Defining a New Classification System for the Surgical Management of Neuroendocrine Tumor Liver Metastases
Abstract
1. Introduction
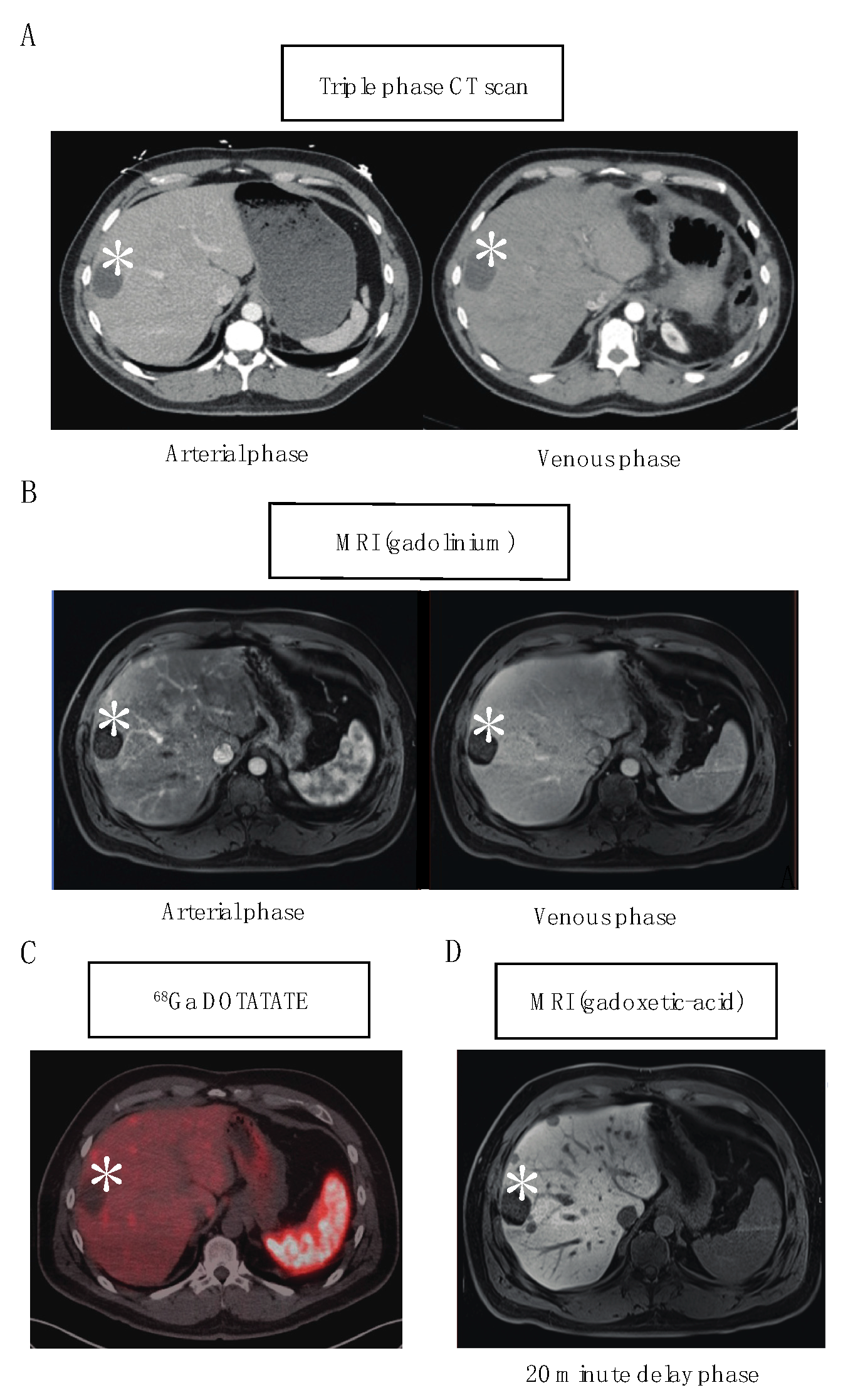
2. Modality of Choice for Optimal Detection of NETLM
3. Hepatic Cytoreduction—Lowering the Debulking Threshold from 90% to 70%
4. Role for Resection of the Primary Tumor with Or without Treatment of NETLM
| Year | Team | Number of Patients | Statistics | Debulking Threshold |
|---|---|---|---|---|
| 1977 | Foster and Berman [25] | 44 | 95% | |
| 1990 | McEntee et al. (Mayo) [26] | 37 | 20-month OS: 83% | 90% |
| 1995 | Que et al. (Mayo) [27] | 74 | 4-year OS: 73% | 90% |
| 2003 | Sarmiento et al. (Mayo) [28] | 170 | 5-year OS: 61% 96% Symptom Improvement | 90% |
| 2008 | Chamber et al. [29] | 66 | 5-year OS: 74% | 70% |
