Prediction of Fluid Responsiveness Using Combined End-Expiratory and End-Inspiratory Occlusion Tests in Cardiac Surgical Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Haemodynamic Monitoring
2.3. Mechanical Ventilation
2.4. Study Design
2.5. Statistical Analysis
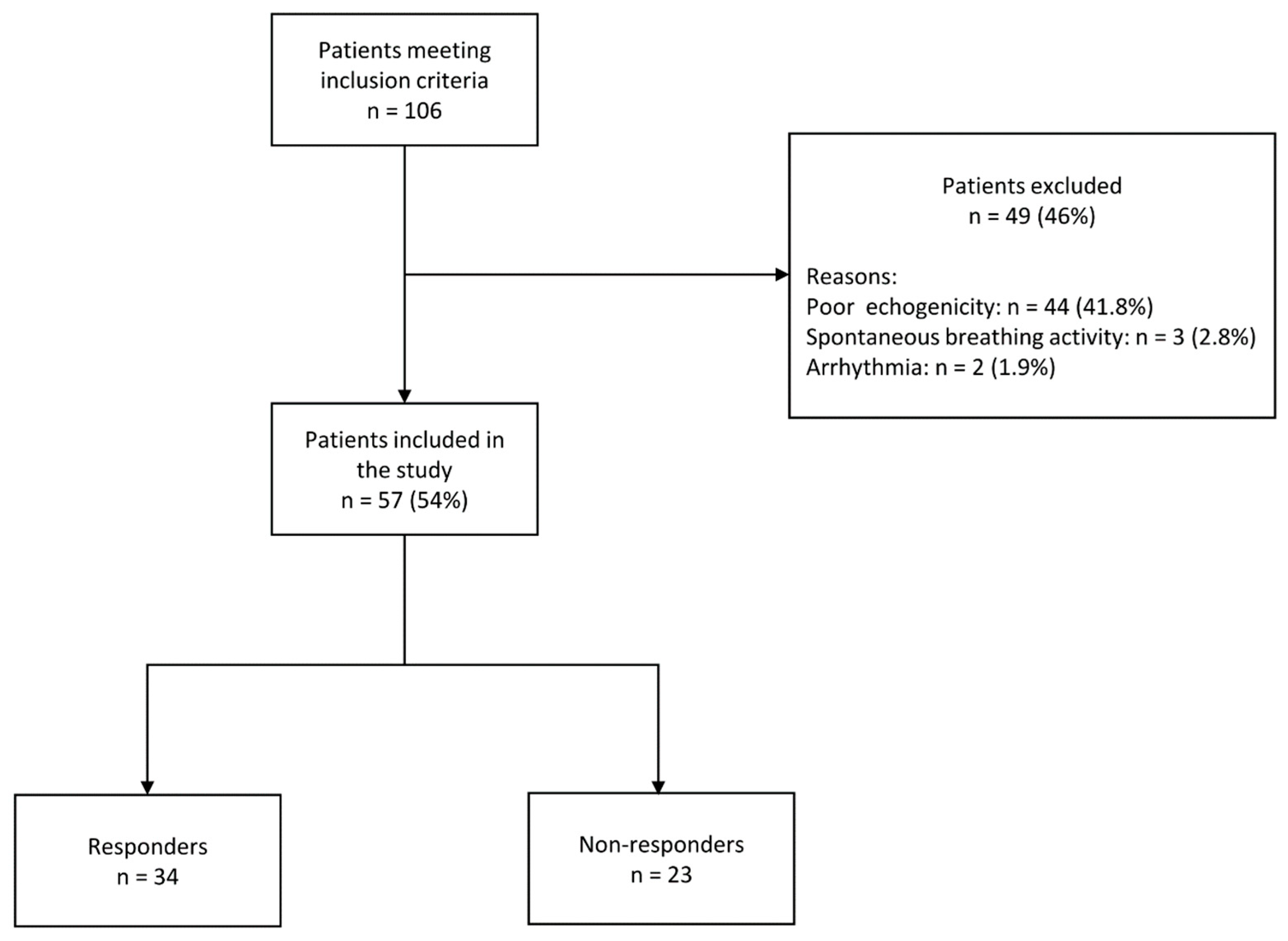
3. Results
3.1. Patient Characteristics
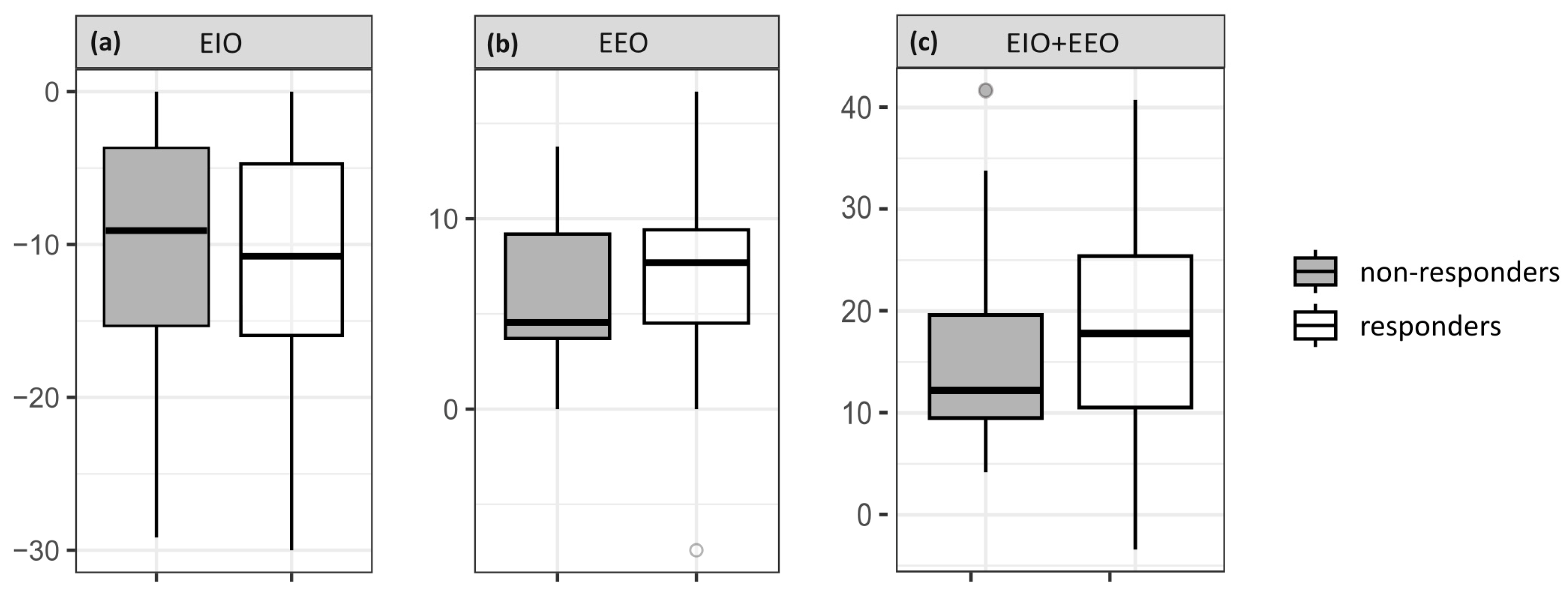
3.2. Hemodynamic Effects of EEO, EIO and Volume Expansion
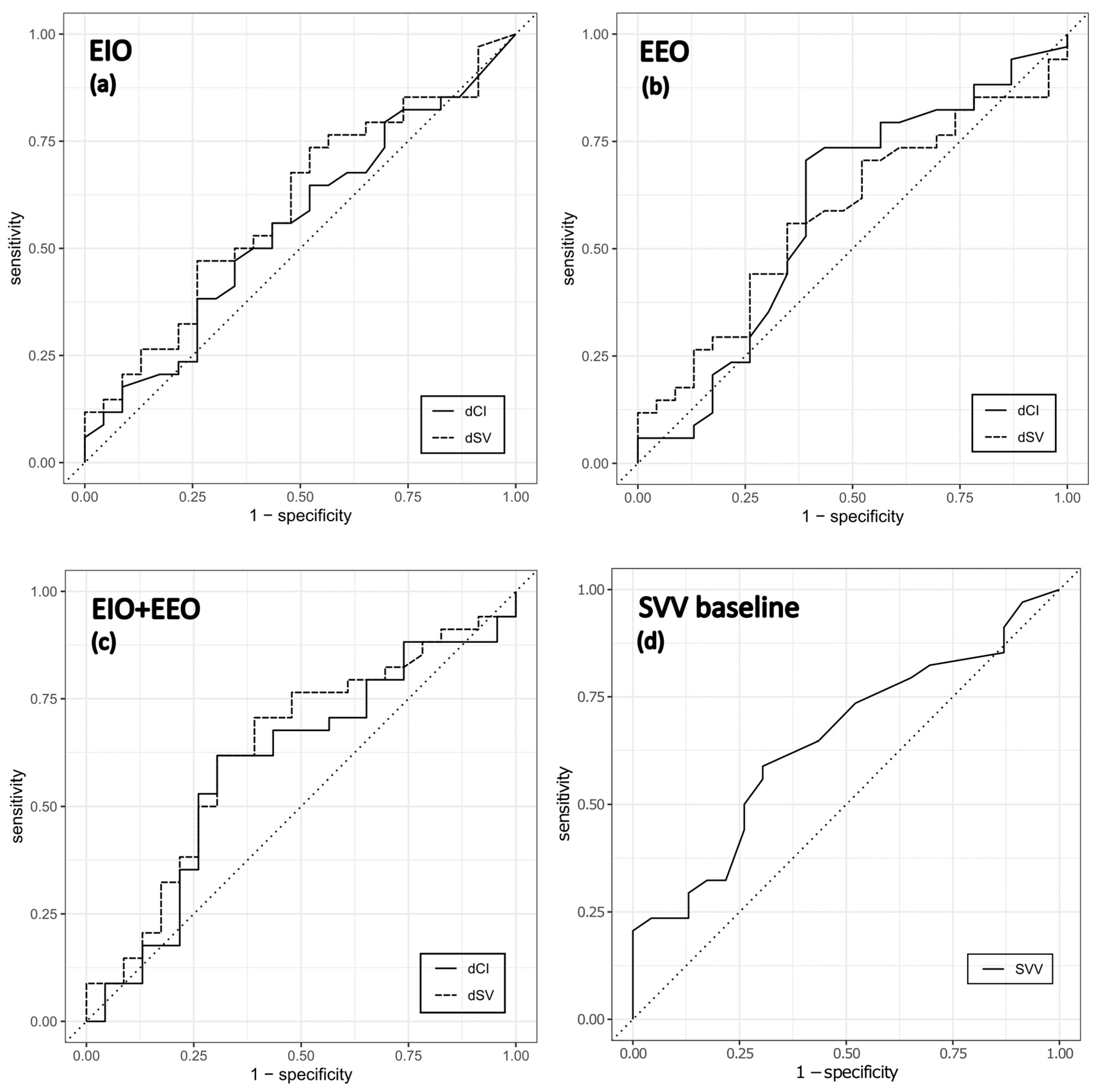
3.3. Prediction of Fluid Responsiveness
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Messina, A.; Longhini, F.; Coppo, C.; Pagni, A.; Lungu, R.; Ronco, C.; Cattaneo, M.A.; Dore, S.; Sotgiu, G.; Navalesi, P. Use of the fluid challenge in critically ill adult patients: A systematic review. Anesth. Analg. 2017, 125, 1532–1543. [Google Scholar] [CrossRef]
- Messina, A.; Pelaia, C.; Bruni, A.; Garofalo, E.; Bonicolini, E.; Longhini, F.; Dellara, E.; Saderi, L.; Romagnoli, S.; Sotgiu, G.; et al. Fluid challenge during anesthesia: A systematic review and meta-analysis. Anesth. Analg. 2018, 127, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, H.; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Hayden, D.; Deboisblanc, B.; Connors, A.F.J.; Hite, R.D.; Harabin, A.L. Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, A.; Vincent, J.L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit. Care 2015, 19, 251. [Google Scholar] [CrossRef]
- Thacker, J.K.; Mountford, W.K.; Ernst, F.R.; Krukas, M.R.; Mythen, M.M.G. Perioperative fluid utilization variability and association with outcomes. Ann. Surg. 2016, 263, 502–510. [Google Scholar] [CrossRef]
- Marik, P.E.; Cavallazzi, R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit. Care Med. 2013, 41, 1774–1781. [Google Scholar] [CrossRef]
- Osman, D.; Osman, D.; Ridel, C.; Ray, P.; Monnet, X.; Anguel, N.; Richard, C.; Teboul, J.L. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit. Care Med. 2007, 35, 64–68. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.E.; Teboul, J.-L. Prediction of fluid responsiveness: An update. Ann. Intensive Care 2016, 6, 111. [Google Scholar] [CrossRef]
