Reproductive and Obstetric Outcomes after Fertility-Sparing Treatments for Cervical Cancer: Current Approach and Future Directions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Fertility-Preserving Interventions in Cervical Cancer Patients
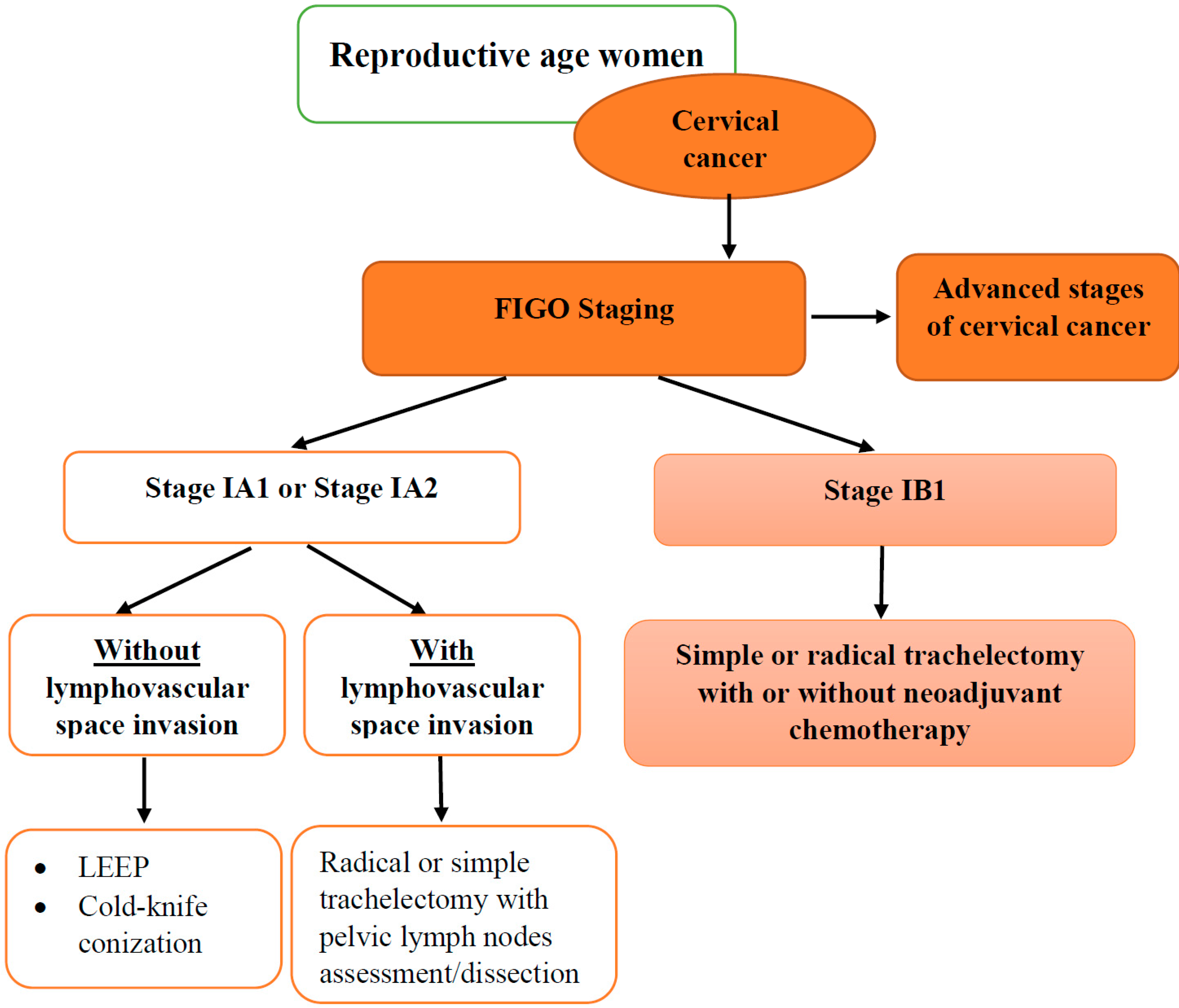
3.1.1. Fertility-Sparing Surgery
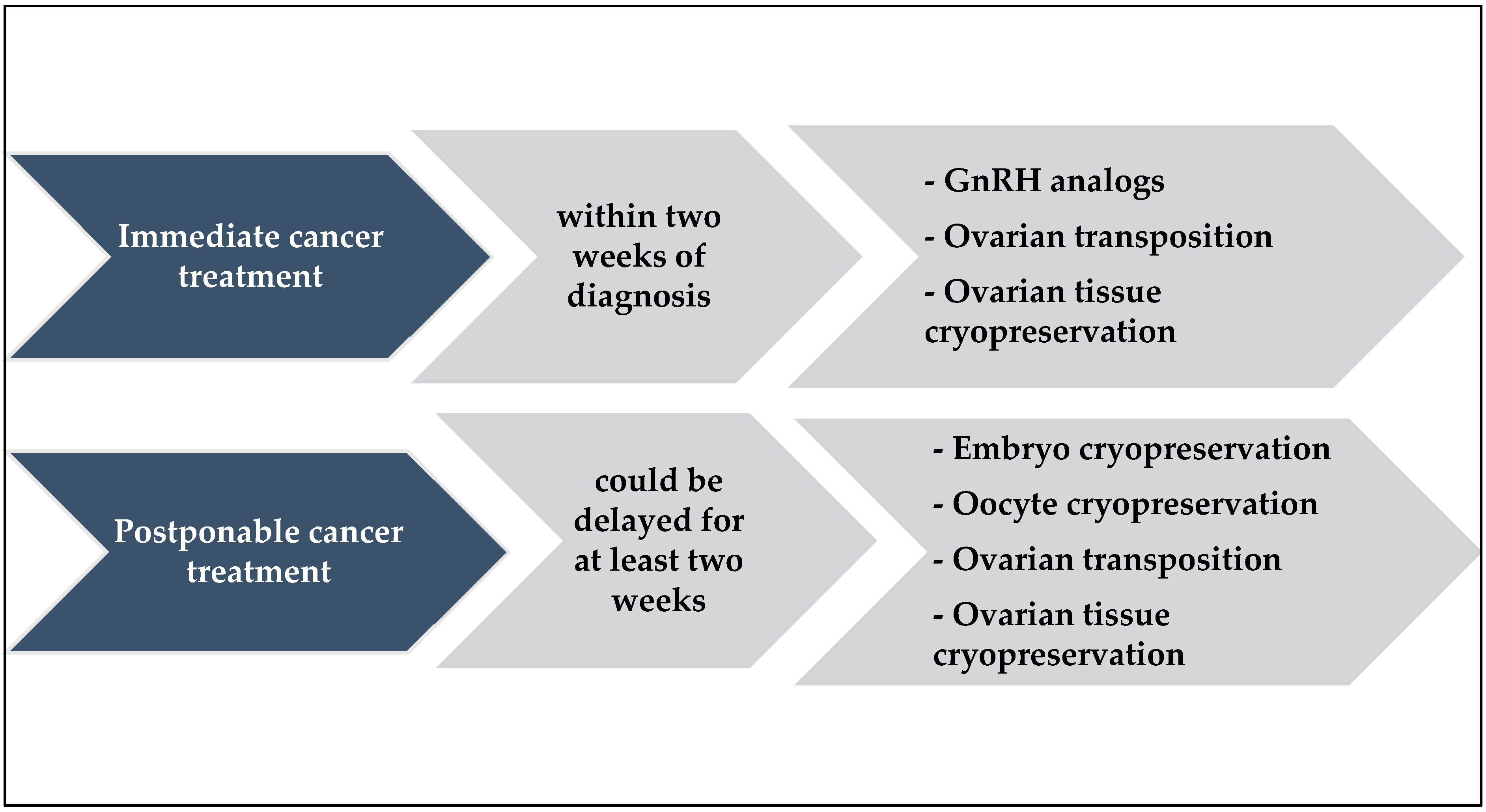
3.1.2. Ovarian Suppression with GnRH Analogs
3.1.3. Ovarian Transposition
3.1.4. Oocyte Cryopreservation
3.1.5. Ovarian Cortex Cryopreservation
3.2. Fertility/Infertility after Cervical Cancer Treatment
3.3. Pregnancy Course and Outcomes after Fertility-Sparing Management of Cervical Cancer
3.4. Prophylactic and Therapeutic HPV Vaccines for Prevention of Cervical Cancer Recurrence after Fertility-Sparing Surgery
3.4.1. Prophylactic HPV Vaccines
3.4.2. Therapeutic HPV Vaccines
3.5. Study Strengths and Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef] [PubMed]
- Greil, A.L. Infertility and psychological distress: A critical review of the literature. Soc. Sci. Med. 1997, 45, 1679–1704. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Sánchez, L.; Alhambra-Borrás, T.; Gallego-Valadés, A.; Garcés-Ferrer, J. Quality of Life and Conformity to Gender Norms in Women Receiving Assisted Reproductive Technologies as a Potential Indicator of Mental Health. Int. J. Environ. Res. Public Health 2022, 19, 10031. [Google Scholar] [CrossRef]
- Bourrion, B.; Panjo, H.; Bithorel, P.L.; de La Rochebrochard, E.; François, M.; Pelletier-Fleury, N. The economic burden of infertility treatment and distribution of expenditures overtime in France: A self-controlled pre-post study. BMC Health Serv. Res. 2022, 22, 512. [Google Scholar] [CrossRef]
- Anderson, K.M.; Sharpe, M.; Rattray, A. Distress and concerns in couples referred to a specialist infertility clinic. J. Psychosom. Res. 2003, 54, 353–355. [Google Scholar] [CrossRef]
- Wdowiak, A.; Anusiewicz, A.; Bakalczuk, G.; Raczkiewicz, D.; Janczyk, P.; Makara-Studzińska, M. Assessment of Quality of Life in Infertility Treated Women in Poland. Int. J. Environ. Res. Public Health 2021, 18, 4275. [Google Scholar] [CrossRef]
- Benedict, C.; Thom, B.; Friedman, D.N.; Pottenger, E.; Raghunathan, N.; Kelvin, J.F. Fertility information needs and concerns post-treatment contribute to lowered quality of life among young adult female cancer survivors. Support. Care Cancer 2018, 26, 2209–2215. [Google Scholar] [CrossRef]
