Endothelial Function Is Preserved in Patients with Wild-Type Transthyretin Amyloid Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Protocol
2.3. Measurements of FMD and NID
2.4. Measurement of Brachial IMT
2.5. Measurement of baPWV
2.6. Echocardiography
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
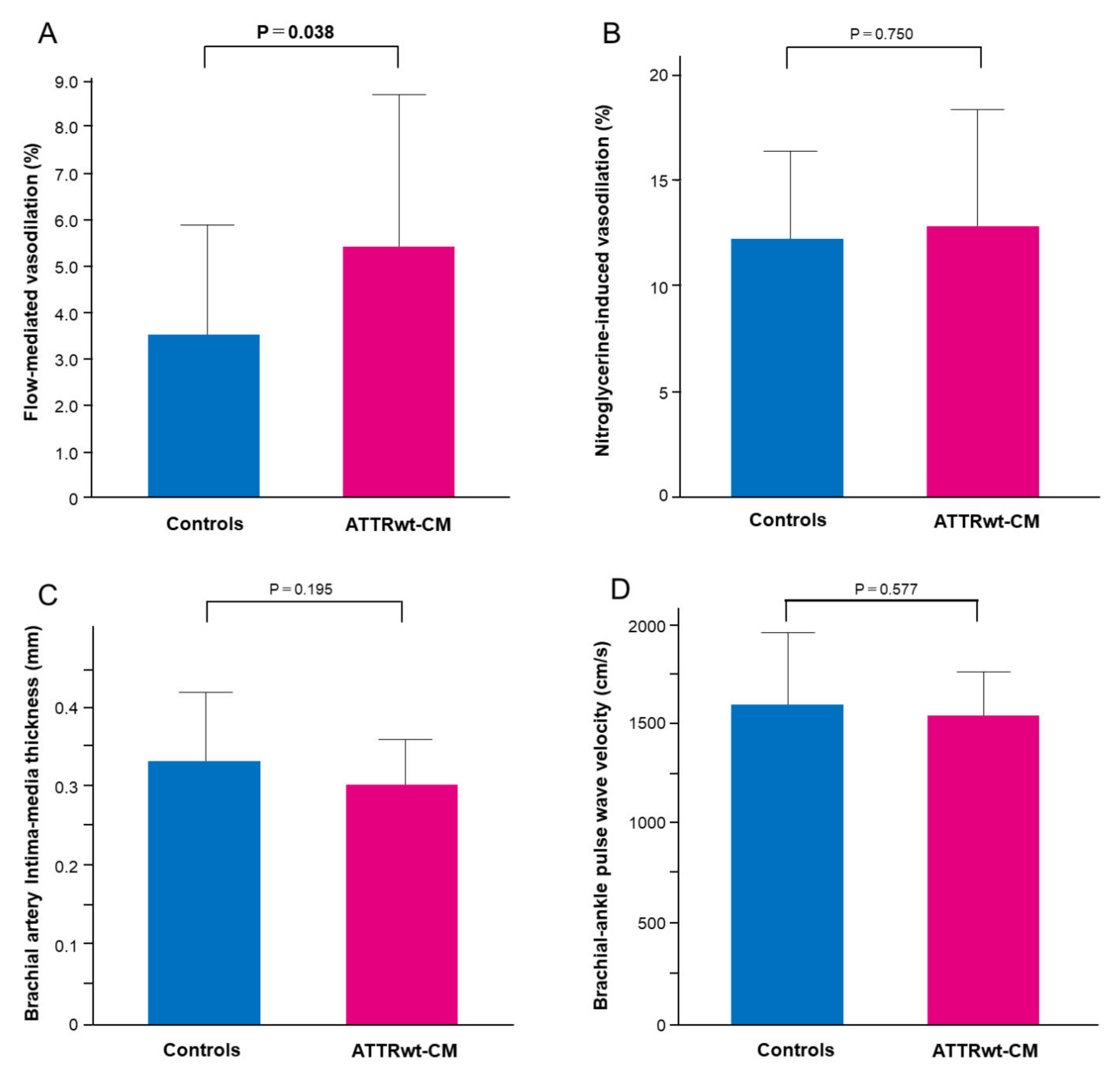
3.2. Vascular Function
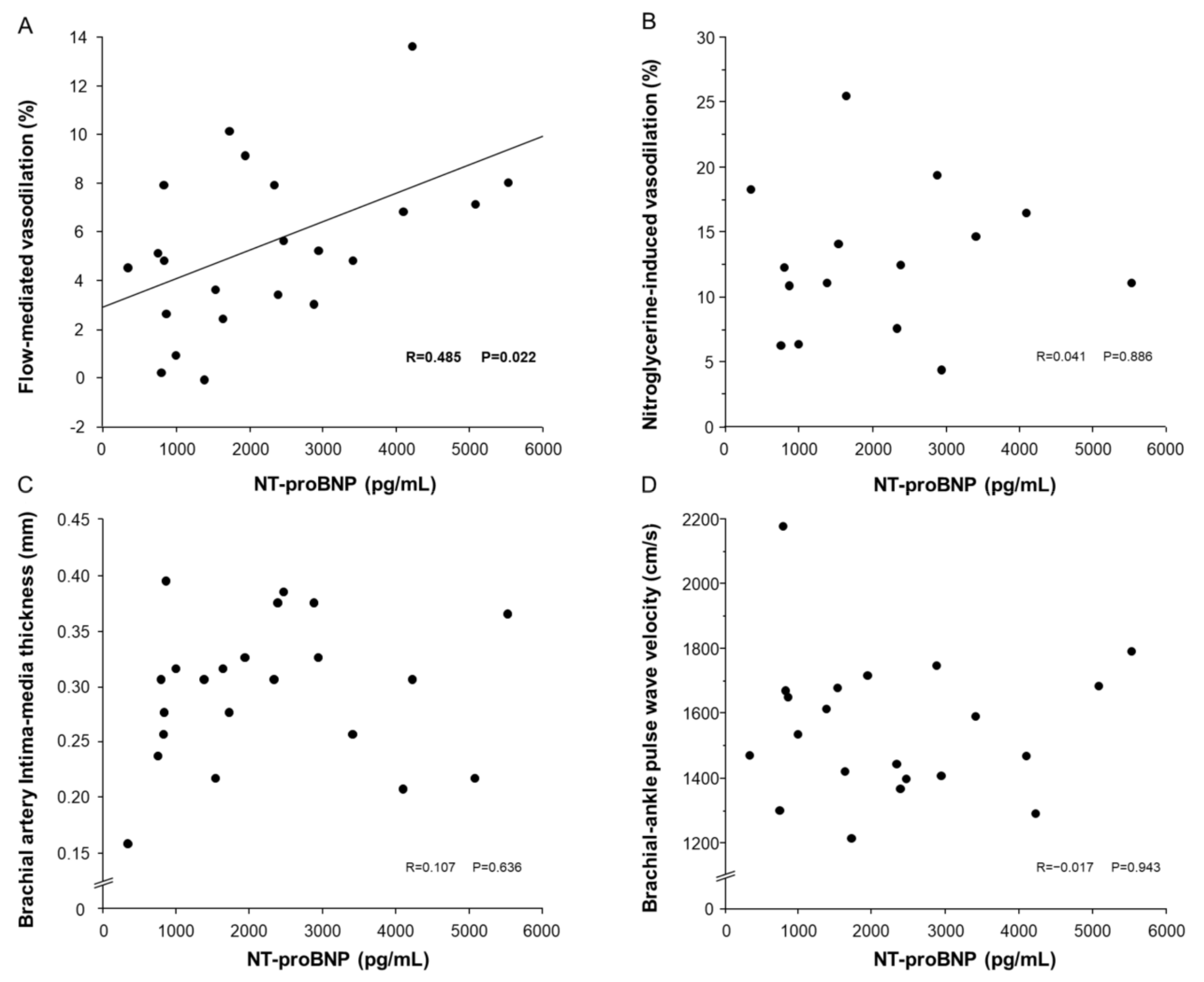
3.3. Relationships of NT-proBNP with FMD, NID, bIMT and baPWV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Benson, M.D.; Dyck, P.J.; Grogan, M.; Coelho, T.; Cruz, M.; Berk, J.L.; Plante-Bordeneuve, V.; Schmidt, H.H.; Merlini, G. Diagnosis, Prognosis, and Therapy of Transthyretin Amyloidosis. J. Am. Coll. Cardiol. 2015, 66, 2451–2466. [Google Scholar] [CrossRef]
- Lane, T.; Fontana, M.; Martinez-Naharro, A.; Quarta, C.C.; Whelan, C.J.; Petrie, A.; Rowczenio, D.M.; Gilbertson, J.A.; Hutt, D.F.; Rezk, T.; et al. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis. Circulation 2019, 140, 16–26. [Google Scholar] [CrossRef]
- Rapezzi, C.; Lorenzini, M.; Longhi, S.; Milandri, A.; Gagliardi, C.; Bartolomei, I.; Salvi, F.; Maurer, M.S. Cardiac amyloidosis: The great pretender. Heart Fail. Rev. 2015, 20, 117–124. [Google Scholar] [CrossRef]
- Tanskanen, M.; Peuralinna, T.; Polvikoski, T.; Notkola, I.-L.; Sulkava, R.; Hardy, J.; Singleton, A.; Kiuru-Enari, S.; Paetau, A.; Tienari, P.J.; et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: A population-based autopsy study. Ann. Med. 2008, 40, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Horibata, Y.; Shono, M.; Misumi, Y.; Oshima, T.; Su, Y.; Tasaki, M.; Shinriki, S.; Kawahara, S.; Jono, H.; et al. Clinicopathological features of senile systemic amyloidosis: An ante- and post-mortem study. Mod. Pathol. 2011, 24, 1533–1544. [Google Scholar] [CrossRef]
