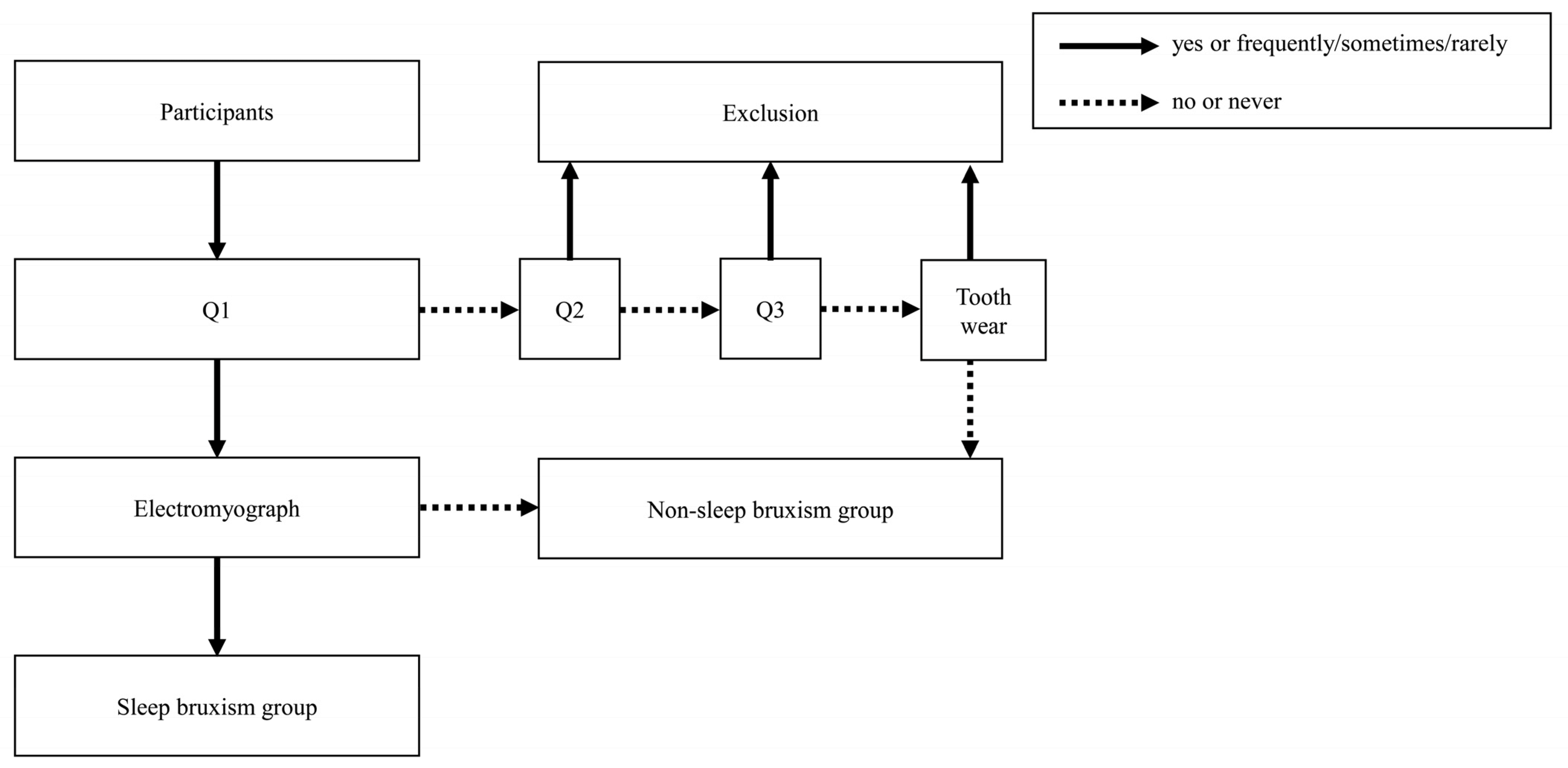
2.4. Screening of the SB and NSB Groups
- Q1: Has anyone heard you grinding your teeth while you sleep at night? (yes/no);
- Q2: How often does your jaw feel fatigued or sore on waking in the morning? (frequently/sometimes/rarely/never);
- Q3: How often do you experience headache in the temples on waking in the morning? (frequently/sometimes/rarely/never).