- Ho, D.; Kim, S.Y.; Kim, S.I.; Lim, W.J. Insomnia, Anxiety, and Depression in Patients First Diagnosed with Female Cancer. Psychiatry Investig. 2021, 18, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Jentschke, M.; Lehmann, R.; Drews, N.; Hansel, A.; Schmitz, M.; Hillemanns, P. Psychological distress in cervical cancer screening: Results from a German online survey. Arch. Gynecol. Obstet. 2020, 302, 699–705. [Google Scholar] [CrossRef]
- Taumberger, N.; Joura, E.A.; Arbyn, M.; Kyrgiou, M.; Sehouli, J.; Gultekin, M. Myths and fake messages about human papillomavirus (HPV) vaccination: Answers from the ESGO Prevention Committee. Int. J. Gynecol. Cancer 2022, 32, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sit, J.W.H.; Chan, D.N.S.; Akingbade, O.; Chan, C.W.H. Educational Interventions to Promote Cervical Cancer Screening among Rural Populations: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 6874. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Pimple, S.; Mishra, G. Cancer cervix: Epidemiology and disease burden. Cytojournal 2022, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Gerstl, B.; Sullivan, E.; Vallejo, M.; Koch, J.; Johnson, M.; Wand, H.; Webber, K.; Ives, A.; Anazodo, A. Reproductive outcomes following treatment for a gynecological cancer diagnosis: A systematic review. J. Cancer Surviv. 2019, 13, 269–281. [Google Scholar] [CrossRef]
- Llueca, A.; Ibañez, M.V.; Torne, A.; Gil-Moreno, A.; Martin-Jimenez, A.; Diaz-Feijoo, B.; Serra, A.; Climent, M.T.; Gil-Ibañez, B.; On Behalf of the Spain-GOG Cervical Cancer Working Group. Fertility-Sparing Surgery versus Radical Hysterectomy in Early Cervical Cancer: A Propensity Score Matching Analysis and Noninferiority Study. J. Pers. Med. 2022, 12, 1081. [Google Scholar] [CrossRef]
- Silvestris, E.; Paradiso, A.V.; Minoia, C.; Daniele, A.; Cormio, G.; Tinelli, R.; D’Oronzo, S.; Cafforio, P.; Loizzi, V.; Dellino, M. Fertility preservation techniques in cervical carcinoma. Medicine 2022, 101, e29163. [Google Scholar] [CrossRef]
- Taylan, E.; Oktay, K. Fertility preservation in gynecologic cancers. Gynecol. Oncol. 2019, 155, 522–529. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, 72–83. [Google Scholar] [CrossRef]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 64–84. [Google Scholar] [CrossRef]
- Cibula, D.; Potter, R.; Planchamp, F. The European Society of Gynaecological Oncology/European Society for Radio-Therapy and Oncology/European Society of pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef]
- Tomao, F.; Maruccio, M.; Preti, E.P. Conization in early stage cervical cancer: Pattern of recurrence in a 10-year sin-gle-institution experience. Int. J. Gynecol. Cancer 2017, 27, 1001–1008. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Guideline Version 1. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 21 September 2022).