- Maurer, M.S.; Bokhari, S.; Damy, T.; Dorbala, S.; Drachman, B.M.; Fontana, M.; Grogan, M.; Kristen, A.V.; Lousada, I.; Nativi-Nicolau, J.; et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ. Heart Fail. 2019, 12, e006075. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Yamada, T.; Takashio, S.; Arima, Y.; Nishi, M.; Morioka, M.; Hirakawa, K.; Hanatani, S.; Fujisue, K.; Yamanaga, K.; Kanazawa, H.; et al. Clinical characteristics and natural history of wild-type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Fail. 2020, 7, 2829–2837. [Google Scholar] [CrossRef]
- Hirooka, Y.; Egashira, K.; Imaizumi, T.; Tagawa, T.; Kai, H.; Sugimachi, M.; Takeshita, A. Effect of l-arginine on acetylcholine-induced endothelium-dependent vasodilation differs between the coronary and forearm vasculatures in humans. J. Am. Coll. Cardiol. 1994, 24, 948–955. [Google Scholar] [CrossRef]
- Nishioka, K.; Nakagawa, K.; Umemura, T.; Jitsuiki, D.; Ueda, K.; Goto, C.; Chayama, K.; Yoshizumi, M.; Higashi, Y. Carvedilol improves endothelium-dependent vasodilation in patients with dilated cardiomyopathy. Heart 2007, 93, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Kajikawa, M.; Maruhashi, T.; Iwamoto, Y.; Matsumoto, T.; Iwamoto, A.; Oda, N.; Matsui, S.; Hidaka, T.; Kihara, Y.; et al. Endothelial dysfunction and abnormal vascular structure are simultaneously present in patients with heart failure with preserved ejection fraction. Int. J. Cardiol. 2017, 231, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, M.; Maruhashi, T.; Kishimoto, S.; Hashimoto, H.; Takaeko, Y.; Yamaji, T.; Harada, T.; Hashimoto, Y.; Han, Y.; Kihara, Y.; et al. Association of Body Mass Index with Endothelial Function in Asian Men. Int. J. Cardiol. 2021, 324, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial Function and Oxidative Stress in Cardiovascular Diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef]
- Takishima, I.; Nakamura, T.; Hirano, M.; Kitta, Y.; Kobayashi, T.; Fujioka, D.; Saito, Y.; Watanabe, K.; Watanabe, Y.; Mishina, H.; et al. Predictive value of serial assessment of endothelial function in chronic heart failure. Int. J. Cardiol. 2012, 158, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Maurer, M.S. Noninvasive Identification of ATTRwt Cardiac Amyloid: The Re-emergence of Nuclear Cardiology. Am. J. Med. 2015, 128, 1275–1280. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef]
