Systematic Review on In Situ Laser Fenestrated Repair for the Endovascular Management of Aortic Arch Pathologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligible Studies
2.2. Ethical Considerations and Approval
2.3. Search Strategy
2.4. Data Extraction
2.5. Quality Assessment
2.6. Outcomes
2.7. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Intra-Operative Details
3.3. 30-Day Outcomes
3.4. Follow-Up Outcomes
3.5. Risk of Bias
4. Discussion
4.1. Findings in the Current Literature
4.2. Technical Aspects
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czerny, M.; Schmidli, J.; Adler, S.; van den Berg, J.C.; Bertoglio, L.; Carrel, T.; Chiesa, R.; Clough, R.E.; Eberle, B.; Etz, C.; et al. Editor’s Choice–Current Options and Recommendations for the Treatment of Thoracic Aortic Pathologies Involving the Aortic Arch: An Expert Consensus Document of the European Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2019, 57, 165–198. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Law, Y.; Rohlffs, F.; Spanos, K.; Debus, E.S.; Kölbel, T. Fenestrated endovascular repair for diseases involving the aortic arch. J. Vasc. Surg. 2020, 71, 1464–1471. [Google Scholar] [CrossRef]
- Tsilimparis, N.; Haulon, S.; Spanos, K.; Rohlffs, F.; Heidemann, F.; Resch, T.; Dias, N.; Kölbel, T. Combined fenestrated-branched endovascular repair of the aortic arch and the thoracoabdominal aorta. J. Vasc. Surg. 2020, 71, 1825–1833. [Google Scholar] [CrossRef]
- Mougin, J.; Charbonneau, P.; Guihaire, J.; Schwein, A.; Tyrrell, M.R.; Maurel, B.; Fabre, D.; Haulon, S. Endovascular management of chronic post-dissection aneurysms of the aortic arch. J. Cardiovasc. Surg. 2020, 61, 402–415. [Google Scholar] [CrossRef]
- Spear, R.; Hertault, A.; Van Calster, K.; Settembre, N.; Delloye, M.; Azzaoui, R.; Sobocinski, J.; Fabre, D.; Tyrrell, M.; Haulon, S. Complex endovascular repair of postdissection arch and thoracoabdominal aneurysms. J. Vasc. Surg. 2018, 67, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Chastant, R.; Belarbi, A.; Ozdemir, B.A.; Alric, P.; Gandet, T.; Canaud, L. Homemade fenestrated physician-modified stent grafts for arch aortic degenerative aneurysms. J. Vasc. Surg. 2022, 76, 1133–1140.e2. [Google Scholar] [CrossRef] [PubMed]
- Bosiers, M.J.; Donas, K.P.; Mangialardi, N.; Torsello, G.; Riambau, V.; Criado, F.J.; Veith, F.J.; Ronchey, S.; Fazzini, S.; Lachat, M. European Multicenter Registry for the Performance of the Chimney/Snorkel Technique in the Treatment of Aortic Arch Pathologic Conditions. Ann. Thorac. Surg. 2016, 101, 2224–2230. [Google Scholar] [CrossRef]
- Li, Y.; He, C.; Chen, X.; Yao, J.; Zhang, T.; Zhang, H. Endovascular In Situ Fenestration Technique of Aortic Arch Pathology: A Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2021, 76, 472–480. [Google Scholar] [CrossRef]
- Ahanchi, S.S.; Almaroof, B.; Stout, C.L.; Panneton, J.M. In situ laser fenestration for revascularization of the left subclavian artery during emergent thoracic endovascular aortic repair. J. Endovasc. Ther. 2012, 19, 226–230. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, J.; Yin, M.; Liu, G.; Liu, X.; Ye, K.; Wang, R.; Shi, H.; Li, W.; Jiang, M.; et al. In Situ Laser Stent Graft Fenestration of the Left Subclavian Artery during Thoracic Endovascular Repair of Type B Aortic Dissection with Limited Proximal Landing Zones: 5-Year Outcomes. J. Vasc. Interv. Radiol. 2020, 31, 1321–1327. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses. 2013. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 30 October 2022).