- AIOM 2018. Preservazione della Fertilità nei Pazienti Oncologici. Available online: https://https:www.aiom.it/linee-guida-aiom-2018-preservazione-della-fertilita-nei-pazienti-oncologici (accessed on 21 September 2022).
- Laios, A.; Portela, S.D.; Papadopoulou, A.; Gallos, I.D.; Otify, M.; Ind, T. Ovarian transposition and cervical cancer. Best Pr. Res. Clin. Obstet. Gynaecol. 2021, 75, 37–53. [Google Scholar] [CrossRef]
- Vyfhuis, M.A.L.; Fellows, Z.; McGovern, N.; Zhu, M.; Mohindra, P.; Wong, J.; Nichols, E.M. Preserving Endocrine Function in Premenopausal Women Undergoing Whole Pelvis Radiation for Cervical Cancer. Int. J. Part. Ther. 2019, 6, 10–17. [Google Scholar] [CrossRef]
- Valsamakis, G.; Valtetsiotis, K.; Charmandari, E.; Lambrinoudaki, I.; Vlahos, N.F. GnRH Analogues as a Co-Treatment to Therapy in Women of Reproductive Age with Cancer and Fertility Preservation. Int. J. Mol. Sci. 2022, 23, 2287. [Google Scholar] [CrossRef]
- Yuan, J.; Lai, Y.; Li, T. Ovarian protection and safety of gonadotropin-releasing hormone agonist after cervical cancer surgery: Systematic review and meta-analysis. Ann. Transl. Med. 2022, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: An Ethics Committee opinion. Fertil. Steril. 2018, 110, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Buonomo, B.; Massarotti, C.; Dellino, M.; Anserini, P.; Ferrari, A.; Campanella, M.; Magnotti, M.; De Stefano, C.; Peccatori, F.A.; Lambertini, M. Reproductive issues in carriers of germline pathogenic variants in the BRCA1/2 genes: An expert meeting. BMC Med. 2021, 19, 205. [Google Scholar] [CrossRef]
- Marin, L.; Ambrosini, G.; Esposito, F.; Capobianco, G.; Laganà, A.S.; Vio, C.; Nuzzi, L.; Rossato, M.; Andrisani, A. Fertility Preservation in Girls and Women: State of Art and Future Possibilities. Clin. Exp. Obstet. Gynecol. 2022, 49, 206. [Google Scholar] [CrossRef]
- Bentivegna, E.; Maulard, A.; Pautier, P.; Chargari, C.; Gouy, S.; Morice, P. Fertility results and pregnancy outcomes after conservative treatment of cervical cancer: A systematic review of the literature. Fertil. Steril. 2016, 106, 1195–1211.e5. [Google Scholar] [CrossRef]
- Moawad, N.S.; Santamaria, E.; Rhoton-Vlasak, A.; Lightsey, J.L. Laparoscopic Ovarian Transposition Before Pelvic Cancer Treatment: Ovarian Function and Fertility Preservation. J. Minim. Invasive Gynecol. 2017, 24, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ghadjar, P.; Budach, V.; Kohler, C.; Jantke, A.; Marnitz, S. Modern radiation therapy and potential fertility preservation strategies in patients with cervical cancer undergoing chemoradiation. Radiat. Oncol. 2015, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Peccatori, F.; Demeestere, I. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 12, 1664–1678. [Google Scholar] [CrossRef]
- Bizzarri, N.; Loverro, M.; Angeles, M.A.; Anchora, L.P.; Fagotti, A.; Fanfani, F.; Ferrandina, G.; Scambia, G.; Querleu, D. Laparoscopic Ovarian Transposition with Extraperitonealization of the Infundibulopelvic Ligament for Cervical Cancer in Ten Steps. Ann. Surg. Oncol. 2022, 29, 5906–5907. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, M.Y.; Mu, Y.; Mo, S.P.; Zheng, A.; Li, J.K. Ovarian metastasis in women with cervical carcinoma in stages IA to IIB. Medicine 2020, 99, e21146. [Google Scholar] [CrossRef]
- Laios, A.; Otify, M.; Papadopoulou, A.; Gallos, I.D.; Ind, T. Outcomes of ovarian transposition in cervical cancer; an updated meta-analysis. BMC Women’s Health 2022, 22, 305. [Google Scholar] [CrossRef]
- Somigliana, E.; Mangili, G.; Martinelli, F.; Noli, S.; Filippi, F.; Bergamini, A.; Bocciolone, L.; Buonomo, B.; Peccatori, F. Fertility preservation in women with cervical cancer. Crit. Rev. Oncol. 2020, 154, 103092. [Google Scholar] [CrossRef] [PubMed]
- Yli-Kuha, A.N.; Gissler, M.; Klemetti, R. Cancer morbidity in a cohort of 9175 Finnish women treated for infertility. Hum. Reprod. 2012, 27, 1149–1155. [Google Scholar] [CrossRef]
- von Wolff, M.; Bruckner, T.; Strowitzki, T.; Germeyer, A. Fertility preservation: Ovarian response to freeze oocytes is not affected by different malignant diseases—An analysis of 992 stimulations. J. Assist. Reprod. Genet. 2018, 35, 1713–1719. [Google Scholar] [CrossRef]
- Campos, A.P.C.; Geber, G.P.; Hurtado, R.; Sampaio, M.; Geber, S. Ovarian response after random-start controlled ovarian stimulation to cryopreserve oocytes in cancer patients. JBRA Assist. Reprod. 2018, 22, 352–354. [Google Scholar] [CrossRef]
- Ozcan, M.C.; Snegovskikh, V.; Adamson, G.D. Oocyte and embryo cryopreservation before gonadotoxic treatments: Principles of safe ovarian stimulation, a systematic review. Women’s Health 2022, 18. [Google Scholar] [CrossRef] [PubMed]
- Akel, R.A.; Guo, X.M.; Moravek, M.B.; Confino, R.; Smith, K.N.; Lawson, A.K.; Klock, S.C.; Tanner, E.J., III; Pavone, M.E. Ovarian Stimulation Is Safe and Effective for Patients with Gynecologic Cancer. J. Adolesc. Young-Adult Oncol. 2020, 9, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Taylan, E.; Oktay, K. Autologous Transplantation of Human Ovarian Tissue. In The Ovary, 3rd ed.; Leung, P., Adashi, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; p. 493500. [Google Scholar]