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Kajikawa, M.; Matsumoto, T.; Hidaka, T.; Kihara, Y.; et al. Nitroglycerine-Induced Vasodilation for Assessment of Vascular Function: A comparison with flow-mediated vasodilation. Arter. Thromb. Vasc. Biol. 2013, 33, 1401–1408. [Google Scholar] [CrossRef]
- Kishimoto, S.; Maruhashi, T.; Kajikawa, M.; Harada, T.; Yamaji, T.; Han, Y.; Mizobuchi, A.; Hashimoto, Y.; Yoshimura, K.; Nakano, Y.; et al. Vascular Dysfunction Predicts Future Deterioration of Left Ventricular Ejection Fraction in Patients with Heart Failure with Mildly Reduced Ejection Fraction. J. Clin. Med. 2021, 10, 5980. [Google Scholar] [CrossRef]
- Volpe, M.; Carnovali, M.; Mastromarino, V. The natriuretic peptides system in the pathophysiology of heart failure: From molecular basis to treatment. Clin. Sci. 2015, 130, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Emdin, M.; Aimo, A.; Castiglione, V.; Vergaro, G.; Georgiopoulos, G.; Saccaro, L.F.; Lombardi, C.M.; Passino, C.; Cerbai, E.; Metra, M.; et al. Targeting Cyclic Guanosine Monophosphate to Treat Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1795–1807. [Google Scholar] [CrossRef]
- Han, B.; Ghanim, D.; Peleg, A.; Uretzky, G.; Hasin, Y. Loss of systemic endothelial function post-PCI. Acute Card. Care 2008, 10, 79–87. [Google Scholar] [CrossRef]
- Rapezzi, C.; Merlini, G.; Quarta, C.C.; Riva, L.; Longhi, S.; Leone, O.; Salvi, F.; Ciliberti, P.; Pastorelli, F.; Biagini, E.; et al. Systemic Cardiac Amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation 2009, 120, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Takemura, G.; Takatsu, Y.; Doyama, K.; Itoh, H.; Saito, Y.; Koshiji, M.; Ando, F.; Fujiwara, T.; Nakao, K.; Fujiwara, H. Expression of Atrial and Brain Natriuretic Peptides and Their Genes in Hearts of Patients With Cardiac Amyloidosis. J. Am. Coll. Cardiol. 1998, 31, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Ihne, S.; Morbach, C.; Sommer, C.; Geier, A.; Knop, S.; Störk, S. Amyloidosis—The Diagnosis and Treatment of an Underdiagnosed Disease. Dtsch. Ärzteblatt Int. 2020, 117, 159–166. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L.; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e7–e22. [Google Scholar] [CrossRef]