| 2014 | Graff-Baker et al. (OHSU) [30] | 52 | 5-year OS: 88% No difference in PFS or DFS between debulking groups (70–89% vs. 90–99% vs. 100%) | 70% |
| 2014 | Boudreaux et al. [33] | 189 | 5-year OS: 87% 10-year OS: 77% | 70% |
| 2015 | Maxwell et al. (Iowa) [31] | 108 | Improved Survival (Median OS NR for >70% vs. 6.5 months <70%; p < 0.05) | 70% |
| 2019 | Scott et al. (Iowa) [32] | 188 | Improved Survival (Median OS 134.3 months >70% vs. 37.6 months <70%) | 70% |
5. Parenchymal-Sparing Techniques
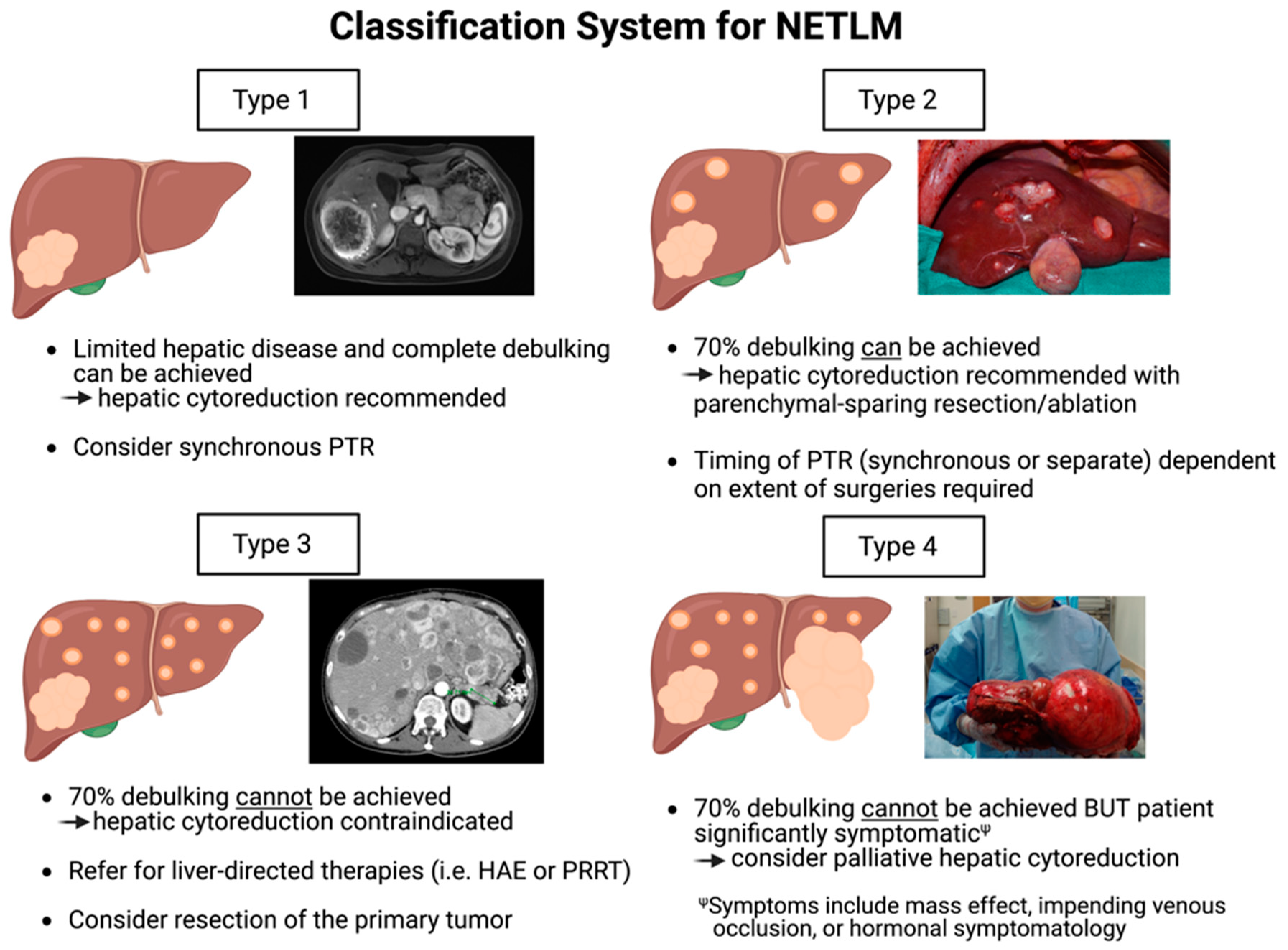
6. A New Classification System to Guide Surgical Management of NETLM
- Type 1: Patients of this category have a limited number of NETLMs that can be completely cleared with hepatic debulking. Depending on the degree of hepatic resection, synchronous PTR can be performed at the time of hepatic cytoreduction as per surgeon discretion.