- Mahjoub, Y.; Lejeune, V.; Muller, L.; Perbet, S.; Zieleskiewicz, L.; Bart, F.; Veber, B.; Paugam-Burtz, C.; Jaber, S.; Ayham, A.; et al. Evaluation of pulse pressure variation validity criteria in critically ill patients: A prospective observational multicentre point-prevalence study. Br. J. Anaesth. 2014, 112, 681–685. [Google Scholar] [CrossRef]
- Cherpanath, T.G.; Hirsch, A.; Geerts, B.F.; Lagrand, W.K.; Leeflang, M.M.; Schultz, M.J.; Groeneveld, A.B. Predicting Fluid Responsiveness by Passive Leg Raising: A Systematic Review and Meta-Analysis of 23 Clinical Trials. Crit. Care Med. 2016, 44, 981–991. [Google Scholar] [CrossRef]
- Monnet, X.; Osman, D.; Ridel, C.; Lamia, B.; Richard, C.; Teboul, J.L. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit. Care Med. 2009, 37, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, M.; Depret, F.; Teboul, J.L.; Alphonsine, J.E.; Lai, C.; Richard, C.; Monnet, X. Predicting Fluid Responsiveness in Critically Ill Patients by Using Combined End-Expiratory and End-Inspiratory Occlusions with Echocardiography. Crit. Care Med. 2017, 45, e1131–e1138. [Google Scholar] [CrossRef]
- Depret, F.; Jozwiak, M.; Teboul, J.L.; Alphonsine, J.E.; Richard, C.; Monnet, X. Esophageal Doppler Can Predict Fluid Responsiveness Through End-Expiratory and End-Inspiratory Occlusion Tests. Crit. Care Med. 2019, 47, e96–e102. [Google Scholar] [CrossRef] [PubMed]
- Gavelli, F.; Shi, R.; Teboul, J.-L.; Azzolina, D.; Monnet, X. The end-expiratory occlusion test for detecting preload responsiveness: A systematic review and meta-analysis. Ann. Intensive Care 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Jozwiak, M.; Teboul, J.L.; Persichini, R.; Richard, C.; Monnet, X. End-expiratory occlusion test predicts preload responsiveness independently of positive end-expiratory pressure during acute respiratory distress syndrome. Crit. Care Med. 2013, 41, 1692–1701. [Google Scholar] [CrossRef]