2.5. Diagnosis of SB
- Phasic episode: burst occurred ≥3 times, and each burst continued ≥0.25 s but <2 s.
- Tonic episode: a burst continued ≥2 s.
- Mixed episode: both phasic and tonic episodes were measured.
- Total number of episodes (phasic, tonic and mixed) >30 times/night.
- Number of episodes (phasic, tonic or mixed) >4 episodes/h.
- Number of bursts >6 bursts/episode.
- Number of bursts >25 bursts/h.
2.6. Assessment of Nutrients
2.7. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishigawa, K.; Bando, E.; Nakano, M. Quantitative Study of Bite Force during Sleep Associated Bruxism. J. Oral Rehabil. 2001, 28, 485–491. [Google Scholar] [CrossRef]
- Murali, R.V.; Rangarajan, P.; Mounissamy, A. Bruxism: Conceptual Discussion and Review. J. Pharm. Bioallied Sci. 2015, 7, S265–S270. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; Colten, H.R., Altevogt, B.M., Eds.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2006; ISBN 978-0-309-10111-0.
- Kato, T.; Rompré, P.; Montplaisir, J.Y.; Sessle, B.J.; Lavigne, G.J. Sleep Bruxism: An Oromotor Activity Secondary to Micro-Arousal. J. Dent. Res. 2001, 80, 1940–1944. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.; Kato, T.; Rompré, P.H.; Okura, K.; Saber, M.; Lanfranchi, P.A.; Montplaisir, J.Y.; Lavigne, G.J. Sleep Bruxism Is Associated to Micro-Arousals and an Increase in Cardiac Sympathetic Activity. J. Sleep Res. 2006, 15, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.J.; Kato, T.; Kolta, A.; Sessle, B.J. Neurobiological Mechanisms Involved in Sleep Bruxism. Crit. Rev. Oral Biol. Med. 2003, 14, 30–46. [Google Scholar] [CrossRef]
- Ribeiro-Lages, M.B.; Jural, L.A.; Magno, M.B.; Vicente-Gomila, J.; Ferreira, D.M.; Fonseca-Gonçalves, A.; Maia, L.C. A World Panorama of Bruxism in Children and Adolescents with Emphasis on Associated Sleep Features: A Bibliometric Analysis. J. Oral Rehabil. 2021, 48, 1271–1282. [Google Scholar] [CrossRef]
- Wieczorek, T.; Michałek-Zrąbkowska, M.; Więckiewicz, M.; Mazur, G.; Rymaszewska, J.; Smardz, J.; Wojakowska, A.; Martynowicz, H. Sleep Bruxism Contributes to Motor Activity Increase during Sleep in Apneic and Nonapneic Patients—A Polysomnographic Study. Biomedicines 2022, 10, 2666. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Bogunia-Kubik, K.; Mazur, G.; Danel, D.; Smardz, J.; Wojakowska, A.; Poreba, R.; Dratwa, M.; Chaszczewska-Markowska, M.; Winocur, E.; et al. Genetic Basis of Sleep Bruxism and Sleep Apnea—Response to a Medical Puzzle. Sci. Rep. 2020, 10, 7497. [Google Scholar] [CrossRef]
- Martynowicz, H.; Smardz, J.; Wieczorek, T.; Mazur, G.; Poreba, R.; Skomro, R.; Zietek, M.; Wojakowska, A.; Michalek, M.; Wieckiewicz, M. The Co-Occurrence of Sexsomnia, Sleep Bruxism and Other Sleep Disorders. J. Clin. Med. 2018, 7, 233. [Google Scholar] [CrossRef]