- Tamma, R.; Limongelli, L.; Maiorano, E.; Pastore, D.; Cascardi, E.; Tempesta, A.; Carluccio, P.; Mastropasqua, M.G.; Capodiferro, S.; Covelli, C.; et al. Vascular density and inflammatory infiltrate in primary oral squamous cell carcinoma and after allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2019, 98, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Terzic, M.; Aimagambetova, G.; Terzic, S.; Kongrtay, K.; Bapayeva, G.; Gullo, G. Chapter 7 Fertility Preservation Management for Ovarian Cancer. In Fertility Preservation in Gynecological Cancer: Current Management and Novel Insights; Nova Science Publishers: Hauppauge, NY, USA, 2021; pp. 183–219. ISBN 978-1-53619-179-0. [Google Scholar]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.H.; Kawamura, N.; Tamura, M.; Hashimoto, S.; et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.A.; Kubo, H.; Handa, N.; Hanna, M.; Laronda, M.M. A Systematic Review of Ovarian Tissue Transplantation Outcomes by Ovarian Tissue Processing Size for Cryopreservation. Front. Endocrinol. 2022, 13, 918899. [Google Scholar] [CrossRef] [PubMed]
- Fraison, E.; Huberlant, S.; Labrune, E.; Cavalieri, M.; Montagut, M.; Brugnon, F.; Courbiere, B. Live birth rate after female fertility preservation for cancer or haematopoietic stem cell transplantation: A systematic review and meta-analysis of the three main techniques; embryo, oocyte and ovarian tissue cryopreservation. Hum. Reprod. 2022, 24, 249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huo, L.; Zong, L.; Kong, Y.; Yang, J.; Xiang, Y. Oncological Outcomes and Safety of Ovarian Preservation for Early Stage Adenocarcinoma of Cervix: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 777. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Zhu, Y.; Lu, A.W.; Zhou, L.; Wang, J.S.; Zhang, Y.; Wang, J.T. The oncological and obstetric results of radical trachelectomy as a fertility-sparing therapy in early-stage cervical cancer patients. BMC Women’s Health 2022, 22, 424. [Google Scholar] [CrossRef]
- Kinjyo, Y.; Nana, Y.; Chinen, Y.; Kinjo, T.; Mekaru, K.; Aoki, Y. Transabdominal cerclage in early pregnancy for cervical shortening after radical trachelectomy: A case report. Case Rep. Women’s Health 2021, 31, e00323. [Google Scholar] [CrossRef]
- Kasuga, Y.; Miyakoshi, K.; Nishio, H.; Akiba, Y.; Otani, T.; Fukutake, M.; Ikenoue, S.; Ochiai, D.; Matsumoto, T.; Tanaka, K.; et al. Mid-trimester residual cervical length and the risk of preterm birth in pregnancies after abdominal radical trachelectomy: A retrospective analysis. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1729–1735. [Google Scholar] [CrossRef]
- Pareja, R.; Rendón, G.J.; Sanz-Lomana, C.M.; Monzón, O.; Ramirez, P.T. Surgical, oncological, and obstetrical outcomes after abdominal radical trachelectomy—A systematic literature review. Gynecol. Oncol. 2013, 131, 77–82. [Google Scholar] [CrossRef]
- Alvarez, R.M.; Biliatis, I.; Rockall, A.; Papadakou, E.; Sohaib, S.A.; DeSouza, N.M.; Butler, J.; Nobbenhuis, M.; Barton, D.J.P.; Shepherd, J.H.; et al. MRI measurement of residual cervical length after radical trachelectomy for cervical cancer and the risk of adverse pregnancy outcomes: A blinded imaging analysis. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1726–1733. [Google Scholar] [CrossRef]
- Harris-Glocker, M.; McLaren, J.F. Role of female pelvic anatomy in infertility. Clin. Anat. 2013, 26, 89–96. [Google Scholar] [CrossRef]
- Anderson, R.A.; Brewster, D.H.; Wood, R.; Nowell, S.; Fischbacher, C.; Kelsey, T.W.; Wallace, W.H.B. The impact of cancer on subsequent chance of pregnancy: A population-based analysis. Hum. Reprod. 2018, 33, 1281–1290. [Google Scholar] [CrossRef]
- Bentivegna, E.; Gouy, S.; Maulard, A.; Chargari, C.; Leary, A.; Morice, P. Oncological outcomes after fertility-sparing surgery for cervical cancer: A systematic review. Lancet Oncol. 2016, 17, e240–e253. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Roman, R.A.; Rambhatla, A.; Nezhat, F. Reproductive and oncologic outcomes after fertility-sparing surgery for early stage cervical cancer: A systematic review. Fertil. Steril. 2020, 113, 685–703. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, K.H.; Suk, H.J.; Chae, H.D.; Kang, B.M.; Kim, C.H. Successful pregnancy by direct intraperitoneal insemination in an infertile patient with failure of recanalization of isthmic stenosis after laparoscopic radical trachelectomy. Obstet. Gynecol. Sci. 2014, 57, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Jooya, N.D.; Woodard, T.L.; Ramirez, P.T.; Fleming, N.D.; Frumovitz, M. Reproductive counseling and pregnancy outcomes after radical trachelectomy for early stage cervical cancer. J. Gynecol. Oncol. 2019, 30, e45. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, L.; Li, J.; Chen, X.; Ju, X.; Wu, X. Reproductive and obstetric outcomes after abdominal radical trachelectomy (ART) for patients with early-stage cervical cancers in Fudan, China. Gynecol. Oncol. 2020, 157, 418–422. [Google Scholar] [CrossRef]
- Bernardini, M.; Barrett, J.; Seaward, G.; Covens, A. Pregnancy outcomes in patients after radical trachelectomy. Am. J. Obstet. Gynecol. 2003, 189, 1378–1382. [Google Scholar] [CrossRef]
- Nakajima, T.; Kasuga, A.; Hara-Yamashita, A.; Ikeda, Y.; Asai-Sato, M.; Nakao, T.; Hayashi, C.; Takeya, C.; Adachi, K.; Tsuruga, T.; et al. Reconstructed uterine length is critical for the prevention of cervical stenosis following abdominal trachelectomy in cervical cancer patients. J. Obstet. Gynaecol. Res. 2020, 46, 328–336. [Google Scholar] [CrossRef]
- Vieira, M.D.A.; de Araújo, R.L.C.; Andrade, C.E.M.D.C.; Schmidt, R.L.; Filho, A.L.; dos Reis, R. A randomized clinical trial of a new anti–cervical stenosis device after conization by loop electrosurgical excision. PLoS ONE 2021, 16, e0242067. [Google Scholar] [CrossRef] [PubMed]