- Myatra, S.N.; Prabu, N.R.; Divatia, J.V.; Monnet, X.; Kulkarni, A.P.; Teboul, J.L. The Changes in Pulse Pressure Variation or Stroke Volume Variation After a “Tidal Volume Challenge” Reliably Predict Fluid Responsiveness During Low Tidal Volume Ventilation. Crit. Care Med. 2017, 45, 415–421. [Google Scholar] [CrossRef]
- Biais, M.; Larghi, M.; Henriot, J.; de Courson, H.; Sesay, M.; Nouette-Gaulain, K. End-Expiratory Occlusion Test Predicts Fluid Responsiveness in Patients With Protective Ventilation in the Operating Room. Anesth. Analg. 2017, 125, 1889–1895. [Google Scholar] [CrossRef]
- Georges, D.; de Courson, H.; Lanchon, R.; Sesay, M.; Nouette-Gaulain, K.; Biais, M. End-expiratory occlusion maneuver to predict fluid responsiveness in the intensive care unit: An echocardiographic study. Crit. Care 2018, 22, 32. [Google Scholar] [CrossRef]
- Messina, A.; Montagnini, C.; Cammarota, G.; De Rosa, S.; Giuliani, F.; Muratore, L.; Della Corte, F.; Navalesi, P.; Cecconi, M. Tidal volume challenge to predict fluid responsiveness in the operating room: An observational study. Eur. J. Anaesthesiol. 2019, 36, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Y.; Tu, G.W.; Cang, J.; Hou, J.Y.; Yu, Y.; Luo, Z.; Guo, K.F. End-expiratory occlusion test predicts fluid responsiveness in cardiac surgical patients in the operating theatre. Ann. Transl. Med. 2019, 7, 315. [Google Scholar] [CrossRef]
- Monnet, X.; Bleibtreu, A.; Ferre, A.; Dres, M.; Gharbi, R.; Richard, C.; Teboul, J.L. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit. Care Med. 2012, 40, 152–157. [Google Scholar] [CrossRef]
- Beurton, A.; Teboul, J.-L.; Girotto, V.; Galarza, L.; Anguel, N.; Richard, C.; Monnet, X. Intra-abdominal hypertension is responsible for false negatives to the passive leg raising test. Crit. Care Med. 2019, 47, e639–e647. [Google Scholar] [CrossRef]
- Guinot, P.G.; Godart, J.; de Broca, B.; Bernard, E.; Lorne, E.; Dupont, H. End-expiratory occlusion manoeuvre does not accurately predict fluid responsiveness in the operating theatre. Br. J. Anaesth. 2014, 112, 1050–1054. [Google Scholar] [CrossRef]