- Smardz, J.; Wieckiewicz, M.; Wojakowska, A.; Michalek-Zrabkowska, M.; Poreba, R.; Gac, P.; Mazur, G.; Martynowicz, H. Incidence of Sleep Bruxism in Different Phenotypes of Obstructive Sleep Apnea. J. Clin. Med. 2022, 11, 4091. [Google Scholar] [CrossRef]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Witkowska, A.M. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients 2022, 14, 1912. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Mikic, A.; Pietrolungo, C.E. Effects of Diet on Sleep Quality. Adv. Nutr. 2016, 7, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodríguez, A.B.; Barriga, C. Tryptophan-Enriched Cereal Intake Improves Nocturnal Sleep, Melatonin, Serotonin, and Total Antioxidant Capacity Levels and Mood in Elderly Humans. Age 2013, 35, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Afaghi, A.; O’Connor, H.; Chow, C.M. High-Glycemic-Index Carbohydrate Meals Shorten Sleep Onset. Am. J. Clin. Nutr. 2007, 85, 426–430. [Google Scholar] [CrossRef]
- Shilo, L.; Sabbah, H.; Hadari, R.; Kovatz, S.; Weinberg, U.; Dolev, S.; Dagan, Y.; Shenkman, L. The Effects of Coffee Consumption on Sleep and Melatonin Secretion. Sleep Med. 2002, 3, 271–273. [Google Scholar] [CrossRef]
- Zhao, M.; Tuo, H.; Wang, S.; Zhao, L. The Effects of Dietary Nutrition on Sleep and Sleep Disorders. Mediat. Inflamm. 2020, 2020, 3142874. [Google Scholar] [CrossRef]
- Bulanda, S.; Ilczuk-Rypuła, D.; Nitecka-Buchta, A.; Nowak, Z.; Baron, S.; Postek-Stefańska, L. Sleep Bruxism in Children: Etiology, Diagnosis, and Treatment—A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 9544. [Google Scholar] [CrossRef]
- Castroflorio, T.; Bargellini, A.; Rossini, G.; Cugliari, G.; Deregibus, A. Sleep Bruxism and Related Risk Factors in Adults: A Systematic Literature Review. Arch. Oral Biol. 2017, 83, 25–32. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Okawa, M.; Kim, K.; Shibui, K.; Kamei, Y. Psychometric Assessment of Subjective Sleep Quality Using the Japanese Version of the Pittsburgh Sleep Quality Index (PSQI-J) in Psychiatric Disordered and Control Subjects. Psychiatry Res. 2000, 97, 165–172. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition (ICSD-3) (Online). Available online: https://learn.aasm.org/Public/Catalog/Details.aspx?id=%2FgqQVDMQIT%2FEDy86PWgqgQ%3D%3D&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3d%252fgqQVDMQIT%252fEDy86PWgqgQ%253d%253d (accessed on 18 April 2019).
- Casett, E.; Réus, J.C.; Stuginski-Barbosa, J.; Porporatti, A.L.; Carra, M.C.; Peres, M.A.; de Luca Canto, G.; Manfredini, D. Validity of Different Tools to Assess Sleep Bruxism: A Meta-Analysis. J. Oral Rehabil. 2017, 44, 722–734. [Google Scholar] [CrossRef]
- Lavigne, G.J.; Rompre, P.H.; Montplaisir, J.Y. Sleep Bruxism: Validity of Clinical Research Diagnostic Criteria in a Controlled Polysomnographic Study. J. Dent. Res. 1996, 75, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Imaeda, N.; Goto, C.; Sasakabe, T.; Mikami, H.; Oze, I.; Hosono, A.; Naito, M.; Miyagawa, N.; Ozaki, E.; Ikezaki, H.; et al. Reproducibility and Validity of Food Group Intake in a Short Food Frequency Questionnaire for the Middle-Aged Japanese Population. Environ. Health Prev. Med. 2021, 26, 28. [Google Scholar] [CrossRef]
- Ministry of Health, Labour Standards. Overview of the Dietary Reference Intakes for Japanese (2020); Ministry of Health, Labour Standards: Tokyo, Japan, 2020.