- Coelho, T.; Adams, D.; Silva, A.; Lozeron, P.; Hawkins, P.N.; Mant, T.; Perez, J.; Chiesa, J.; Warrington, S.; Tranter, E.; et al. Safety and Efficacy of RNAi Therapy for Transthyretin Amyloidosis. N. Engl. J. Med. 2013, 369, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Maurer, M.S.; Judge, D.; Zeldenrust, S.; Skinner, M.; Kim, A.Y.; Falk, R.H.; Cheung, K.N.; Patel, A.; Pano, A.; et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: The Transthyretin Amyloidosis Cardiac Study (TRACS). Am. Heart J. 2012, 164, 222–228.e1. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Kajikawa, M.; Matsumoto, T.; Hidaka, T.; Kihara, Y.; et al. Relationship between flow-mediated vasodilation and cardiovascular risk factors in a large community-based study. Heart 2013, 99, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Huang, S.; Daniels, L.B.; Yeboah, J.; Lima, J.A.C.; Cannone, V.; Burnett, J.C., Jr.; Beckman, J.; Carr, J.J.; Wang, T.; et al. NT-proBNP, race and endothelial function in the Multi-Ethnic Study of Atherosclerosis. Heart 2019, 105, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
| Variables | Matched Controls (n = 22) | ATTRwt-CM (n = 22) | p Value |
|---|---|---|---|
| Age, yrs. | 74 ± 11 | 77 ± 6 | 0.365 |
| Men, n (%) | 16 (72.7) | 17 (77.3) | 0.728 |
| Body mass index, kg/m2 | 22.3 ± 3.2 | 22.4 ± 3.0 | 0.905 |
| Heart rate, bpm | 71 ± 15 | 71 ± 13 | 0.942 |
| Systolic blood pressure, mmHg | 115 ± 19 | 117 ± 19 | 0.678 |
| Diastolic blood pressure, mmHg | 70 ± 8 | 72 ± 7 | 0.505 |
| Total cholesterol, mg/dL | 179 ± 30 | 183 ± 38 | 0.682 |
| Triglycerides, mg/dL | 105 ± 51 | 113 ± 80 | 0.678 |
| HDL-C, mg/dL | 68 ± 20 | 71 ± 15 | 0.553 |
| LDL-C, mg/dL | 90 ± 25 | 89 ± 28 | 0.849 |
| eGFR, mL/min/1.73 m2 | 66.4 ± 16.0 | 56.3 ± 19.6 | 0.072 |
| Fasting blood glucose, mg/dL | 120 ± 47 | 117 ± 34 | 0.860 |
| Hemoglobin A1c, % | 6.0 ± 0.7 | 6.0 ± 0.8 | 0.945 |