- R Development Core Team, R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 15 September 2022).
- RStudio Team, RStudio: Integrated Development Environment for R. 2022. Available online: http://www.rstudio.com/ (accessed on 15 September 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Thiele, C.; Hirschfeld, G. Cutpointr: Improved Estimation and Validation of Optimal Cutpoints in R. J. Stat. Softw. 2021, 98, 1–27. [Google Scholar] [CrossRef]
- Flynn, B.C.; Spellman, J.; Bodian, C.; Moitra, V.K. Inadequate visualization and reporting of ventricular function from transthoracic echocardiography after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2010, 24, 280–284. [Google Scholar] [CrossRef]
- Beaulieu, Y.; Marik, P.E. Bedside ultrasonography in the ICU: Part 1. Chest 2005, 128, 881–895. [Google Scholar] [CrossRef]
- Lamia, B.; Ochagavia, A.; Monnet, X.; Chemla, D.; Richard, C.; Teboul, J.L. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007, 33, 1125–1132. [Google Scholar] [CrossRef]
- Hadian, M.; Kim, H.K.; Severyn, D.A.; Pinsky, M.R. Cross-comparison of cardiac output trending accuracy of LiDCO, PiCCO, FloTrac and pulmonary artery catheters. Crit. Care 2010, 14, R212. [Google Scholar] [CrossRef] [PubMed]
- Fougères, E.; Teboul, J.-L.; Richard, C.; Osman, D.; Chemla, D.; Monnet, X. Hemodynamic impact of a positive end-expiratory pressure setting in acute respiratory distress syndrome: Importance of the volume status. Crit. Care Med. 2010, 38, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Thiele, R.H.; Bartels, K.; Gan, T.J. Cardiac output monitoring: A contemporary assessment and review. Crit. Care Med. 2015, 43, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, O.; Monnet, X.; Richard, C.; Osman, D.; Chemla, D.; Teboul, J.-L. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit. Care Med. 2008, 36, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Slagt, C.; Malagon, I.; Groeneveld, A.B. Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation. Br. J. Anaesth. 2014, 112, 626–637. [Google Scholar] [CrossRef]