- Hue, H.J.; Choi, H.J.; Park, J.Y.; Suh, D.H.; Lee, J.R.; Jee, B.C.; Kim, S.K. Successful pregnancy following transmyometrial embryo transfer after robot-assisted radical trachelectomy. Clin. Exp. Reprod. Med. 2021, 48, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Olympios, N.; Lequesne, J.; Calbrix, C.; Fontanilles, M.; Loeb, A.; Leheurteur, M.; Demeestere, I.; Di Fiore, F.; Perdrix, A.; et al. Impact of Taxanes, Endocrine Therapy, and Deleterious Germline BRCA Mutations on Anti-Müllerian Hormone Levels in Early Breast Cancer Patients Treated with Anthracycline- and Cyclophosphamide-Based Chemotherapy. Front. Oncol. 2019, 9, 575. [Google Scholar] [CrossRef]
- Wang, C.; Chen, M.; Fu, F.; Huang, M. Gonadotropin-Releasing Hormone Analog Cotreatment for the Preservation of Ovarian Function during Gonadotoxic Chemotherapy for Breast Cancer: A Meta-Analysis. PLoS ONE 2013, 8, e66360. [Google Scholar] [CrossRef] [PubMed]
- Black, K.Z.; Nichols, H.B.; Eng, E.; Rowley, D.L. Prevalence of preterm, low birthweight, and small for gestational age delivery after breast cancer diagnosis: A population-based study. Breast Cancer Res. 2017, 19, 11. [Google Scholar] [CrossRef]
- van de Loo, L.E.; Berg, M.H.V.D.; Overbeek, A.; van Dijk, M.; Damen, L.; Lambalk, C.B.; Ronckers, C.M.; Heuvel-Eibrink, M.M.V.D.; Kremer, L.C.; van der Pal, H.J.; et al. Uterine function, pregnancy complications, and pregnancy outcomes among female childhood cancer survivors. Fertil. Steril. 2019, 111, 372–380. [Google Scholar] [CrossRef]
- Muñoz, E.; Fernandez, I.; Martinez, M.; Tocino, A.; Portela, S.; Pellicer, A.; García-Velasco, J.A.; Garrido, N. Oocyte donation outcome after oncological treatment in cancer survivors. Fertil. Steril. 2015, 103, 205–213. [Google Scholar] [CrossRef]
- Teh, W.T.; Stern, C.; Chander, S.; Hickey, M. The Impact of Uterine Radiation on Subsequent Fertility and Pregnancy Outcomes. BioMed Res. Int. 2014, 2014, 482968. [Google Scholar] [CrossRef]
- Beneventi, F.; Locatelli, E.; Giorgiani, G.; Zecca, M.; Mina, T.; Simonetta, M.; Cavagnoli, C.; Albanese, M.; Spinillo, A. Adolescent and adult uterine volume and uterine artery Doppler blood flow among subjects treated with bone marrow transplantation or chemotherapy in pediatric age: A case-control study. Fertil. Steril. 2015, 103, 455–461. [Google Scholar] [CrossRef]
- Larsen, E.C.; Schmiegelow, K.; Rechnitzer, C.; Loft, A.; Müller, J.; Andersen, A.N. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet. Gynecol. Scand. 2004, 83, 96–102. [Google Scholar] [CrossRef]
- Jakobsson, M.; Gissler, M.; Tiitinen, A.; Paavonen, J.; Tapper, A.M. Treatment for cervical intraepithelial neoplasia and subsequent IVF deliveries. Hum. Reprod. 2008, 23, 2252–2255. [Google Scholar] [CrossRef]
- Wise, L.A.; Willis, S.K.; Perkins, R.B.; Wesselink, A.K.; Klann, A.; Crowe, H.M.; Hahn, K.A.; Mikkelsen, E.M.; Hatch, E.E. A prospective study of treatments for cervical intraepithelial neoplasia and fecundability. Am. J. Obstet. Gynecol. 2019, 223, 96.e1–96.e15. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, J.; Wang, M.; Li, Z.; Huang, B.; Zhu, L.; Xi, Q.; Jin, L. Early Cervical Lesions Affecting Ovarian Reserve and Reproductive Outcomes of Females in Assisted Reproductive Cycles. Front. Oncol. 2022, 12, 761219. [Google Scholar] [CrossRef]
- Tamauchi, S.; Kajiyama, H.; Osuka, S.; Moriyama, Y.; Yoshihara, M.; Kikkawa, F. Reduced response to controlled ovarian stimulation after radical trachelectomy: A pitfall of fertility-sparing surgery for cervical cancer. Int. J. Gynecol. Obstet. 2021, 154, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Fujii, T.; Sugiyama, J.; Kuji, N.; Tanaka, M.; Hamatani, T.; Miyakoshi, K.; Minegishi, K.; Tsuda, H.; Iwata, T.; et al. Reproductive and obstetric outcomes after radical abdominal trachelectomy for early-stage cervical cancer in a series of 31 pregnancies. Hum. Reprod. 2013, 28, 1798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tong, J.; Ma, X.; Yu, H.; Guan, X.; Li, J.; Yang, J. Evaluation of cervical length and optimal timing for pregnancy after cervical conization in patients with cervical intraepithelial neoplasia. Medicine 2020, 99, e23411. [Google Scholar] [CrossRef]
- Iwata, T.; Machida, H.; Matsuo, K.; Okugawa, K.; Saito, T.; Tanaka, K.; Morishige, K.; Kobayashi, H.; Yoshino, K.; Tokunaga, H.; et al. The validity of the subsequent pregnancy index score for fertility-sparing trachelectomy in early-stage cervical cancer. Fertil. Steril. 2021, 115, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Šimják, P.; Cibula, D.; Pařízek, A.; Sláma, J. Management of pregnancy after fertility-sparing surgery for cervical cancer. Acta Obstet. Gynecol. Scand. 2020, 99, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, S.; Ishioka, S.; Mariya, T.; Fujibe, Y.; Kim, M.; Someya, M.; Saito, T. Pregnancies after vaginal radical trachelectomy (RT) in patients with early invasive uterine cervical cancer: Results from a single institute. BMC Pregnancy Childbirth 2020, 20, 248. [Google Scholar] [CrossRef]
- Tesfai, F.M.; Kroep, J.R.; Gaarenstroom, K.; De Kroon, C.; Van Loenhout, R.; Smit, V.; Trimbos, B.; Nout, R.A.; van Poelgeest, M.I.E.; Beltman, J.J. Fertility-sparing surgery of cervical cancer >2 cm (International Federation of Gynecology and Obstetrics 2009 stage IB1–IIA) after neoadjuvant chemotherapy. Int. J. Gynecol. Cancer 2019, 30, 115–121. [Google Scholar] [CrossRef]
- Rendón, G.J.; Blanco, A.L.; Aragona, A.; Saadi, J.M.; Di Guilmi, J.; Eblen, C.A.; Muñoz, F.H.; Pareja, R. Oncological and obstetrical outcomes after neo-adjuvant chemotherapy followed by fertility-sparing surgery in patients with cervical cancer ≥2 cm. Int. J. Gynecol. Cancer 2020, 31, 462–467. [Google Scholar] [CrossRef]
