The SALINE Technique for the Treatment of the No-Reflow Phenomenon during Percutaneous Coronary Intervention in STEMI
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Definitions
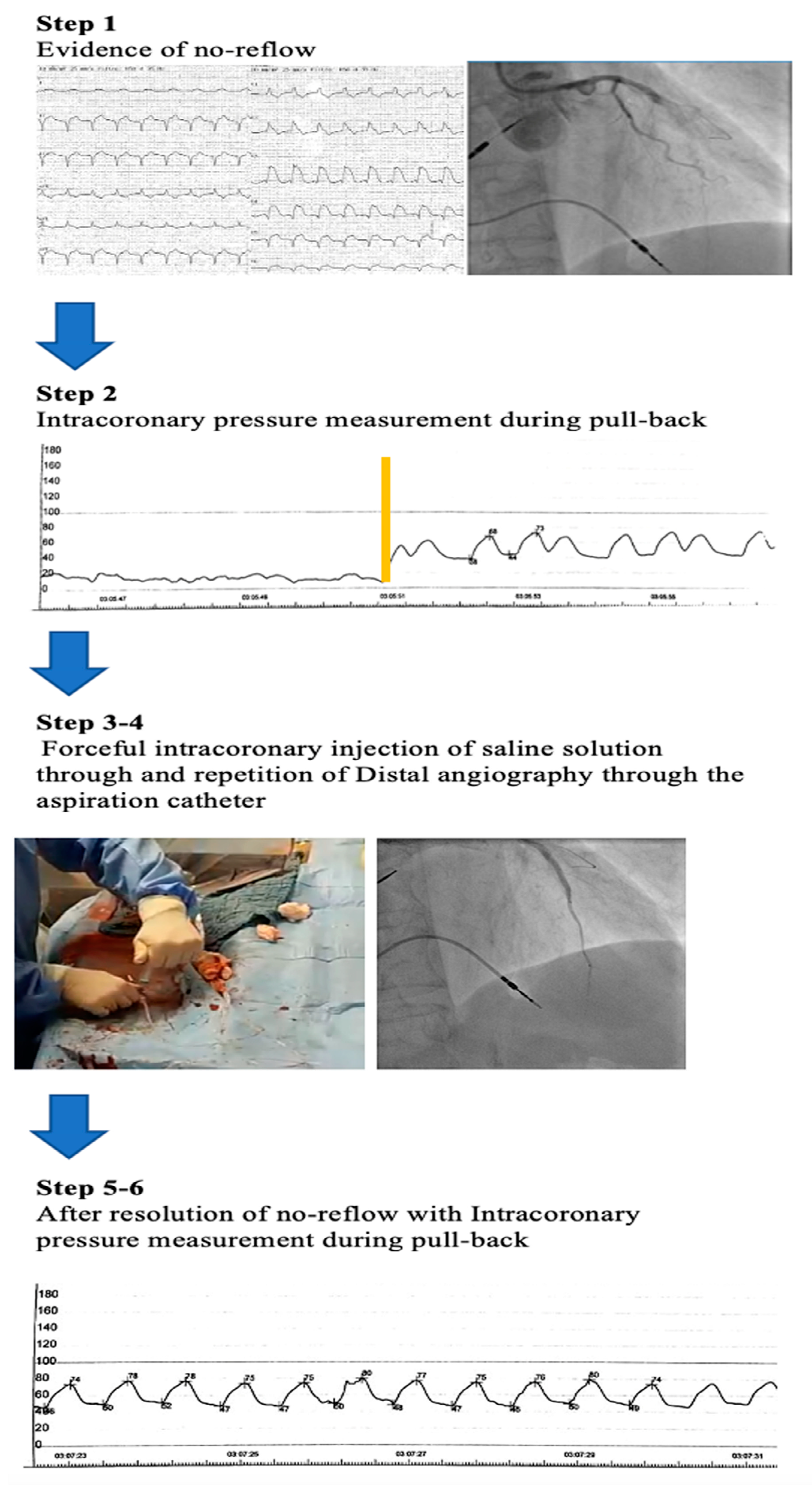
2.2. The SALINE Technique
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
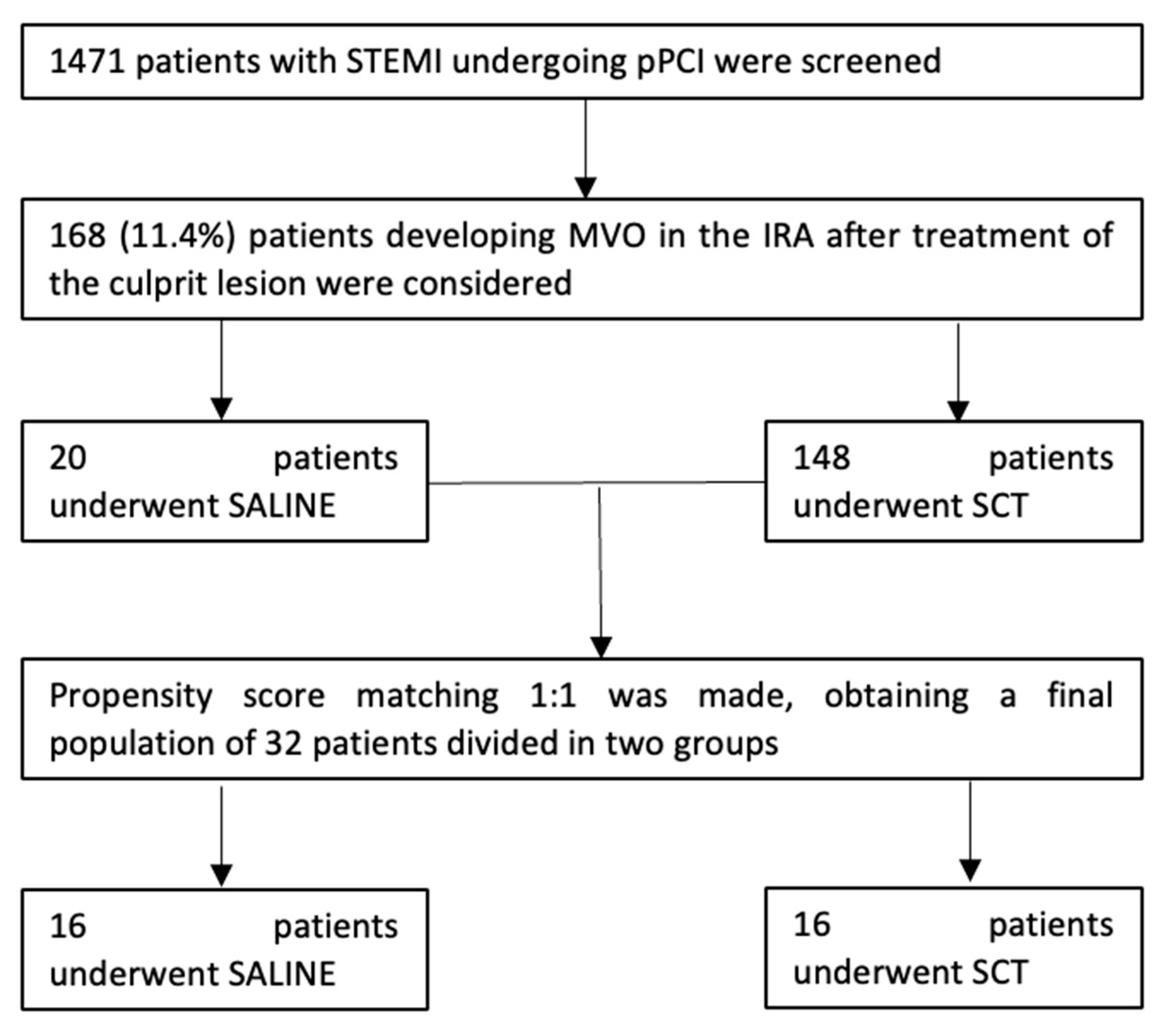
3.1. Study Population
3.2. Primary Endpoint and In-Hospital Outcomes
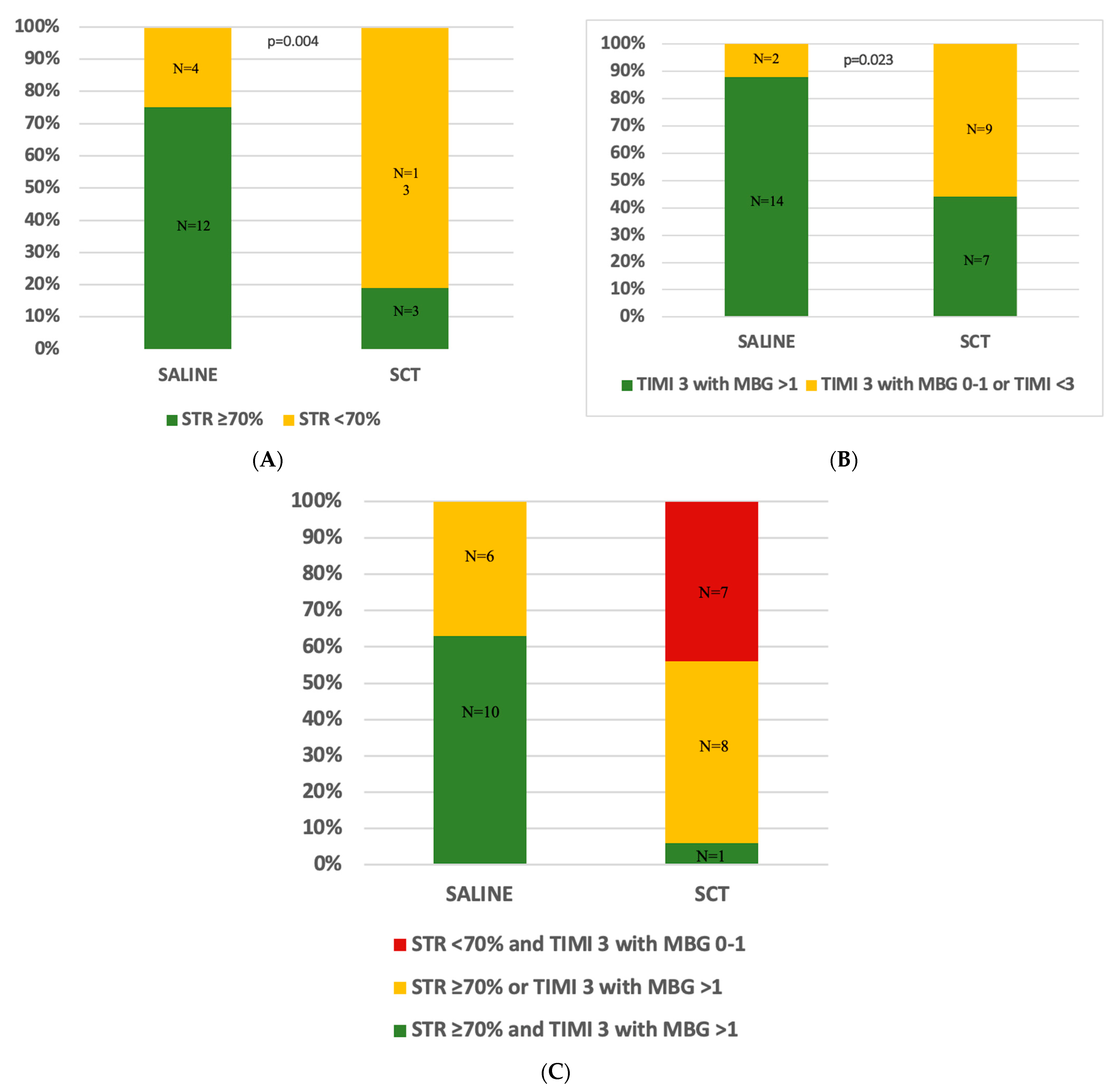
3.3. Secondary Endpoints
4. Discussion
Limitations of the Study
5. Conclusions
Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Ibáñez, B.; Heusch, G.; Ovize, M.; Van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef]
- Heusch, G. The Coronary Circulation as a Target of Cardioprotection. Circ. Res. 2016, 118, 1643–1658. [Google Scholar] [CrossRef] [PubMed]
- Morishima, I.; Sone, T.; Okumura, K.; Tsuboi, H.; Kondo, J.; Mukawa, H.; Matsui, H.; Toki, Y.; Ito, T.; Hayakawa, T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J. Am. Coll. Cardiol. 2000, 36, 1202–1209. [Google Scholar] [CrossRef]
- Piana, R.N.; Paik, G.Y.; Moscucci, M.; Cohen, D.J.; Gibson, C.M.; Kugelmass, A.D.; Carrozza, J.P., Jr.; Kuntz, R.E.; Baim, D.S. Incidence and treatment of ‘no-reflow’ after percutaneous coronary intervention. Circulation 1994, 89, 2514–2518. [Google Scholar] [CrossRef]
- Taniyama, Y.; Ito, H.; Iwakura, K.; Masuyama, T.; Hori, M.; Takiuchi, S.; Nishikawa, N.; Higashino, Y.; Fujii, K.; Minamino, T. Beneficial effect of intracoronary verapamil on microvascular and myocardial salvage in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 1997, 30, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Suryapranata, H.; Stone, G.W.; Antoniucci, D.; Tcheng, J.E.; Neumann, F.J.; Van de Werf, F.; Antman, E.M.; Topol, E.J. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: A meta-analysis of randomized trials. JAMA 2005, 293, 1759–1765. [Google Scholar] [CrossRef]