- St-Onge, M.-P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J. Clin. Sleep Med. 2016, 12, 19–24. [Google Scholar] [CrossRef]
- Tang, M.; Song, X.; Zhong, W.; Xie, Y.; Liu, Y.; Zhang, X. Dietary Fiber Ameliorates Sleep Disturbance Connected to the Gut–Brain Axis. Food Funct. 2022, 13, 12011–12020. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.; Huang, J.; Zhang, H.; Lin, Q.; Han, L.; Liu, J.; Wang, J.; Liu, H. Insoluble Dietary Fiber from Soy Hulls Regulates the Gut Microbiota in Vitro and Increases the Abundance of Bifidobacteriales and Lactobacillales. J. Food Sci. Technol. 2020, 57, 152–162. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The Microbiota-Gut-Brain Axis in Sleep Disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef]
- Kato, T.; Velly, A.M.; Nakane, T.; Masuda, Y.; Maki, S. Age Is Associated with Self-Reported Sleep Bruxism, Independently of Tooth Loss. Sleep Breath 2012, 16, 1159–1165. [Google Scholar] [CrossRef]
- Strausz, T.; Ahlberg, J.; Lobbezoo, F.; Restrepo, C.C.; Hublin, C.; Ahlberg, K.; Könönen, M. Awareness of Tooth Grinding and Clenching from Adolescence to Young Adulthood: A Nine-Year Follow-Up. J. Oral Rehabil. 2010, 37, 497–500. [Google Scholar] [CrossRef]
- Wetselaar, P.; Vermaire, E.J.H.; Lobbezoo, F.; Schuller, A.A. The Prevalence of Awake Bruxism and Sleep Bruxism in the Dutch Adolescent Population. J. Oral Rehabil. 2021, 48, 143–149. [Google Scholar] [CrossRef]
- Wetselaar, P.; Vermaire, E.; Lobbezoo, F.; Schuller, A.A. The Prevalence of Awake Bruxism and Sleep Bruxism in the Dutch Adult Population. J. Oral Rehabil. 2019, 46, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Pontes, L.D.S.; Prietsch, S.O.M. Sleep Bruxism: Population Based Study in People with 18 Years or More in the City of Rio Grande, Brazil. Rev. Bras. Epidemiol. 2019, 22, e190038. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, P.; Carpinelli, L.; Savarese, G. Perceived Stress and Bruxism in University Students. BMC Res. Notes 2016, 9, 514. [Google Scholar] [CrossRef]
- Smardz, J.; Wieckiewicz, M.; Gac, P.; Poreba, R.; Wojakowska, A.; Mazur, G.; Martynowicz, H. Influence of Age and Gender on Sleep Bruxism and Snoring in Non-Apneic Snoring Patients: A Polysomnographic Study. J. Sleep Res. 2021, 30, e13178. [Google Scholar] [CrossRef] [PubMed]
- Macek, P.; Wieckiewicz, M.; Poreba, R.; Gac, P.; Bogunia-Kubik, K.; Dratwa, M.; Wojakowska, A.; Mazur, G.; Martynowicz, H. Assessment of Telomerase Reverse Transcriptase Single Nucleotide Polymorphism in Sleep Bruxism. J. Clin. Med. 2022, 11, 525. [Google Scholar] [CrossRef]
- Guo, H.; Wang, T.; Niu, X.; Wang, H.; Yang, W.; Qiu, J.; Yang, L. The Risk Factors Related to Bruxism in Children: A Systematic Review and Meta-Analysis. Arch. Oral Biol. 2018, 86, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Kazubowska-Machnowska, K.; Jodkowska, A.; Michalek-Zrabkowska, M.; Wieckiewicz, M.; Poreba, R.; Dominiak, M.; Gac, P.; Mazur, G.; Kanclerska, J.; Martynowicz, H. The Effect of Severity of Obstructive Sleep Apnea on Sleep Bruxism in Respiratory Polygraphy Study. Brain Sci. 2022, 12, 828. [Google Scholar] [CrossRef]