- Redlinger, R.E.; Ahanchi, S.S.; Panneton, J.M. In situ laser fenestration during emergent thoracic endovascular aortic repair is an effective method for left subclavian artery revascularization. J. Vasc. Surg. 2013, 58, 1171–1177. [Google Scholar] [CrossRef]
- Liu, G.; Qin, J.; Cui, C.; Zhao, Z.; Ye, K.; Shi, H.; Liu, X.; Yin, M.; Yang, G.; Huang, S.; et al. Endovascular repair of aortic arch intramural hematoma and penetrating ulcers with 810 nm in situ laser-assisted fenestration: Preliminary results of a single-center. Lasers Surg. Med. 2018, 50, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Sonesson, B.; Dias, N.; Abdulrasak, M.; Resch, T. Midterm results of laser generated in situ fenestration of the left subclavian artery during thoracic endovascular aneurysm repair. J. Vasc. Surg. 2019, 69, 1664–1669. [Google Scholar] [CrossRef]
- Yan, D.; Shi, H.; Qin, J.; Zhao, Z.; Yin, M.; Liu, X.; Ye, K.; Liu, G.; Li, W.; Lu, X. Outcomes of emergency in situ laser fenestration-assisted thoracic endovascular aortic repair in patients with acute Stanford type A aortic dissection unfit for open surgery. J. Vasc. Surg. 2020, 71, 1472–1479.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, P.; Hua, Z.; Jiao, Z.; Cao, H.; Liu, S.; Zhang, W.W.; Li, Z. Early and midterm outcomes of in situ laser fenestration during thoracic endovascular aortic repair for acute and subacute aortic arch diseases and analysis of its complications. J. Vasc. Surg. 2020, 72, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Veeraswamy, R.; Zeigler, S.; Wooster, M. Laser in situ Fenestration in Thoracic Endovascular Aortic Repair: A Single-Center Analysis. Ann. Vasc. Surg. 2021, 76, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Glorion, M.; Coscas, R.; McWilliams, R.; Javerliat, I.; Goëau-Brissonniere, O.; Coggia, M. A Comprehensive Review of In Situ Fenestration of Aortic Endografts. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.A.; Sanford, R.M.; Forbes, T.L.; Amon, C.H.; Doyle, M.G. Clinical outcomes and material properties of in situ fenestration of endovascular stent grafts. J. Vasc. Surg. 2016, 64, 244–250. [Google Scholar] [CrossRef]