- Lamia, B.; Kim, H.K.; Severyn, D.A.; Pinsky, M.R. Cross-comparisons of trending accuracies of continuous cardiac-output measurements: Pulse contour analysis, bioreactance, and pulmonary-artery catheter. J. Clin. Monit. Comput. 2018, 32, 33–43. [Google Scholar] [CrossRef]
- Monnet, X.; Anguel, N.; Naudin, B.; Jabot, J.; Richard, C.; Teboul, J.L. Arterial pressure-based cardiac output in septic patients: Different accuracy of pulse contour and uncalibrated pressure waveform devices. Crit. Care 2010, 14, R109. [Google Scholar] [CrossRef]
- De Backer, D.; Marx, G.; Tan, A.; Junker, C.; Van Nuffelen, M.; Hüter, L.; Ching, W.; Michard, F.; Vincent, J.L. Arterial pressure-based cardiac output monitoring: A multicenter validation of the third-generation software in septic patients. Intensive Care Med. 2011, 37, 233–240. [Google Scholar] [CrossRef]
- Monnet, X.; Anguel, N.; Jozwiak, M.; Richard, C.; Teboul, J.L. Third-generation FloTrac/Vigileo does not reliably track changes in cardiac output induced by norepinephrine in critically ill patients. Br. J. Anaesth. 2012, 108, 615–622. [Google Scholar] [CrossRef]
- Suehiro, K.; Tanaka, K.; Mikawa, M.; Uchihara, Y.; Matsuyama, T.; Matsuura, T.; Funao, T.; Yamada, T.; Mori, T.; Nishikawa, K. Improved Performance of the Fourth-Generation FloTrac/Vigileo System for Tracking Cardiac Output Changes. J. Cardiothorac. Vasc. Anesth. 2015, 29, 656–662. [Google Scholar] [CrossRef]
- Ji, F.; Li, J.; Fleming, N.; Rose, D.; Liu, H. Reliability of a new 4th generation FloTrac algorithm to track cardiac output changes in patients receiving phenylephrine. J. Clin. Monit. Comput. 2015, 29, 467–473. [Google Scholar] [CrossRef]
- Kaufmann, T.; Clement, R.P.; Hiemstra, B.; Vos, J.J.; Scheeren, T.W.L.; Keus, F.; van der Horst, I.C.C.; Koster, G.; Keus, F.; van der Horst, I.C.C.; et al. Disagreement in cardiac output measurements between fourth-generation FloTrac and critical care ultrasonography in patients with circulatory shock: A prospective observational study. J. Intensive Care 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Gavelli, F.; Teboul, J.L.; Monnet, X. The end-expiratory occlusion test: Please, let me hold your breath! Crit. Care 2019, 23, 274. [Google Scholar] [CrossRef] [PubMed]