- Fanfani, F.; Anchora, L.P.; Di Martino, G.; Bizzarri, N.; Di Meo, M.L.; Carbone, V.; Paderno, M.; Fedele, C.; Paniga, C.; Fagotti, A.; et al. Oncologic and obstetric outcomes after simple conization for fertility-sparing surgery in FIGO 2018 stage IB1 cervical cancer. Int. J. Gynecol. Cancer 2021, 31, 452–456. [Google Scholar] [CrossRef]
- Yamamoto, M.; Motohara, T.; Iwagoi, Y.; Tayama, S.; Tashiro, H.; Kondoh, E.; Katabuchi, H. Fertility-sparing surgery for early-stage cervical cancer: A case series study on the efficacy and feasibility of cervical conization followed by pelvic lymphadenectomy. J. Obstet. Gynaecol. Res. 2022, 48, 1444–1450. [Google Scholar] [CrossRef]
- Kasuga, Y.; Nishio, H.; Miyakoshi, K.; Sato, S.; Sugiyama, J.; Matsumoto, T.; Tanaka, K.; Ochiai, D.; Minegishi, K.; Hamatani, T.; et al. Pregnancy Outcomes After Abdominal Radical Trachelectomy for Early-Stage Cervical Cancer: A 13-Year Experience in a Single Tertiary-Care Center. Int. J. Gynecol. Cancer 2016, 26, 163–168. [Google Scholar] [CrossRef]
- Ekdahl, L.; Paraghamian, S.; Eoh, K.J.; Thumuluru, K.M.; Butler-Manuel, S.A.; Kim, Y.T.; Boggess, J.F.; Persson, J.; Falconer, H. Long term oncologic and reproductive outcomes after robot-assisted radical trachelectomy for early-stage cervical cancer. An international multicenter study. Gynecol. Oncol. 2021, 164, 529–534. [Google Scholar] [CrossRef]
- Tsaousidis, C.; Kraemer, B.; Kommoss, S.; Hartkopf, A.; Brucker, S.; Neis, K.; Andress, J.; Neis, F. Large Conization—Retrospective Monocentric Results for Fertility Preservation in Young Women with Early Stage Cervical Cancer. Reprod. Sci. 2021, 29, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Bevis, K.S.; Biggio, J.R. Cervical conization and the risk of preterm delivery. Am. J. Obstet. Gynecol. 2011, 205, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Robova, H.; Rob, L.; Halaska, M.J.; Pluta, M.; Skapa, P. Review of Neoadjuvant Chemotherapy and Trachelectomy: Which Cervical Cancer Patients Would Be Suitable for Neoadjuvant Chemotherapy Followed by Fertility-Sparing Surgery? Curr. Oncol. Rep. 2015, 17, 446. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Athanasiou, A.; Paraskevaidi, M.; Mitra, A.; Kalliala, I.; Martin-Hirsch, P.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: Systematic review and meta-analysis. BMJ 2016, 354, i3633. [Google Scholar] [CrossRef]
- Arbyn, M.; Kyrgiou, M.; Simoens, C.; Raifu, A.O.; Koliopoulos, G.; Martin-Hirsch, P.; Prendiville, W.; Paraskevaidis, E. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: Meta-analysis. BMJ 2008, 337, a1284. [Google Scholar] [CrossRef]
- Shepherd, J.; Spencer, C.; Herod, J.; Ind, T. Radical vaginal trachelectomy as a fertility-sparing procedure in women with early-stage cervical cancer-cumulative pregnancy rate in a series of 123 women. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 719–724. [Google Scholar] [CrossRef]
- Kim, C.H.; Abu-Rustum, N.R.; Chi, D.S.; Gardner, G.J.; Leitao, M.M., Jr.; Carter, J.; Barakat, R.R.; Sonoda, Y. Reproductive outcomes of patients undergoing radical trachelectomy for early-stage cervical cancer. Gynecol. Oncol. 2012, 125, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Petignat, P.; Stan, C.; Megevand, E.; Dargent, D. Pregnancy after trachelectomy: A high-risk condition of preterm delivery. Report of a case and review of the literature. Gynecol. Oncol. 2004, 94, 575–577. [Google Scholar] [CrossRef]
- Ishioka, S.; Endo, T.; Hayashi, T.; Baba, T.; Umemura, K.; Saito, T. Pregnancy-related complications after vaginal radical trachelectomy for early-stage invasive uterine cervical cancer. Int. J. Clin. Oncol. 2007, 12, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, L.; Eoh, K.J.; Thumuluru, K.M.; Butler-Manuel, S.A.; Kim, Y.T.; Lönnerfors, C.; Falconer, H.; Persson, J. A combination of second trimester oral metronidazole and no sexual intercourse during second and third trimester may reduce late miscarriage and premature delivery after fertility sparing radical trachelectomy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 265, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Maulard, A.; Scherier, S.; Sanson, C.; Zarokian, J.; Zaccarini, F.; Espenel, S.; Pautier, P.; Leary, A.; Genestie, C.; et al. Oncologic results of fertility sparing surgery of cervical cancer: An updated systematic review. Gynecol. Oncol. 2022, 165, 169–183. [Google Scholar] [CrossRef]
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef]
- Frazer, I.H. Cervical cancer vaccine development. Sex. Health 2010, 7, 230–234. [Google Scholar] [CrossRef]
- Ferrall, L.; Lin, K.Y.; Roden, R.B.; Hung, C.F.; Wu, T.C. Cervical Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2021, 27, 4953–4973. [Google Scholar] [CrossRef]
- Akhatova, A.; Azizan, A.; Atageldiyeva, K.; Ashimkhanova, A.; Marat, A.; Iztleuov, Y.; Suleimenova, A.; Shamkeeva, S.; Aimagambetova, G. Prophylactic Human Papillomavirus Vaccination: From the Origin to the Current State. Vaccines 2022, 10, 1912. [Google Scholar] [CrossRef]
- Aimagambetova, G.; Azizan, A. Human Papillomavirus Vaccination: Past, Present and Future. Vaccines 2022, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Kamolratanakul, S.; Pitisuttithum, P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Gardasil 9. Package Insert. Available online: https://www.fda.gov/media/90064/download (accessed on 13 October 2021).