- Type 2: Patients have multiple lesions diffusely throughout the liver, and >70% debulking can be achieved utilizing parenchymal-sparing techniques and ablation. PTR should be considered and can be performed synchronously or as a separate procedure depending on the extent of operation required.
- Type 3: Patients have extensive, bilobar hepatic involvement, but unlike Type 2 patients, >70% debulking clearance cannot be achieved and cytoreduction should not be performed. These patients are better candidates for liver-directed therapies such as HAE with radioembolization. However, they should be evaluated for PTR as survival benefit is demonstrated even without liver-directed interventions as discussed previously in this review.
- Type 4: Patients have extensive hepatic involvement that is profoundly symptomatic from either the mass effect from large, bulky tumors (often from impending venous occlusion due to tumor compression of the IVC, hepatic veins, or portal vein), or from hormonal symptomatology that cannot be medically mitigated. Although >70% debulking cannot be achieved, select patients may have improved quality of life with palliative hepatic debulking of these symptomatic lesions. As survival is unlikely to be substantially prolonged, palliative cytoreduction must be carefully weighed with potential surgical morbidity.
7. Liver-Directed Therapies
8. Peptide Receptor Radionuclide Therapy
9. Liver Transplantation
10. Conclusions
Funding
Conflicts of Interest
References
- Hallet, J.; Law, C.H.L.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, E.; Boffetta, P.; Shafir, M.; Aleksovska, K.; Boccia, S.; Rindi, G. Increased incidence trend of low-grade and high-grade neuroendocrine neoplasms. Endocrine 2017, 58, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Díez, M.; Teulé, A.; Salazar, R. Gastroenteropancreatic neuroendocrine tumors: Diagnosis and treatment. Ann. Gastroenterol. 2013, 26, 29–36. [Google Scholar]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Öberg, K.; Steinmüller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The epidemiology of metastases in neuroendocrine tumors. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.M.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Sayed, B.A.; Wei, A.C. Liver Resection for Neuroendocrine Metastases and the Obligation to Individualize Care. Ann. Surg. Oncol. 2018, 25, 3787–3789. [Google Scholar] [CrossRef]
- Basturk, O.; Tang, L.; Hruban, R.H.; Adsay, N.V.; Yang, Z.; Krasinskas, A.M.; Vakiani, E.; La Rosa, S.; Jang, K.T.; Frankel, W.L.; et al. Poorly Differentiated Neuroendocrine Carcinomas of the Pancreas: A Clinicopathologic Analysis of 44 Cases. Am. J. Surg. Pathol. 2014, 38, 437. [Google Scholar] [CrossRef]
- Saxena, A.; Chua, T.C.; Perera, M.; Chu, F.; Morris, D.L. Surgical resection of hepatic metastases from neuroendocrine neoplasms: A systematic review. Surg. Oncol. 2012, 21, e131–e141. [Google Scholar] [CrossRef] [PubMed]
- Uri, I.; Grozinsky-Glasberg, S. Current treatment strategies for patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Clin. Diabetes Endocrinol. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Camus, B.; Cottereau, A.-S.; Palmieri, L.-J.; Dermine, S.; Tenenbaum, F.; Brezault, C.; Coriat, R. Indications of Peptide Receptor Radionuclide Therapy (PRRT) in Gastroenteropancreatic and Pulmonary Neuroendocrine Tumors: An Updated Review. J. Clin. Med. 2021, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Leon Pachter, H.; Sarpel, U. Hepatic arterial embolization for the treatment of metastatic neuroendocrine tumors. Int. J. Hepatol. 2012, 2012, 471203. [Google Scholar] [CrossRef]