- Pratt, B.; Roteliuk, L.; Hatib, F.; Frazier, J.; Wallen, R.D. Calculating Arterial Pressure-Based Cardiac Output Using a Novel Measurement and Analysis Method. Biomed. Instrum. Amp Technol. 2007, 41, 403–411. [Google Scholar] [CrossRef]
| Responders n = 34 | Non-Responders n = 23 | p-Value | |
|---|---|---|---|
| Age (years) | 65 ± 9 | 66 ± 8 | 0.3 |
| Sex (male/female) | 26/8 | 19/4 | 0.7 |
| BMI | 30.5 (26.4–32.7) | 28.7 (25.7–32.9) | 0.4 |
| EuroSCORE II (%) | 0.89 (0.68–1.47) | 1.25 (0.91–1.62) | 0.04 |
| Hypertension, n | 32 (97%) | 23 (100%) | >0.9 |
| Diabetes mellitus, n | 10 (29%) | 7 (30%) | >0.9 |
| COPD, n | 7 (21%) | 2 (9%) | 0.3 |
| PVD, n | 6 (18%) | 8 (35%) | 0.2 |
| Renal insufficiency, n | 1 (3%) | 2 (9%) | 0.6 |
| LV EF (%) | 61 ± 5.7 | 61 ± 7.5 | >0.9 |
| RV FAC (%) | 47.2 ± 10.5 | 45.1 ± 10.9 | 0.5 |
| Use of CPB, n (%) | 4 (12%) | 2 (9%) | >0.9 |
| Norepinephrine support, n | 19 (56%) | 16 (70%) | 0.6 |
| Norepinephrine dose (µg/kg/min) | 0.02 (0–0.07) | 0.04 (0.01–0.08) | 0.3 |
| Mechanical ventilation parameters | |||
| Tidal volume (mL) | 530 (500–568) | 520 (500–560) | 0.7 |
| Respiratory rate (breaths/minute) | 12 (12–12) | 12 (12–12) | 0.3 |
| PEEP (cm H2O) | 5 (5–5) | 5 (5–5) | 0.6 |
| Peak inspiratory pressure (cm H2O) | 17 (16–20) | 17 (15.5–20) | >0.9 |
| Static compliance (mL/cm H2O) | 56 ± 15 | 52 ± 10 | 0.2 |
| Baseline 1 | EIO | Baseline 2 | EEO | Baseline 3 | FC | |
|---|---|---|---|---|---|---|
| HR (min−1) | ||||||
| responders | 74.2 ± 13.6 | 74.6 ± 13.9 | 74.2 ± 14 | 73.7 ± 14 | 74.6 ± 13.6 | 72.4 ± 12.9 |
| non-responders | 74 ± 13.4 | 73.9 ± 13.9 | 73.8 ± 13.5 | 73.4 ± 13.4 | 74.3 ± 13.5 | 72.5 ± 13.5 |
| MAP (mmHg) | ||||||
| responders | 74.6 ± 8.5 | 71.4 ± 9.5 | 74.7 ± 8.7 | 76.1 ± 9.7 | 75.6 ± 7.6 | 82.2 ± 11.8 |