- Jayet, J.; Heim, F.; Coggia, M.; Chakfe, N.; Coscas, R. An Experimental Study of Laser in situ Fenestration of Current Aortic Endografts. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 68–77. [Google Scholar] [CrossRef]
- Nana, P.; Spanos, K.; Dakis, K.; Giannoukas, A.; Kölbel, T.; Haulon, S. Systematic Review on Customized and Non-customized Device Techniques for the Endovascular Repair of the Aortic Arch. J. Endovasc. Ther. 2022, 15266028221133701. [Google Scholar] [CrossRef]
- Nana, P.; Tyrrell, M.R.; Guihaire, J.; Le Houérou, T.; Gaudin, A.; Fabre, D.; Haulon, S. A Review: Single and MultiBranch Devices for the Treatment of Aortic Arch Pathologies with Proximal Sealing in Ishimaru Zone. Ann. Vasc. Surg. 2022; In Press. [Google Scholar] [CrossRef]
- Trimarchi, S.; Nienaber, C.A.; Rampoldi, V.; Myrmel, T.; Suzuki, T.; Bossone, E.; Tolva, V.; Deeb, M.G.; Upchurch, G.R.; Cooper, J.V.; et al. Role and results of surgery in acute type B aortic dissection: Insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006, 114, 357–364. [Google Scholar] [CrossRef]
- Matsumura, J.S.; Melissano, G.; Cambria, R.P.; Dake, M.D.; Mehta, S.; Svensson, L.G.; Moore, R.D. Five-year results of thoracic endovascular aortic repair with the Zenith TX2. J. Vasc. Surg. 2014, 60, 1–10. [Google Scholar] [CrossRef]
- Haulon, S.; Greenberg, R.K.; Spear, R.; Eagleton, M.; Abraham, C.; Lioupis, C.; Verhoeven, E.; Ivancev, K.; Kölbel, T.; Stanley, B.; et al. Global experience with an inner branched arch endograft. J. Thorac. Cardiovasc. Surg. 2014, 148, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Spear, R.; Sobocinski, J.; Settembre, N.; Tyrrell, M.; Malikov, S.; Maurel, B.; Haulon, S. Early Experience of Endovascular Repair of Post-dissection Aneurysms Involving the Thoraco-abdominal Aorta and the Arch. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 488–497. [Google Scholar] [CrossRef]
- Makaloski, V.; Tsilimparis, N.; Rohlffs, F.; Heidemann, F.; Debus, E.S.; Kölbel, T. Endovascular total arch replacement techniques and early results. Ann. Cardiothorac. Surg. 2018, 7, 380–388. [Google Scholar] [CrossRef]