- Mayo, S.C.; De Jong, M.C.; Pulitano, C.; Clary, B.M.; Reddy, S.K.; Gamblin, T.C.; Celinksi, S.A.; Kooby, D.A.; Staley, C.A.; Stokes, J.B.; et al. Surgical management of hepatic neuroendocrine tumor metastasis: Results from an international multi-institutional analysis. Ann. Surg. Oncol. 2010, 17, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Lefevre, J.H.; Duvillard, P.; Goéré, D.; Dromain, C.; Dumont, F.; Baudin, E. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: They are many more than you think. Ann. Surg. 2010, 251, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Ejaz, A.; Konda, B.; Makary, M.S.; Pawlik, T.M. Neuroendocrine liver metastases: A contemporary review of treatment strategies. HepatoBiliary Surg. Nutr. 2020, 9, 440–451. [Google Scholar] [CrossRef]
- Ronot, M.; Clift, A.K.; Baum, R.P.; Singh, A.; Kulkarni, H.R.; Frilling, A.; Vilgrain, V. Morphological and Functional Imaging for Detecting and Assessing the Resectability of Neuroendocrine Liver Metastases. Neuroendocrinology 2017, 106, 74–88. [Google Scholar] [CrossRef]
- Morin, C.; Drolet, S.; Daigle, C.; Deshaies, I.; Ouellet, J.-F.; Ball, C.G.; Dixon, E.; Marceau, J. Additional value of gadoxetic acid-enhanced MRI to conventional extracellular gadolinium-enhanced MRI for the surgical management of colorectal and neuroendocrine liver metastases. HPB 2019, 22, 710–715. [Google Scholar] [CrossRef]
- Haider, M.; Jiang, B.G.; Parker, J.A.; Bullock, A.J.; Goehler, A.; Tsai, L.L. Use of MRI and Ga-68 DOTATATE for the detection of neuroendocrine liver metastases. Abdom. Imaging 2021, 47, 586–595. [Google Scholar] [CrossRef]
- Attiyeh, M.; Malhotra, G.; Li, D.; Manoukian, S.; Motarjem, P.; Singh, G. Defining MRI superiority over CT for neuroendocrine liver metastases. In Proceedings of the NANETS 2021 Symposium, Chicago, IL, USA, 4–6 November 2021. [Google Scholar]
- Malhotra, G.; Attiyeh, M.; Manoukian, S.; Motarjem, P.; Singh, G. MRI has improved detection of small neuroendocrine liver metastasis compared with Ga-68 DOTATATE. In Proceedings of the NANETS 2021 Symposium, Chicago, IL, USA, 4–6 November 2021. [Google Scholar]
- Albanus, D.R.; Apitzsch, J.; Erdem, Z.; Erdem, O.; Verburg, F.A.; Behrendt, F.F.; Mottaghy, F.M.; Heinzel, A. Clinical value of 68Ga-DOTATATE-PET/CT compared to stand-alone contrast enhanced CT for the detection of extra-hepatic metastases in patients with neuroendocrine tumours (NET). Eur. J. Radiol. 2015, 84, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Wang, W.M.; Yusuf, S.; Evans, J.; Ramaswami, R.; Wernig, F.; Frilling, A.; Mauri, F.; Al-Nahhas, A.; Aboagye, E.O.; et al. 68Ga-DOTATATE PET/CT parameters predict response to peptide receptor radionuclide therapy in neuroendocrine tumours. Radiother. Oncol. 2019, 141, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.; Berman, M. Palliative liver resection to relieve symptoms of the malignant carcinoid and other endocrine syndromes. Solid liver tumors. Solid Liver Tumors 1977, 22, 235–245. [Google Scholar]
- McEntee, G.P.; Nagorney, D.M.; Kvols, L.K.; Moertel, C.G.; Grant, C.S. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery 1990, 108, 1091–1096. [Google Scholar]
- Que, F.G.; Nagorney, D.M.; Batts, K.P.; Linz, L.J.; Kvols, L.K. Hepatic resection for metastatic neuroendocrine carcinomas. Am. J. Surg. 1995, 169, 36–43. [Google Scholar] [CrossRef]