| non-responders | 73.5 ± 8.6 | 71.8 ± 10.5 | 72.4 ± 8 | 74 ± 8.4 | 72.8 ± 7.4 | 78.4 ± 11.5 |
| CVP (mmHg) | ||||||
| responders | 5.4 ± 2.4 | 6.4 ± 2.6 | 5.3 ± 2.2 | 4.6 ± 2.3 | 5.4 ± 2.2 | 7.2 ± 2.3 |
| non-responders | 6.4 ± 2.4 | 7.3 ± 3 | 6.2 ± 2.6 | 6.2 ± 3.1 | 6.3 ± 2.7 | 7.9 ± 2.8 |
| CI (L/min/m2) | ||||||
| responders | 2.5 ± 0.5 | 2.2 ± 0.6 | 2.5 ± 0.5 | 2.6 ± 0.6 | 2.4 ± 0.6 | 3.1 ± 0.7 |
| non-responders | 2.8 ± 0.7 | 2.5 ± 0.7 | 2.8 ± 0.7 | 2.9 ± 0.8 | 2.9 ± 0.8 a | 3.1 ± 0.8 |
| SV (mL) | ||||||
| responders | 66.9 ± 16.6 | 58.4 ± 16.3 | 66.7 ± 16.4 | 71.4 ± 15.9 | 66.7 ± 16.7 | 84.7 ± 16.9 |
| non-responders | 76.6 ± 25.7 | 69.9 ± 26.5 | 75.7 ± 24.9 | 80.3 ± 25.5 | 78.2 ± 26.8 | 86.2 ± 27.4 |
| SVV (%) | ||||||
| responders | 17.1 ± 7.9 | 16.8 ± 7 | 17.1 ± 8.3 | 10.5 ± 6.8 | 16.6 ± 8.3 | 7.6 ± 3.1 |
| non-responders | 12.9 ± 5.1 a | 12.8 ± 6.1 a | 12.9 ± 5.5 a | 8.2 ± 6.2 | 12.3 ± 5.1 | 6.6 ± 2.7 |
| EIO | EEO | EIO + EEO | FC | |
|---|---|---|---|---|
| ΔHeart rate (%) | ||||
| responders | 1 ± 3 | −1 ± 2 | −1 ± 4 | −2 ± 6 |
| non-responders | 0 ± 3 | −1 ± 2 | 0 ± 4 | −3 ± 6 |
| ΔMAP (%) | ||||
| responders | −4 ± 7 | 2 ± 5 | 3 ± 9 | 9 ± 13 |
| non-responders | −3 ± 6 | 2 ± 4 | 3 ± 7 | 8 ± 14 |
| ΔCVP (%) | ||||
| responders | 19 ± 26 | −16 ± 24 | −34 ± 38 | 36 ± 30 |
| non-responders | 14 ± 23 | −5 ± 24 | −18 ± 36 | 48 ± 90 a |
| ΔCI (%) | ||||
| responders | −12 ± 9 | 7 ± 4 | 19 ± 11 | 26 ± 8 |
| non-responders | −10 ± 8 | 6 ± 4 | 16 ± 10 | 7 ± 4 a |
| ΔSV (%) | ||||
| responders | −13 ± 9 | 8 ± 6 | 20 ± 12 | 29 ± 13 |
| non-responders | −10 ± 8 | 7 ± 4 | 16 ± 10 | 11 ± 7 a |
| ΔSVV (%) | ||||
| responders | 2 ± 19 | −31 ± 42 | 33 ± 40 | −50 ± 20 |
| non-responders | 0 ± 24 | −34 ± 45 | 34 ± 53 | −33 ± 55 |
| Independent Variable | OR (95% Confidence Interval) | AUC | p-Value |
|---|---|---|---|
| ΔCI (%) during EIO | 0.98 (0.92, 1.04) | 0.55 | 0.5 |
| ΔSV (%) during EIO | 0.96 (0.89, 1.02) | 0.60 | 0.2 |
| ΔCI (%) during EEO | 1.06 (0.93, 1.12) | 0.59 | 0.4 |
| ΔSV (%) during EEO | 1.04 (0.94, 1.17) | 0.58 | 0.4 |