- Tish, S.; Chase, J.-A.; Scoville, C.; Vogel, T.R.; Cheung, S.; Bath, J. A Systematic Review of Contemporary Outcomes from Aortic Arch In Situ Laser Fenestration during Thoracic Endovascular Aortic Repair. Ann. Vasc. Surg. 2023, 91, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Eleshra, A.; Heo, W.; Lee, K.-H.; Kim, T.-H.; Sim, S.A.; Sharafeldin, H.; Song, S.-W. Mid-term outcomes of hybrid debranching endovascular aortic arch repair in landing zones 0–2. Vascular 2022, 17085381211068230. [Google Scholar] [CrossRef]
- Qin, J.; Wu, X.; Li, W.; Ye, K.; Yin, M.; Liu, G.; Cui, C.; Zhao, Z.; Liu, X.; Lu, X. Laser fenestration of aortic arch stent grafts for endovascular treatment of retrograde type A dissection. Int. J. Cardiol. 2021, 328, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhao, Z.; Liu, G.; Ye, K.; Yin, M.; Cui, C.; Shi, H.; Peng, Z.; Jiang, M.; Liu, X.; et al. In situ diode laser fenestration of aortic arch stent grafts during thoracic endovascular aortic repair of Stanford type A aortic dissection. Eurointervention 2019, 14, e1854–e1860. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M.; Nienaber, C.A.; Peterson, M.D.; Woznicki, E.M.; Braverman, A.C.; Trimarchi, S.; Myrmel, T.; Pyeritz, R.; Hutchison, S.; Strauss, C.; et al. Early Mortality in Type A Acute Aortic Dissection: Insights From the International Registry of Acute Aortic Dis-section. JAMA Cardiol. 2022, 7, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, R.G.; Murphy, M.; Hartley, D.; Lawrence-Brown, M.M.D.; Harris, P.L. In situ stent-graft fenestration to preserve the left subclavian artery. J. Endovasc. Ther. 2004, 11, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.H.; Dimaio, J.M.; Dean, W.; Jessen, M.E.; Arko, F.R. Endovascular Repair of Acute Traumatic Thoracic Aortic Transection With Laser-Assisted In-Situ Fenestration of a Stent-Graft Covering the Left Subclavian Artery. J. Endovasc. Ther. 2009, 16, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Pernot, M.; D’Ostrevy, N.; Piperata, A.; Busuttil, O.; Labrousse, L. Percutaneous Total Aortic Arch Repair With In Situ Laser Fenestration. Ann. Thorac. Surg. 2022, 114, e389–e392. [Google Scholar] [CrossRef] [PubMed]