- Viarengo, N.O.; Gonçalves, M.L.L.; Bruno, L.H.; Fossati, A.L.; Sertaje, M.R.F.; Santos, E.M.; Sobral, A.P.T.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S.; Horliana, A.C.R.T.; et al. Evaluation of Intravascular Irradiation of Blood in Children with Sleep Bruxism: Study Protocol for a Randomized Controlled Clinical Trial. Medicine 2022, 101, e31230. [Google Scholar] [CrossRef]
- Ahlberg, J.; Lobbezoo, F.; Hublin, C.; Piirtola, M.; Kaprio, J. Self-Reported Sleep Bruxism in 1990 and 2011 in a Nationwide Twin Cohort: Evidence of Trait Persistence and Genetic Liability. J. Oral Rehabil. 2022. [Google Scholar] [CrossRef]
- Stuginski-Barbosa, J.; Porporatti, A.L.; Costa, Y.M.; Svensson, P.; Conti, P.C.R. Agreement of the International Classification of Sleep Disorders Criteria with Polysomnography for Sleep Bruxism Diagnosis: A Preliminary Study. J. Prosthet. Dent. 2017, 117, 61–66. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Mikami, S.; Maeda, M.; Saito, T.; Nakajima, T.; Yachida, W.; Gotouda, A. Portable and Wearable Electromyographic Devices for the Assessment of Sleep Bruxism and Awake Bruxism: A Literature Review. CRANIO® 2020, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Campello, C.P.; Moraes, S.L.D.; Vasconcelos, B.C.D.E.; de Lima, E.L.S.; Pellizzer, E.P.; Lemos, C.A.A.; Muniz, M.T.C. Polymorphisms of the Serotonin Receptors Genes in Patients with Bruxism: A Systematic Review. J. Appl. Oral Sci. 2022, 29, e20210262. [Google Scholar] [CrossRef] [PubMed]
| SB Group | NSB Group | p | ||
|---|---|---|---|---|
| n = 58 | n = 85 | |||
| Sex | Male | 24 (41.4) * | 20 (23.5) | 0.023 † |
| Underlying systemic disease | Yes | 6 (10.3) | 5 (5.9) | 0.353 |
| Medicines taken regularly | Yes | 17 (29.3) | 21 (24.7) | 0.567 |
| Smoking status | Current | 1 (1.7) | 2 (2.4) | 0.355 |
| Past | 3 (5.2) | 1 (1.2) | ||
| Age | 21.9 ± 2.5 ‡ | 20.7 ± 1.6 | 0.002 § | |
| PSQI score | 5.2 ± 2.1 | 5.0 ± 1.9 | 0.560 | |
| Height | 163.7 ± 8.7 | 160.8 ± 8.0 | 0.039 | |
| Weight | 55.3 ± 8.5 | 52.6 ± 8.3 | 0.068 | |
| BMI | 20.5 ± 1.7 | 20.3 ± 2.1 | 0.492 | |
| Nutrients | ||||
| Alcohol (g) | 4.9 ± 8.2 | 2.0 ± 4.2 | 0.014 | |
| Biotin (μg) | 29.5 ± 11.3 | 29.7 ± 8.5 | 0.908 | |
| Caffeine (mg) | 1.4 ± 2.7 | 2.1 ± 3.1 | 0.219 | |
| Calcium (mg) | 448.0 ± 230.8 | 445.9 ± 162.6 | 0.947 | |
| Carbohydrate (g) | 236.5 ± 90.9 | 237.6 ± 79.2 | 0.938 | |
| Cholesterol (mg) | 352.7 ± 162.1 | 331.6 ± 108.2 | 0.387 | |
| Chromium (μg) | 6.8 ± 2.2 | 7.4 ± 2.2 | 0.123 | |
| Copper (mg) | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.319 | |
| Dietary fiber (g) | 10.6 ± 3.7 | 11.9 ± 4.3 | 0.056 | |
| Energy (kcal) | 1872.7 ± 569.2 | 1852.3 ± 479.0 | 0.818 | |
| Folic acid (μg) | 221.0 ± 92.7 | 243.3 ± 105.0 | 0.193 | |