- Food and Drug Administration. Cervarix Package Insert. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/cervarix (accessed on 6 December 2022).
- Food and Drug Administartion. Gardasil Package Insert. Available online: https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Package-Insert---Gardasil.pdf (accessed on 6 December 2022).
- Cecolin Wantai BioPharm Official Web. Available online: http://www.ystwt.cn/cecolin/ (accessed on 8 December 2022).
- Bei, L.; Zhang, X.; Meng, D.; Gao, S.; Jia, J.; Zhao, D.; Luo, C.; Li, X.; Qiu, H.; Xie, L. Immunogenicity correlation in cynomolgus monkeys between Luminex-based total IgG immunoassay and pseudovirion-based neutralization assay for a 14-valent recombinant human papillomavirus vaccine. J. Med. Virol. 2022, 94, 3946–3955. [Google Scholar] [CrossRef]
- Arbyn, M.; Xu, L. Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Rev. Vaccines 2018, 17, 1085–1091. [Google Scholar] [CrossRef]
- Jentschke, M.; Kampers, J.; Becker, J.; Sibbertsen, P.; Hillemanns, P. Prophylactic HPV vaccination after conization: A systematic review and meta-analysis. Vaccine 2020, 38, 6402–6409. [Google Scholar] [CrossRef]
- Zhuang, C.-L.; Lin, Z.-J.; Bi, Z.-F.; Qiu, L.-X.; Hu, F.-F.; Liu, X.-H.; Lin, B.-Z.; Su, Y.-Y.; Pan, H.-R.; Zhang, T.-Y.; et al. In-flammation-related adverse reactions following vaccination potentially indicate a stronger immune response. Emerg. Microbes Infect. 2021, 10, 365–375. [Google Scholar] [CrossRef]
- Castle, P.E.; Maza, M. Prophylactic HPV Vaccination: Past, present, and future. Epidemiol. Infect. 2016, 144, 449–468. [Google Scholar] [CrossRef]
- Brotherton, J.M. Impact of HPV vaccination: Achievements and future challenges. Papillomavirus Res. 2019, 7, 138–140. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, M.; Kim, K.; Kim, H.J.; Lee, K.H.; Kim, J.W. Major clinical research advances in gynecologic cancer in 2016: 10-year special edition. J. Gynecol. Oncol. 2017, 28, e45. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, C.M.; Skinner, S.R.; Del Rosario-Raymundo, M.R.; Garland, S.M.; Chatterjee, A.; Lazcano-Ponce, E.; Salmerón, J.; McNeil, S.; Stapleton, J.T.; Bouchard, C.; et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016, 16, 1154–1168. [Google Scholar] [CrossRef]
- Issanov, A.; Karim, M.E.; Aimagambetova, G.; Dummer, T.J.B. Does Vaccination Protect against Human Papillomavirus-Related Cancers? Preliminary Findings from the United States National Health and Nutrition Examination Survey (2011–2018). Vaccines 2022, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 2020, CD009069. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Arbyn, M.; Ronco, G.; Anttila, A.; Meijer, C.J.; Poljak, M.; Ogilvie, G.; Koliopoulos, G.; Naucler, P.; Sankaranarayanan, R.; Peto, J. Evidence Regarding Human Papillomavirus Testing in Secondary Prevention of Cervical Cancer. Vaccine 2012, 30, F88–F99. [Google Scholar] [CrossRef]
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination—Review of Current Perspectives. J. Oncol. 2019, 2019, 3257939. [Google Scholar] [CrossRef]
- Di Donato, V.; Caruso, G.; Petrillo, M.; Kontopantelis, E.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Muzii, L.; Panici, P.B.; et al. Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines 2021, 9, 410. [Google Scholar] [CrossRef]
- Sand, F.L.; Kjaer, S.K.; Frederiksen, K.; Dehlendorff, C. Risk of cervical intraepithelial neoplasia grade 2 or worse after conization in relation to HPV vaccination status. Int. J. Cancer 2019, 147, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Lichter, K.; Krause, D.; Xu, J.; Tsai, S.H.L.; Hage, C.; Weston, E.; Eke, A.; Levinson, K. Adjuvant Human Papillomavirus Vaccine to Reduce Recurrent Cervical Dysplasia in Unvaccinated Women: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2020, 135, 1070–1083. [Google Scholar] [CrossRef]
- Petrillo, M.; Dessole, M.; Tinacci, E.; Saderi, L.; Muresu, N.; Capobianco, G.; Cossu, A.; Dessole, S.; Sotgiu, G.; Piana, A. Efficacy of HPV Vaccination in Women Receiving LEEP for Cervical Dysplasia: A Single Institution’s Experience. Vaccines 2020, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Robles, C.; Díaz, M.; Arbyn, M.; Baussano, I.; Clavel, C.; Ronco, G.; Dillner, J.; Lehtinen, M.; Petry, K.-U.; et al. HPV-FASTER: Broadening the scope for prevention of HPV-related cancer. Nat. Rev. Clin. Oncol. 2015, 13, 119–132. [Google Scholar] [CrossRef]
- Yadav, R.; Zhai, L.; Tumban, E. Virus-like Particle-Based L2 Vaccines against HPVs: Where Are We Today? Viruses 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Zhai, L.; Kunda, N.; Muttil, P.; Tumban, E. Mixed Bacteriophage MS2-L2 VLPs Elicit Long-Lasting Protective Antibodies against HPV Pseudovirus 51. Viruses 2021, 13, 1113. [Google Scholar] [CrossRef] [PubMed]
- Rossi, I.; Spagnoli, G.; Buttini, F.; Sonvico, F.; Stellari, F.; Cavazzini, D.; Chen, Q.; Müller, M.; Bolchi, A.; Ottonello, S.; et al. A respirable HPV-L2 dry-powder vaccine with GLA as amphiphilic lubricant and immune-adjuvant. J. Control. Release 2021, 340, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, W.; Jin, L. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. 2020, 471, 88–102. [Google Scholar] [CrossRef]
- Akhatova, A.; Chan, C.K.; Azizan, A.; Aimagambetova, G. The Efficacy of Therapeutic DNA Vaccines Expressing the Human Papillomavirus E6 and E7 Oncoproteins for Treatment of Cervical Cancer: Systematic Review. Vaccines 2021, 10, 53. [Google Scholar] [CrossRef]