| ΔCI (%) during EIO + EEO | 1.03 (0.97, 1.084) | 0.59 | 0.4 |
| ΔSV (%) during EIO + EEO | 1.04 (0.99, 1.096) | 0.63 | 0.2 |
| Baseline SVV | 1.1 (1.01, 1.23) | 0.65 | 0.05 |
| Threshold | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ROC AUC | |
|---|---|---|---|---|---|---|
| ΔCI | ||||||
| EIO | −8.3 | 64.7 | 47.8 | 55.3 | 57.5 | 0.552 |
| EEO | 5.3 | 70.6 | 60.9 | 64.3 | 67.4 | 0.590 |
| EIO + EEO | 16.7 | 61.8 | 69.6 | 67 | 65 | 0.593 |
| ΔSV | ||||||
| EIO | −6 | 73.5 | 47.8 | 58.4 | 64.3 | 0.598 |
| EEO | 7 | 55.9 | 65.2 | 61.6 | 59.7 | 0.580 |
| EIO + EEO | 15.8 | 70.6 | 60.9 | 64.3 | 67.4 | 0.631 |
| SVV | ||||||
| baseline | 14 | 58.8 | 69.6 | 65.9 | 62.8 | 0.645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horejsek, J.; Balík, M.; Kunstýř, J.; Michálek, P.; Brožek, T.; Kopecký, P.; Fink, A.; Waldauf, P.; Pořízka, M. Prediction of Fluid Responsiveness Using Combined End-Expiratory and End-Inspiratory Occlusion Tests in Cardiac Surgical Patients. J. Clin. Med. 2023, 12, 2569. https://doi.org/10.3390/jcm12072569
Horejsek J, Balík M, Kunstýř J, Michálek P, Brožek T, Kopecký P, Fink A, Waldauf P, Pořízka M. Prediction of Fluid Responsiveness Using Combined End-Expiratory and End-Inspiratory Occlusion Tests in Cardiac Surgical Patients. Journal of Clinical Medicine. 2023; 12(7):2569. https://doi.org/10.3390/jcm12072569
Chicago/Turabian StyleHorejsek, Jan, Martin Balík, Jan Kunstýř, Pavel Michálek, Tomáš Brožek, Petr Kopecký, Adam Fink, Petr Waldauf, and Michal Pořízka. 2023. "Prediction of Fluid Responsiveness Using Combined End-Expiratory and End-Inspiratory Occlusion Tests in Cardiac Surgical Patients" Journal of Clinical Medicine 12, no. 7: 2569. https://doi.org/10.3390/jcm12072569
APA StyleHorejsek, J., Balík, M., Kunstýř, J., Michálek, P., Brožek, T., Kopecký, P., Fink, A., Waldauf, P., & Pořízka, M. (2023). Prediction of Fluid Responsiveness Using Combined End-Expiratory and End-Inspiratory Occlusion Tests in Cardiac Surgical Patients. Journal of Clinical Medicine, 12(7), 2569. https://doi.org/10.3390/jcm12072569