- Svilaas, T.; Vlaar, P.J.; van der Horst, I.C.; Diercks, G.F.; de Smet, B.J.; van den Heuvel, A.F.; Anthonio, R.L.; Jessurun, G.A.; Tan, E.S.; Suurmeijer, A.J.; et al. Thrombus aspiration during primary percutaneous coronary intervention. N. Engl. J. Med. 2008, 358, 557–567. [Google Scholar] [CrossRef]
- Ito, H. No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 499–506. [Google Scholar] [CrossRef]
- Burzotta, F.; Crea, F. Thrombus-aspiration: A victory in the war against no reflow. Lancet 2008, 371, 1889–1890. [Google Scholar] [CrossRef]
- Niccoli, G.; Rigattieri, S.; De Vita, M.R.; Valgimigli, M.; Corvo, P.; Fabbiocchi, F.; Romagnoli, E.; De Caterina, A.R.; La Torre, G.; Lo Schiavo, P.; et al. Open-label, randomized, placebo-controlled evaluation of intracoronary adenosine or nitroprusside after thrombus aspiration during primary percutaneous coronary intervention for the prevention of microvascular obstruction in acute myocardial infarction: The REOPEN-AMI study (Intracoronary Nitroprusside Versus Adenosine in Acute Myocardial Infarction). JACC Cardiovasc. Interv. 2013, 6, 580–589. [Google Scholar]
- Nazir, S.A.; Khan, J.N.; Mahmoud, I.Z.; Greenwood, J.P.; Blackman, D.J.; Kunadian, V.; Been, M.; Abrams, K.R.; Wilcox, R.; Adgey, A.A.J.; et al. Efficacy and Mechanism Evaluation. The REFLO-STEMI (REperfusion Facilitated by LOcal Adjunctive Therapy in ST-Elevation Myocardial Infarction) Trial: A Randomised Controlled Trial Comparing Intracoronary Administration of Adenosine or Sodium Nitroprusside with Control for Attenuation of Microvascular Obstruction during Primary Percutaneous Coronary Intervention; NIHR Journals Library: Southampton, UK, 2016. [Google Scholar]