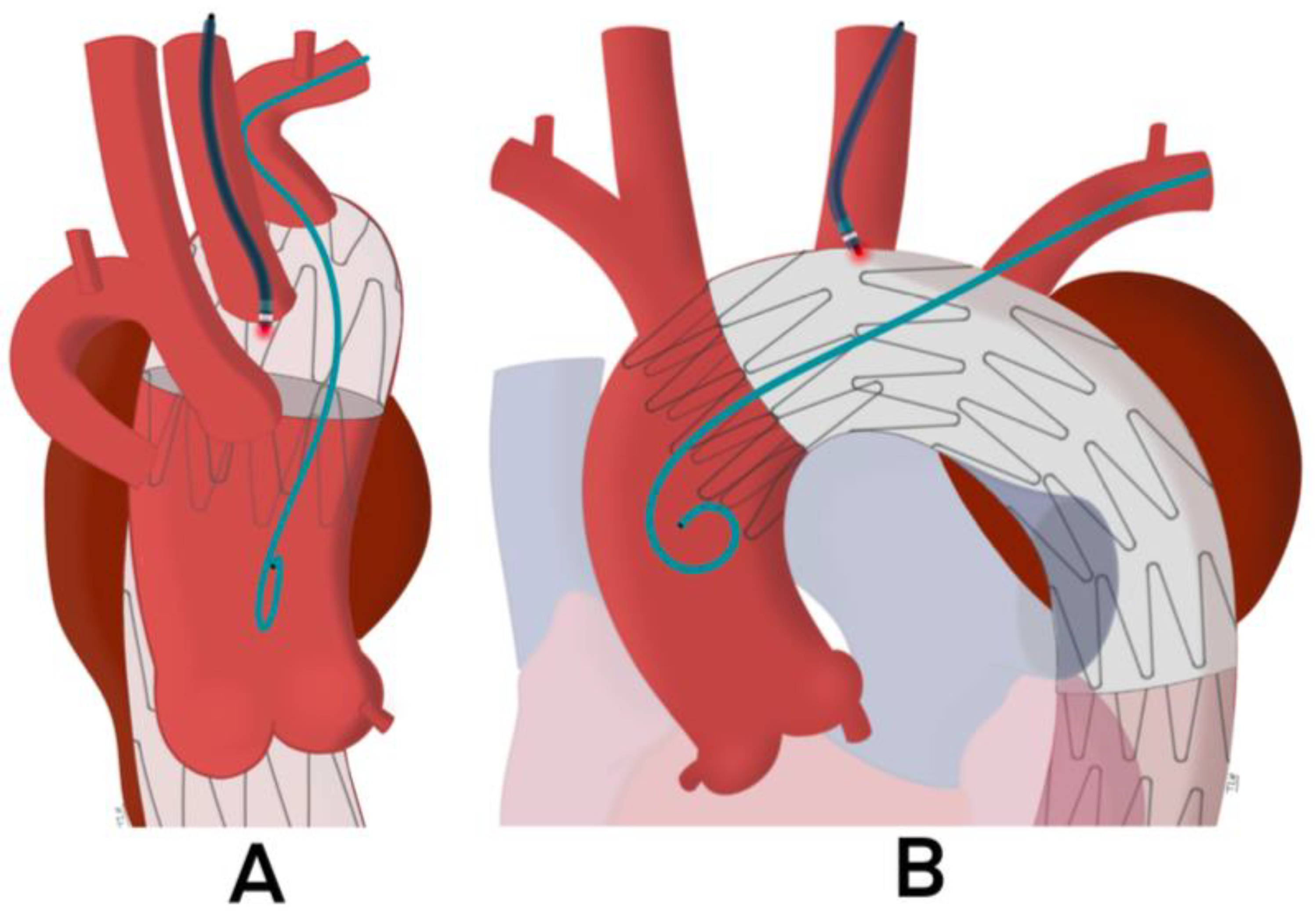
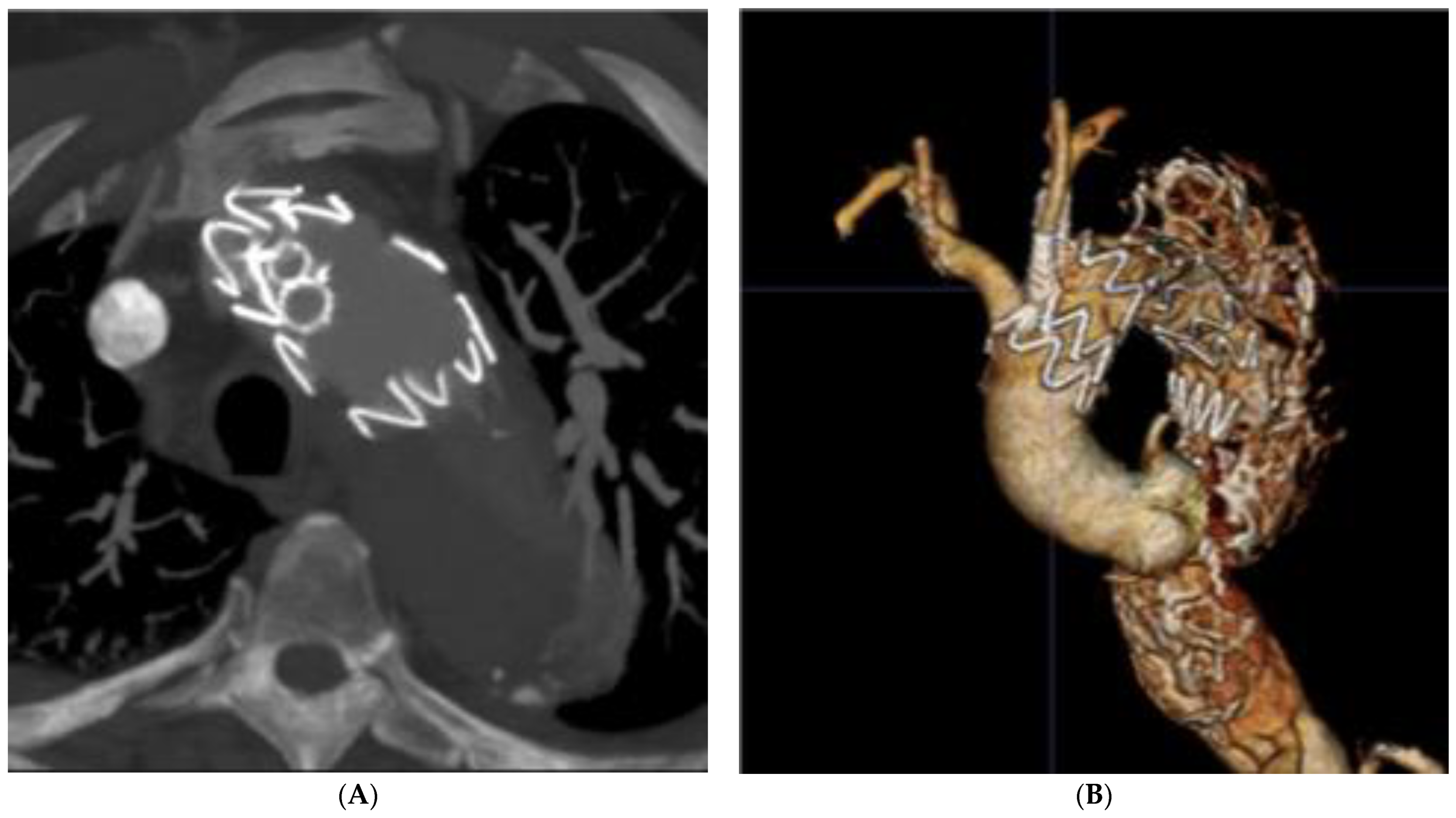
| Author | Year | Country | Type of Study | Timespan | No. of Patients | Age | Aortic Arch Aneurysm | Dissections Involving the Aortic Arch | IMH or PAU |
|---|---|---|---|---|---|---|---|---|---|
| Redlinger et al. [14] | 2013 | USA | Retrospective, single center | 2009–2012 | 22 | 57 (37–83) | 12 (8 chronic dissections) | 6 | |
| Liu et al. [15] | 2018 | China | Retrospective, single center | 2014–2017 | 23 | 66 ± 10.8 (41–82) | 7 | 4 | |
| Sonesson et al. [16] | 2019 | Sweden | Retrospective, single center | 2014–2016 | 10 | 68 | 7 | 2 | 1 |
| Yan et al. [17] | 2020 | China | Retrospective, single center | 2016–2018 | 20 | 67 | 20 (20 type A) | ||
| Li et al. [18] | 2020 | China | Retrospective of prospective data, single center | 2017–2019 | 148 | 54.9 ± 12.9 | 17 | 120 (13 type A) | 11 |
| Evans et al. [19] | 2021 | USA | Retrospective, single center | 2017–2020 | 24 | 62 (24–82) | 16 (8 chronic dissections) | 4 |
| Author | Type of Laser | Main Graft | 3 Fenestrations | 2 Fenestrations | 1 Fenestration | Bridging Stents |
|---|---|---|---|---|---|---|
| Redlinger et al. [14] | 2.0- to 2.5-mm Turbo Elite laser catheter, Spectranetics, Colorado Springs, CO, USA | Talent or Valiant (Medtronic, Santa Rosa, CA, USA), Zenith TX2 (Cook Inc., Bloomington, IN, USA) | 0 | 0 | 22 | iCAST (Atrium, Hudson, NH, USA) |
| Liu et al. [15] | 810 nm wavelength laser, Gigaa Optronics Technology Co. Ltd., Wuhan, China, application of 18 W for 3–5 s | TAG/cTAG (W.L. Gore & Associates, Flagstaff, AZ, USA), Valiant (Medtronic, Santa Rosa, CA, USA), Ankura (Lifetech, Shenzhen, China) | 2 | 3 | 6 | Covered and bare metal stents |
| Sonesson et al. [16] | 308 nm wavelength laser, 0.9 mm Turbo Elite over the wire, Spectranetics, Colorado Springs, CO, USA | Zenith Alpha (Cook Inc., Bloomington, IN, USA) | 0 | 0 | 10 | Begraft (Bentley, Hechingen, Germany), Fluency (Bard Peripheral Vascular, Tempe, AZ, USA), Visipro (Medtronic, Dublin, Ireland), Advanta (Atrium Europe B.V, Mijdrecht, the Netherlands), Lifestream (Bard Peripheral Vascular, Tempe, AZ, USA), Protégé (ev3 Endovascular Inc., Plymouth, MN, USA) |