| Iodine (μg) | 590.4 ± 560.0 | 626.8 ± 626.3 | 0.722 | |
| Iron (mg) | 6.3 ± 2.1 | 6.8 ± 1.9 | 0.217 | |
| Lipid (g) | 66.5 ± 19.8 | 65.9 ± 17.4 | 0.851 | |
| Magnesium (mg) | 208.4 ± 69.2 | 218.4 ± 60.0 | 0.359 | |
| Manganese (mg) | 2.3 ± 0.9 | 2.5 ± 0.7 | 0.417 | |
| Molybdenum (μg) | 157.9 ± 76.4 | 157.8 ± 55.1 | 0.997 | |
| Monounsaturated fatty acid (g) | 24.0 ± 6.9 | 23.9 ± 6.5 | 0.88 | |
| n-3 Polyunsaturated fatty acid (g) | 2.0 ± 0.8 | 1.9 ± 0.6 | 0.909 | |
| n-6 Polyunsaturated fatty acid (g) | 11.3 ± 3.7 | 11.3 ± 3.4 | 0.995 | |
| Niacin equivalents (mg) | 27.0 ± 8.0 | 27.5 ± 7.1 | 0.675 | |
| Pantothenic acid (mg) | 5.4 ± 1.7 | 5.4 ± 1.3 | 0.952 | |
| Phosphorus (mg) | 933.9 ± 301.2 | 931.7 ± 218.9 | 0.959 | |
| Polyunsaturated fatty acid(g) | 13.3 ± 3.9 | 13.3 ± 3.9 | 0.988 | |
| Potassium (mg) | 1967.7 ± 657.8 | 2076.8 ± 626.5 | 0.318 | |
| Protein (g) | 65.9 ± 17.4 | 67.0 ± 16.9 | 0.733 | |
| Retinol activity equivalents (μg) | 371.9 ± 234.3 | 407.9 ± 247.3 | 0.384 | |
| Saturated fatty acid (g) | 21.8 ± 7.8 | 21.4 ± 6.3 | 0.699 | |
| Selenium (μg) | 60.1 ± 17.2 | 60.9 ± 15.1 | 0.775 | |
| Sodium (g) | 7.5 ± 2.7 | 8.1 ± 2.7 | 0.167 | |
| Vitamin B1 (mg) | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.414 | |
| Vitamin B12 (μg) | 3.8 ± 2.0 | 3.8 ± 1.6 | 0.938 | |
| Vitamin B2 (mg) | 1.1 ± 0.4 | 1.1 ± 0.3 | 0.967 | |
| Vitamin B6 (mg) | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.487 | |
| Vitamin C (mg) | 58.1 ± 28.9 | 66.4 ± 34.6 | 0.137 | |
| Vitamin D (μg) | 3.5 ± 2.0 | 3.5 ± 1.6 | 0.903 | |
| Vitamin K (μg) | 186.1 ± 99.7 | 204.4 ± 114.5 | 0.322 | |
| Water (g) | 845.7 ± 298.1 | 815.3 ± 244.4 | 0.505 | |
| Zinc (mg) | 7.9 ± 2.5 | 7.9 ± 2.0 | 0.901 | |
| α-Tocopherol (mg) | 6.2 ± 2.0 | 6.5 ± 2.0 | 0.379 |
| Odds Ratio | p | 95%CI | ||
|---|---|---|---|---|
| Sex | Female | Ref. | ||
| Male | 2.11 | 0.058 | 0.97–4.58 | |
| Age | 1.32 | 0.005 | 1.09–1.60 | |
| Dietary fiber | 0.91 | 0.059 | 0.83–1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyama, N.; Ekuni, D.; Fukuhara, D.; Sawada, N.; Yamashita, M.; Komiyama, M.; Nagahama, T.; Morita, M. Nutrients Associated with Sleep Bruxism. J. Clin. Med. 2023, 12, 2623. https://doi.org/10.3390/jcm12072623
Toyama N, Ekuni D, Fukuhara D, Sawada N, Yamashita M, Komiyama M, Nagahama T, Morita M. Nutrients Associated with Sleep Bruxism. Journal of Clinical Medicine. 2023; 12(7):2623. https://doi.org/10.3390/jcm12072623
Chicago/Turabian StyleToyama, Naoki, Daisuke Ekuni, Daiki Fukuhara, Nanami Sawada, Miho Yamashita, Momoe Komiyama, Takahiko Nagahama, and Manabu Morita. 2023. "Nutrients Associated with Sleep Bruxism" Journal of Clinical Medicine 12, no. 7: 2623. https://doi.org/10.3390/jcm12072623
APA StyleToyama, N., Ekuni, D., Fukuhara, D., Sawada, N., Yamashita, M., Komiyama, M., Nagahama, T., & Morita, M. (2023). Nutrients Associated with Sleep Bruxism. Journal of Clinical Medicine, 12(7), 2623. https://doi.org/10.3390/jcm12072623