- Hausenloy, D.J.; Chilian, W.; Crea, F.; Davidson, S.M.; Ferdinandy, P.; Garcia-Dorado, D.; van Royen, N.; Schulz, R.; Heusch, G. The coronary circulation in acute myocardial ischaemia/reperfusion injury: A target for cardioprotection. Cardiovasc. Res. 2019, 115, 1143–1155. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients with ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2016, 133, 1135–1147. [Google Scholar] [PubMed]
- Cimmino, G.; Gallinoro, E.; Di Serafino, L.; De Luca, N.; Cirillo, P. Antiplatelet Therapy in Acute Coronary Syndromes. Lights and Shadows of Platelet Function Tests to Guide the Best Therapeutic Approach. Curr. Vasc. Pharmacol. 2020, 18, 262–272. [Google Scholar] [CrossRef]
- Gibson, C.M.; Cannon, C.P.; Murphy, S.A.; Marble, S.J.; Barron, H.V.; Braunwald, E. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation 2002, 105, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, M.; Vlaar, P.J.; Svilaas, T.; Amo, D.; Nijsten, M.W.; Zijlstra, F. Computer-assisted myocardial blush quantification after percutaneous coronary angioplasty for acute myocardial infarction: A substudy from the TAPAS trial. Eur. Heart J. 2009, 30, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Schröder, R. Prognostic impact of early ST-segment resolution in acute ST-elevation myocardial infarction. Circulation 2004, 110, e506–e510. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Stone, G.W.; Selker, H.P.; Thiele, H.; Patel, M.R.; Udelson, J.E.; Ohman, E.M.; Maehara, A.; Eitel, I.; Granger, C.B.; Jenkins, P.L.; et al. Relationship between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis from 10 Randomized Trials. J. Am. Coll. Cardiol. 2016, 67, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance as a Tool to Characterize Microvascular Obstruction and to Predict Infarct Size Regression in Patients with STEMI Undergoing Primary PCI. JACC Cardiovasc. Imaging 2019, 12, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Kodeboina, M.; Nagumo, S.; Munhoz, D.; Sonck, J.; Mileva, N.; Gallinoro, E.; Candreva, A.; Mizukami, T.; Van Durme, F.; Heyse, A.; et al. Simplified Assessment of the Index of Microvascular Resistance. J. Interv. Cardiol. 2021, 2021, 9971874. [Google Scholar] [CrossRef]