| Yan et al. [17] | 810 nm wavelength diode laser, Eufoton Company, Trieste, Italy, application of 18 W for 3 s | cTAG (W.L. Gore & Associates, Flagstaff, AZ, USA) | 18 | 2 | 0 | Fluency (Bard Peripheral Vascular, Tempe, AZ, USA) for LCCA, Endurant limb if TBA >13 mm (Medtronic, Santa Rosa, CA, USA) |
| Li et al. [18] | 980 nm wavelength laser VELAS, GIGAA Laser, Wuhan, China, application of 18 W for 3 s | 90 Valiant (Medtroni, Santa Rosa, CA, USA), 30 cTAG (W.L. Gore & Associates, Flagstaff, AZ, USA), 19 Zenith (Cook Inc., Bloomington, IN, USA), 6 Ankura (Lifetech, Shenzhen, China), 2 Hercules (Microport, Shanghai, China), 1 E-vita Thoracic 3 g (Jotec, Hechingen, Germany) | 11 | 13 | 124 | 119 Fluency (Bard Peripheral Vascular Tempe, AZ, USA), 58 Viabahn (W.L. Gore & Associates, Flagstaff, AZ, USA), 2 Endurant limbs (Medtronic, Santa Rosa, CA, USA) |
| Evans et al. [19] | 308 nm wavelength, 2.3 mm 0.035 Spectranetics laser (Philips, Amsterdam, Netherlands), with a fluency setting of 40 mJ/mm2 and repetition rate of 60 Hz | cTAG (W.L. Gore & Associates, Flagstaff, AZ, USA), Valiant (Medtronic, Santa Rosa, CA, USA) | 0 | 6 | 18 | VBX (W.L. Gore & Associates, Flagstaff, AZ, USA) |
| Author | Early Outcomes | Technical Success | Mortality | Stroke | Spinal Cord Ischemia | Re-Interventions | Acute Kidney Injury |
|---|---|---|---|---|---|---|---|
| Redlinger et al. [14] | 30-day | 100% | 1 | 0 | 1 | NA | 0 |
| Liu et al. [15] | 30-day | 100% | 0 | 1 | NA | 1 | NA |
| Sonesson et al. [16] | 30-day | 90% | 0 | 1 | NA | NA | NA |
| Yan et al. [17] | 30-day | 100% | 2 | 1 | NA | NA | NA |
| Li et al. [18] | 30-day | 97.8% | 3 | 5 | 0 | NA | NA |
| Evans et al. [19] | 30-day | 100% | 2 | 3 | 2 | NA | 4 |
| Author | Follow-Up Duration | Mortality | Aorta-Related Death | TV Patency | Re-Interventions | Endoleak | Reasons for Re-Intervention |
|---|---|---|---|---|---|---|---|
| Redlinger et al. [14] | 11 (1–40) | 2 | 0 | 100% | 2 | 2 | Coils for ET II from LSA |