| NT-pro BNP, pg/mL | 470 ± 677 | 2202 ± 1478 | <0.001 |
| Medical history, n (%) | |||
| Hypertension | 7 (31.8) | 7 (31.8) | 1.000 |
| Dyslipidemia | 9 (40.9) | 8 (36.3) | 0.757 |
| Diabetes mellitus | 7 (31.8) | 7 (31.8) | 1.000 |
| Hyperuricemia | 6 (27.3) | 7 (33.3) | 0.665 |
| Cerebrovascular disease | 1 (4.8) | 3 (14.3) | 0.283 |
| Current Smokers, n (%) | 14 (66.7) | 12 (54.6) | 0.416 |
| Medication, n (%) | |||
| Calcium channel blockers | 2 (9.5) | 3 (13.6) | 0.673 |
| β blockers | 7 (33.3) | 9 (40.9) | 0.607 |
| ARBs/ACEIs | 9 (42.9) | 6 (27.3) | 0.283 |
| Statins | 8 (36.4) | 7 (31.8) | 0.666 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, Y.; Yamaji, T.; Kitagawa, T.; Nakano, Y.; Kajikawa, M.; Yoshimura, K.; Chayama, K.; Goto, C.; Tanigawa, S.; Mizobuchi, A.; et al. Endothelial Function Is Preserved in Patients with Wild-Type Transthyretin Amyloid Cardiomyopathy. J. Clin. Med. 2023, 12, 2534. https://doi.org/10.3390/jcm12072534
Hashimoto Y, Yamaji T, Kitagawa T, Nakano Y, Kajikawa M, Yoshimura K, Chayama K, Goto C, Tanigawa S, Mizobuchi A, et al. Endothelial Function Is Preserved in Patients with Wild-Type Transthyretin Amyloid Cardiomyopathy. Journal of Clinical Medicine. 2023; 12(7):2534. https://doi.org/10.3390/jcm12072534
Chicago/Turabian StyleHashimoto, Yu, Takayuki Yamaji, Toshiro Kitagawa, Yukiko Nakano, Masato Kajikawa, Kenichi Yoshimura, Kazuaki Chayama, Chikara Goto, Syunsuke Tanigawa, Aya Mizobuchi, and et al. 2023. "Endothelial Function Is Preserved in Patients with Wild-Type Transthyretin Amyloid Cardiomyopathy" Journal of Clinical Medicine 12, no. 7: 2534. https://doi.org/10.3390/jcm12072534
APA StyleHashimoto, Y., Yamaji, T., Kitagawa, T., Nakano, Y., Kajikawa, M., Yoshimura, K., Chayama, K., Goto, C., Tanigawa, S., Mizobuchi, A., Harada, T., Yusoff, F. M., Kishimoto, S., Maruhashi, T., Fujita, A., Uchiki, T., Nakashima, A., & Higashi, Y. (2023). Endothelial Function Is Preserved in Patients with Wild-Type Transthyretin Amyloid Cardiomyopathy. Journal of Clinical Medicine, 12(7), 2534. https://doi.org/10.3390/jcm12072534