- Sarmiento, J.M.; Heywood, G.; Rubin, J.; Ilstrup, D.M.; Nagorney, D.M.; Que, F.G. Surgical treatment of neuroendocrine metastases to the liver: A plea for resection to increase survival. J. Am. Coll. Surg. 2003, 197, 29–37. [Google Scholar] [CrossRef]
- Chambers, A.J.; Pasieka, J.L.; Dixon, E.; Rorstad, O. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery 2008, 144, 645–653. [Google Scholar] [CrossRef]
- Graff-Baker, A.N.; Sauer, D.A.; Pommier, S.J.; Pommier, R.F. Expanded criteria for carcinoid liver debulking: Maintaining survival and increasing the number of eligible patients. Surgery 2014, 156, 1369–1377. [Google Scholar] [CrossRef]
- Maxwell, J.E.; Sherman, S.K.; O’Dorisio, T.M.; Bellizzi, A.M.; Howe, J.R. Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery 2015, 159, 320–335. [Google Scholar] [CrossRef]
- Scott, A.T.; Breheny, P.J.; Keck, K.J.; Bellizzi, A.; Dillon, J.S.; O’Dorisio, T.M.; Howe, J.R. Effective cytoreduction can be achieved in patients with numerous neuroendocrine tumor liver metastases (NETLMs). Surgery 2018, 165, 166–175. [Google Scholar] [CrossRef]
- Boudreaux, P.J.; Wang, Y.-Z.; Diebold, A.E.; Frey, D.J.; Anthony, L.; Uhlhorn, A.P.; Ryan, P.; Woltering, E.A. A Single Institution’s Experience with Surgical Cytoreduction of Stage IV, Well-Differentiated, Small Bowel Neuroendocrine Tumors. J. Am. Coll. Surg. 2014, 218, 837–844. [Google Scholar] [CrossRef]
- Capurso, G.; Rinzivillo, M.; Bettini, R.; Boninsegna, L.; Fave, G.D.; Falconi, M. Systematic review of resection of primary midgut carcinoid tumour in patients with unresectable liver metastases. J. Br. Surg. 2012, 99, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Raoof, M.; Ituarte, P.H.G.; Williams, J.; Melstrom, L.; Li, D.; Lee, B.; Singh, G. Resection of the Primary Gastrointestinal Neuroendocrine Tumor Improves Survival With or Without Liver Treatment. Ann. Surg. 2019, 270, 1131–1137. [Google Scholar] [CrossRef]
- Franko, J.; Feng, W.; Yip, L.; Genovese, E.; Moser, A.J. Non-functional Neuroendocrine Carcinoma of the Pancreas: Incidence, Tumor Biology, and Outcomes in 2,158 Patients. J. Gastrointest. Surg. 2009, 14, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Glazer, E.S.; Tseng, J.F.; Al-Refaie, W.; Solorzano, C.C.; Liu, P.; Willborn, K.A.; Abdalla, E.K.; Vauthey, J.N.; Curley, S.A. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB 2010, 12, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Taner, T.; Atwell, T.D.; Zhang, L.; Oberg, T.N.; Harmsen, W.S.; Slettedahl, S.W.; Kendrick, M.L.; Nagorney, D.M.; Que, F.G. Adjunctive radiofrequency ablation of metastatic neuroendocrine cancer to the liver complements surgical resection. HPB 2013, 15, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Auloge, P.; Cazzato, R.L.; Koch, G.; Caudrelier, J.; De Marini, P.; Garnon, J.; Gangi, A. Percutaneous tumor ablation. Presse Med. 2019, 48, 1146–1155. [Google Scholar] [CrossRef]
- Vogl, T.J.; Nour-Eldin, N.A.; Hammerstingl, R.M.; Panahi, B.; Naguib, N.N.N. Microwave Ablation (MWA): Basics, Technique and Results in Primary and Metastatic Liver Neoplasms—Review Article. RöFo-Fortschritte Gebiet R 2017, 189, 1055–1066. [Google Scholar] [CrossRef]
- Spiliotis, A.E.; Gäbelein, G.; Holländer, S.; Scherber, P.-R.; Glanemann, M.; Patel, B. Microwave ablation compared with radiofrequency ablation for the treatment of liver cancer: A systematic review and meta-analysis. Radiol. Oncol. 2021, 55, 247–258. [Google Scholar] [CrossRef]