| Liu et al. [15] | 10.5 ± 5.7 | 0 | 0 | 100% | NA | 0 | |
| Sonesson et al. [16] | 27 | 2 | 1 | 100% | 1 | 2 | Type Ic from LSA |
| Yan et al. [17] | 16 (3–26) | 1 | NA | NA | 0 | 3 | |
| Li et al. [18] | 15 ± 5 | 2 | 0 | 100% | 1 | 7 | Type Ib endoleak, extension LSA |
| Evans et al. [19] | 9 (1–29) | 3 | 0 | 9 | 6 | 6 embolization and extension for ET III, 1 angioplasty for bypass stenosis, 1 bypass for endoleak, 1 hematoma |
| Studies | Year of Publication | Selection | Comparability | Outcome |
|---|---|---|---|---|
| Redlinger et al. [14] | 2013 | ** | ** | * |
| Liu et al. [15] | 2018 | ** | ** | * |
| Sonesson et al. [16] | 2019 | ** | ** | * |
| Yan et al. [17] | 2020 | ** | ** | * |
| Li et al. [18] | 2020 | *** | ** | ** |
| Evans et al. [19] | 2021 | ** | ** | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houérou, T.L.; Nana, P.; Pernot, M.; Guihaire, J.; Gaudin, A.; Lerisson, E.; Costanzo, A.; Fabre, D.; Haulon, S. Systematic Review on In Situ Laser Fenestrated Repair for the Endovascular Management of Aortic Arch Pathologies. J. Clin. Med. 2023, 12, 2496. https://doi.org/10.3390/jcm12072496
Houérou TL, Nana P, Pernot M, Guihaire J, Gaudin A, Lerisson E, Costanzo A, Fabre D, Haulon S. Systematic Review on In Situ Laser Fenestrated Repair for the Endovascular Management of Aortic Arch Pathologies. Journal of Clinical Medicine. 2023; 12(7):2496. https://doi.org/10.3390/jcm12072496
Chicago/Turabian StyleHouérou, Thomas Le, Petroula Nana, Mathieu Pernot, Julien Guihaire, Antoine Gaudin, Erol Lerisson, Alessandro Costanzo, Dominique Fabre, and Stephan Haulon. 2023. "Systematic Review on In Situ Laser Fenestrated Repair for the Endovascular Management of Aortic Arch Pathologies" Journal of Clinical Medicine 12, no. 7: 2496. https://doi.org/10.3390/jcm12072496
APA StyleHouérou, T. L., Nana, P., Pernot, M., Guihaire, J., Gaudin, A., Lerisson, E., Costanzo, A., Fabre, D., & Haulon, S. (2023). Systematic Review on In Situ Laser Fenestrated Repair for the Endovascular Management of Aortic Arch Pathologies. Journal of Clinical Medicine, 12(7), 2496. https://doi.org/10.3390/jcm12072496