- Hancock, G.; Hellner, K.; Dorrell, L. Therapeutic HPV vaccines. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 47, 59–72. [Google Scholar] [CrossRef]
- Garbuglia, A.R.; Lapa, D.; Sias, C.; Capobianchi, M.R.; Del Porto, P. The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease. Front. Immunol. 2020, 11, 188. [Google Scholar] [CrossRef]
- Gardella, B.; Gritti, A.; Soleymaninejadian, E. New Perspectives in Therapeutic Vaccines for HPV: A Critical Review. Medicina 2022, 58, 860. [Google Scholar] [CrossRef]
| Author | Year | Study Design | Cohort Size, Patients (n) | Patients’ Age (Years) | Cervical Cancer FIGO Stage | Procedure | Main Findings | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Anderson et al. [57] | 2018 | Retrospective | 23,201 | ≤39 | early stages of cervical cancer | “Details of treatments received were not available” | Cancer was associated with a lower chance of pregnancy, adjusted HR 0.57 (95% CI: 0.53, 0.61) for women >5 years after cancer diagnosis. | The proportion of first singleton pregnancies after cancer that ended in a live birth was higher compared with the general population. |
| Shah et al. [61] | 2018 | Cross-sectional | 39 | 25–37 | IA1–IB1 | Vaginal radical trachelectomy | Significant proportion of women with early-stage CC do not receive adequate reproductive counseling before ART, and many women undergoing ART experience complications that can negatively impact their fertility. |
|
| Li et al. [62] | 2020 | Retrospective | 360 | 16–53 | IA1–IB1 | Surgery + adjuvant therapy | Cervical stenosis or fallopian tube obstruction led to a low pregnancy rate after ART following the fertility-sparing treatment for CC. |
|
| Shinkai et al. [83] | 2020 | Retrospective | 71 | 23–46 | IA2–IB1 | Vaginal radical trachelectomy + pelvic lymphadenectomy | Both the obstetrical prognosis and oncological prognosis after vaginal RT have become favorable for pregnant patients after vaginal RT. |
|
| Tesfai et al. [84] | 2020 | Retrospective | 19 | 19–36 | IB–IIA | Neoadjuvant chemotherapy + fertility-sparing surgery (trachelectomy) | Neoadjuvant chemotherapy with fertility-sparing surgery is a feasible and safe option in select patients CC IB–IIA. Unfavorable prognostic factors:
|
|
| Tamauchi et al. [78] | 2021 | Case-control | 14 patients and 30 controls | 29–40 | early stages of cervical cancer | Vaginal radical trachelectomy | The response to controlled ovarian stimulation worsens after radical trachelectomy. | Cancer survivors after radical trachelectomy had lower mean estradiol levels during controlled ovarian stimulation and a smaller number of retrieved oocytes, and a higher dosage of gonadotropins compared to the control group. |
| Rendón et al. [85] | 2021 | Retrospective | 23 | 20–37 | cervical cancer of ≥2 cm to ≤6 cm (IB1–IIA2) | Neo-adjuvant chemotherapy + fertility-sparing surgery (abdominal and vaginal radical trachelectomy) | Neo-adjuvant chemotherapy followed by abdominal or vaginal radical trachelectomy in early-stage CC is a good option for fertility sparing in well-selected patients with cervical tumors ≥2 cm. |
|
| Fanfani et al. [86] | 2021 | Retrospective | 42 | 19–44 | IA2–IB1 | Cervical conization and pelvic lymphadenectomy | Cervical conization is feasible for the conservative management of women with stage IB1 cervical cancer desiring fertility. |
|
| Yamamoto et al. [87] | 2022 | Retrospective | 42 | 28–36 | IA1–IB1 | Cervical conization followed by pelvic lymphadenectomy | Cervical conization combined with pelvic lymphadenectomy represents a feasible conservative management for well-selected patients with early-stage cervical cancer. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzic, M.; Makhadiyeva, D.; Bila, J.; Andjic, M.; Dotlic, J.; Aimagambetova, G.; Sarria-Santamera, A.; Laganà, A.S.; Chiantera, V.; Vukovic, I.; et al. Reproductive and Obstetric Outcomes after Fertility-Sparing Treatments for Cervical Cancer: Current Approach and Future Directions. J. Clin. Med. 2023, 12, 2614. https://doi.org/10.3390/jcm12072614
Terzic M, Makhadiyeva D, Bila J, Andjic M, Dotlic J, Aimagambetova G, Sarria-Santamera A, Laganà AS, Chiantera V, Vukovic I, et al. Reproductive and Obstetric Outcomes after Fertility-Sparing Treatments for Cervical Cancer: Current Approach and Future Directions. Journal of Clinical Medicine. 2023; 12(7):2614. https://doi.org/10.3390/jcm12072614
Chicago/Turabian StyleTerzic, Milan, Dinara Makhadiyeva, Jovan Bila, Mladen Andjic, Jelena Dotlic, Gulzhanat Aimagambetova, Antonio Sarria-Santamera, Antonio Simone Laganà, Vito Chiantera, Ivana Vukovic, and et al. 2023. "Reproductive and Obstetric Outcomes after Fertility-Sparing Treatments for Cervical Cancer: Current Approach and Future Directions" Journal of Clinical Medicine 12, no. 7: 2614. https://doi.org/10.3390/jcm12072614
APA StyleTerzic, M., Makhadiyeva, D., Bila, J., Andjic, M., Dotlic, J., Aimagambetova, G., Sarria-Santamera, A., Laganà, A. S., Chiantera, V., Vukovic, I., Kocijancic Belovic, D., Aksam, S., Bapayeva, G., & Terzic, S. (2023). Reproductive and Obstetric Outcomes after Fertility-Sparing Treatments for Cervical Cancer: Current Approach and Future Directions. Journal of Clinical Medicine, 12(7), 2614. https://doi.org/10.3390/jcm12072614