- Woo, S.; Chung, J.W.; Hur, S.; Joo, S.M.; Kim, H.C.; Jae, H.J.; Park, J.H. Liver abscess after transarterial chemoembolization in patients with bilioenteric anastomosis: Frequency and risk factors. AJR Am. J. Roentgenol. 2013, 200, 1370–1377. [Google Scholar] [CrossRef]
- Kennedy, A.; Bester, L.; Salem, R.; Sharma, R.A.; Parks, R.W.; Ruszniewski, P. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): Guidelines from the NET-Liver-Metastases Consensus Conference. HPB 2015, 17, 29–37. [Google Scholar] [CrossRef]
- Lewis, A.; Li, D.; Williams, J.; Singh, G. Pancreatic Neuroendocrine Tumors: State-of-the-Art Diagnosis and Management. Oncology 2017, 31, e1–e12. [Google Scholar] [PubMed]
- Cazzato, R.L.; Hubelé, F.; De Marini, P.; Ouvrard, E.; Salvadori, J.; Addeo, P.; Garnon, J.; Kurtz, J.-E.; Greget, M.; Mertz, L.; et al. Liver-Directed Therapy for Neuroendocrine Metastases: From Interventional Radiology to Nuclear Medicine Procedures. Cancers 2021, 13, 6368. [Google Scholar] [CrossRef] [PubMed]
- Barat, M.; Cottereau, A.-S.; Kedra, A.; Dermine, S.; Palmieri, L.-J.; Coriat, R.; Dautry, R.; Tselikas, L.; Soyer, P.; Dohan, A. The Role of Interventional Radiology for the Treatment of Hepatic Metastases from Neuroendocrine Tumor: An Updated Review. J. Clin. Med. 2020, 9, 2302. [Google Scholar] [CrossRef]
- Tai, E.; Kennedy, S.; Farrell, A.; Jaberi, A.; Kachura, J.; Beecroft, R. Comparison of Transarterial Bland and Chemoembolization for Neuroendocrine Tumours: A Systematic Review and Meta-Analysis. Curr. Oncol. 2020, 27, 537–546. [Google Scholar] [CrossRef]
- Walker, L.A. Radioactive Yttrium 90: A Review of its properties, biological behavior, and clinical uses. Acta Radiol. Ther. Phys. Biol. 1964, 2, 302–314. [Google Scholar]
- Jia, Z.; Wang, W. Yttrium-90 radioembolization for unresectable metastatic neuroendocrine liver tumor: A systematic review. Eur. J. Radiol. 2018, 100, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Clift, A.K.; Braat, A.J.; Alsafi, A.; Wasan, H.S.; Al-Nahhas, A.; Thomas, R.; Drymousis, P.; Habib, N.; Tait, P.N. Radioembolisation with 90Y microspheres for neuroendocrine liver metastases: An institutional case series, systematic review and meta-analysis. HPB 2019, 21, 773–783. [Google Scholar] [CrossRef]
- Braat, A.J.A.T.; Ahmadzadehfar, H.; Kappadath, S.C.; Stothers, C.L.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with 90Y Resin Microspheres of Neuroendocrine Liver Metastases After Initial Peptide Receptor Radionuclide Therapy. Cardiovasc. Interv. Radiol. 2019, 43, 246–253. [Google Scholar] [CrossRef]
- Kessler, J.; Lewis, A.; Gagandeep, S.; Ituarte, P.H.; Park, J.J. Radioembolization following Liver Resection: Safety and Dosing Considerations. J. Vasc. Interv. Radiol. 2016, 27, 46–51. [Google Scholar] [CrossRef]
- Jeyarajah, D.R.; Doyle, M.B.M.; Espat, N.J.; Hansen, P.D.; Iannitti, D.A.; Kim, J.; Thambi-Pillai, T.; Visser, B.C. Role of yttrium-90 selective internal radiation therapy in the treatment of liver-dominant metastatic colorectal cancer: An evidence-based expert consensus algorithm. J. Gastrointest. Oncol. 2020, 11, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, L.; Zhang, W. Neoadjuvant treatment strategies for hepatocellular carcinoma. World J. Gastrointest. Surg. 2021, 13, 1550–1566. [Google Scholar] [CrossRef]