- Mizukami, T.; Sonck, J.; Gallinoro, E.; Kodeboina, M.; Canvedra, A.; Nagumo, S.; Bartunek, J.; Wyffels, E.; Vanderheyden, M.; Shinke, T.; et al. Duration of Hyperemia with Intracoronary Administration of Papaverine. J. Am. Heart Assoc. 2021, 10, e018562. [Google Scholar] [CrossRef]
- Thiele, H.; Kappl, M.J.; Linke, A.; Erbs, S.; Boudriot, E.; Lembcke, A.; Kivelitz, D.; Schuler, G. Influence of time-to-treatment, TIMI-flow grades, and ST-segment resolution on infarct size and infarct transmurality as assessed by delayed enhancement magnetic resonance imaging. Eur. Heart J. 2007, 28, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Vlaar, P.J.; Svilaas, T.; van der Horst, I.C.; Diercks, G.F.; Fokkema, M.L.; de Smet, B.J.; van den Heuvel, A.F.; Anthonio, R.L.; Jessurun, G.A.; Tan, E.S.; et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): A 1-year follow-up study. Lancet 2008, 371, 1915–1920. [Google Scholar] [CrossRef]
- Navarese, E.P.; Frediani, L.; Kandzari, D.E.; Caiazzo, G.; Cenname, A.M.; Cortese, B.; Piva, T.; Muçaj, A.; Tumscitz, C.; Paparoni, F.; et al. Efficacy and safety of intracoronary epinephrine versus conventional treatments alone in STEMI patients with refractory coronary no-reflow during primary PCI: The RESTORE observational study. Catheter. Cardiovasc. Interv. 2021, 97, 602–611. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B.; Adjedj, J.; Xaplanteris, P.; Ferrara, A.; Mo, Y.; Penicka, M.; Floré, V.; Pellicano, M.; Toth, G.; Barbato, E.; et al. Saline-Induced Coronary Hyperemia: Mechanisms and Effects on Left Ventricular Function. Circ. Cardiovasc. Interv. 2017, 10. [Google Scholar] [CrossRef]