- Teo, J.Y.; Allen, J.C., Jr.; Ng, D.C.; Choo, S.P.; Tai, D.W.; Chang, J.P.; Cheah, F.K.; Chow, P.K.; Goh, B.K. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB 2016, 18, 7–12. [Google Scholar] [CrossRef]
- Melstrom, L.G.; Eng, O.S.; Raoof, M.; Singh, G.; Fong, Y.; Latorre, K.; Choi, G.H.; Salem, R.; Bentrem, D.J. Is hepatectomy safe following Yttrium-90 therapy? A multi-institutional international experience. HPB 2019, 21, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Christante, D.; Pommier, S.; Givi, B.; Pommier, R. Hepatic artery chemoinfusion with chemoembolization for neuroendocrine cancer with progressive hepatic metastases despite octreotide therapy. Surgery 2008, 144, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. (177)Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Partelli, S.; Bertani, E.; Bartolomei, M.; Perali, C.; Muffatti, F.; Grana, C.M.; Lena, M.S.; Doglioni, C.; Crippa, S.; Fazio, N.; et al. Peptide receptor radionuclide therapy as neoadjuvant therapy for resectable or potentially resectable pancreatic neuroendocrine neoplasms. Surgery 2018, 163, 761–767. [Google Scholar] [CrossRef]
- Opalińska, M.; Sowa-Staszczak, A.; Grochowska, A.; Olearska, H.; Hubalewska-Dydejczyk, A. Value of Peptide Receptor Radionuclide Therapy as Neoadjuvant Treatment in the Management of Primary Inoperable Neuroendocrine Tumors. Front. Oncol. 2021, 11, 687925. [Google Scholar] [CrossRef]
- Parghane, R.V.; Bhandare, M.; Chaudhari, V.; Ostwal, V.; Ramaswamy, A.; Talole, S.; Shrikhande, S.V.; Basu, S. Surgical Feasibility, Determinants, and Overall Efficacy of Neoadjuvant (177)Lu-DOTATATE PRRT for Locally Advanced Unresectable Gastroenteropancreatic Neuroendocrine Tumors. J. Nucl. Med. 2021, 62, 1558–1563. [Google Scholar] [CrossRef]
- Fan, S.T.; Le Treut, Y.P.; Mazzaferro, V.; Burroughs, A.K.; Olausson, M.; Breitenstein, S.; Frilling, A. Liver transplantation for neuroendocrine tumour liver metastases. HPB 2015, 17, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Le Treut, Y.P.; Grégoire, E.; Klempnauer, J.; Belghiti, J.; Jouve, E.; Lerut, J.; Castaing, D.; Soubrane, O.; Boillot, O.; Mantion, G.; et al. Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: A 213-case European liver transplant registry study. Ann. Surg. 2013, 257, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Beal, E.W.; Felekouras, E.; Vernadakis, S.; Fung, J.J.; Pawlik, T.M. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery 2017, 162, 525–536. [Google Scholar] [CrossRef]
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.-Y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahuron, K.M.; Singh, G. Defining a New Classification System for the Surgical Management of Neuroendocrine Tumor Liver Metastases. J. Clin. Med. 2023, 12, 2456. https://doi.org/10.3390/jcm12072456
Mahuron KM, Singh G. Defining a New Classification System for the Surgical Management of Neuroendocrine Tumor Liver Metastases. Journal of Clinical Medicine. 2023; 12(7):2456. https://doi.org/10.3390/jcm12072456
Chicago/Turabian StyleMahuron, Kelly M., and Gagandeep Singh. 2023. "Defining a New Classification System for the Surgical Management of Neuroendocrine Tumor Liver Metastases" Journal of Clinical Medicine 12, no. 7: 2456. https://doi.org/10.3390/jcm12072456
APA StyleMahuron, K. M., & Singh, G. (2023). Defining a New Classification System for the Surgical Management of Neuroendocrine Tumor Liver Metastases. Journal of Clinical Medicine, 12(7), 2456. https://doi.org/10.3390/jcm12072456