- Adjedj, J.; Picard, F.; Collet, C.; Bruneval, P.; Fournier, S.; Bize, A.; Sambin, L.; Berdeaux, A.; Varenne, O.; De Bruyne, B.; et al. Intracoronary Saline-Induced Hyperemia during Coronary Thermodilution Measurements of Absolute Coronary Blood Flow: An Animal Mechanistic Study. J. Am. Heart Assoc. 2020, 9, e015793. [Google Scholar] [CrossRef] [PubMed]
- Candreva, A.; Gallinoro, E.; van’t Veer, M.; Sonck, J.; Collet, C.; Di Gioia, G.; Kodeboina, M.; Mizukami, T.; Nagumo, S.; Keulards, D.; et al. Basics of Coronary Thermodilution. JACC Cardiovasc. Interv. 2021, 14, 595–605. [Google Scholar] [CrossRef]
- Gallinoro, E.; Candreva, A.; Fernandez-Peregrina, E.; Bailleul, E.; Meeus, P.; Sonck, J.; Bermpeis, K.; Bertolone, D.T.; Esposito, G.; Paolisso, P.; et al. Saline-induced coronary hyperemia with continuous intracoronary thermodilution is mediated by intravascular hemolysis. Atherosclerosis 2022, 352, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Gallinoro, E.; Candreva, A.; Colaiori, I.; Kodeboina, M.; Fournier, S.; Nelis, O.; Di Gioia, G.; Sonck, J.; Van’t Veer, M.; Pijls, N.H.; et al. Thermodilution-derived volumetric resting coronary blood flow measurement in humans. EuroIntervention 2021, 17, e672–e679. [Google Scholar] [CrossRef] [PubMed]
| SALINE (n = 16) | SCT (n = 16) | p-Value | |
|---|---|---|---|
| Age, years | 71.4 ± 15.8 | 69.8 ± 12.3 | 0.758 |
| Male gender | 11 (68.8%) | 11 (68.8%) | 1.000 |
| BMI, kg/m2 | 25.8 ± 5.0 | 25.9 ± 2.9 | 0.903 |
| Hypertension | 12 (75.0%) | 10 (62.5%) | 0.704 |
| Hyperlipidemia | 7 (43.8%) | 8 (50.0%) | 1.000 |
| Diabetes | 3 (18.8%) | 2 (12.5%) | 1.000 |
| COPD | 1 (6.3%) | 1 (6.3%) | 1.000 |
| CAD | 3 (18.8%) | 3 (18.8 %) | 1.000 |
| Current smoking | 4 (25.0%) | 1 (6.3%) | 0.333 |
| eGFR < 60 mL/min | 3 (18.8%) | 3 (18.8%) | 1.000 |
| Killip class > I | 3 (18.8%) | 2 (12.5%) | 1.000 |
| Time from symptoms onset to balloon, minutes | 155 [IQR 96, 344] | 228 [IQR 130, 405] | 0.597 |
| Cardiogenic shock | 2 (12.5%) | 1 (6.3%) | 1.000 |
| Baseline maximal sum of ST-segment elevation, mm | 10 [IQR 3-19] | 11 [IQR 3-20] | 0.934 |
| LVEF at admission, % | 46.0 ± 9.3 | 49.5 ± 12.2 | 0.380 |
| Therapy at admission | |||
| Aspirin | 5 (31.3%) | 3 (18.8%) | 0.683 |
| Beta-blockers | 5 (31.3%) | 7 (43.8%) | 0.715 |
| ACE inhibitors | 4 (25.0%) | 1 (6.3%) | 0.330 |
| ARBs | 4 (25.0%) | 6 (37.5%) | 0.703 |
| Statins | 5 (31.3%) | 4 (25%) | 1.000 |
| Culprit lesion | |||
| LAD | 8 (50.0%) | 8 (50.0%) | 1.000 |
| LCX | 3 (18.8%) | 2 (12.5%) | 1.000 |
| RCA | 4 (25.0%) | 5 (31.3%) | 1.000 |
| SVG | 1 (6.3%) | 1 (6.3%) | 1.000 |
| Location of the lesion in the culprit vessel | |||
| Proximal | 7 (43.8%) | 4 (25.0%) | 0.458 |
| Medial/Distal | 9 (56.3%) | 12 (75.0%) | 0.458 |
| Number of diseased vessels | |||
| 1-vessel CAD | 3 (18.8%) | 2 (12.5%) | 1.000 |
| 2-vessel CAD | 6 (37.5%) | 8 (50.0%) | 0.722 |
| 3-vessel CAD | 6 (37.5%) | 5 (31.3%) | 1.000 |
| SVG | 1 (6.3%) | 1 (6.3%) | 1.000 |
| TIMI flow grade at admission | |||
| 0 | 13 (81.3%) | 13 (81.3%) | 1.000 |
| 1 | 2 (12.5%) | 2 (12.5%) | 1.000 |
| 2 | 1 (6.3%) | 1 (6.3%) | 1.000 |
| 3 | 0 | 0 | - |
| Presence of intracoronary thrombus | 15 (93.8%) | 11 (68.8%) | 0.172 |
| SALINE (n = 16) | SCT (n = 16) | p-Value | |
|---|---|---|---|
| Oral drugs administration | |||
| Aspirin | 16 (100%) | 15 (93.8%) | 1.000 |
| Clopidrogel | 5 (31.3%) | 4 (25.0%) | 1.000 |
| Ticagrelor | 2 (12.5%) | 7 (43.8%) | 0.112 |
| Prasugrel | 9 (56.3%) | 5 (31.3%) | 0.285 |
| IV bolus administration | |||
| Cangrelor | 0 | 0 | - |
| Unfractionated heparin (5000 IU) | 16 (100%) | 16 (100.0%) | - |
| Abciximab (0.25 mg/kg) | 0 | 0 | - |
| Radial access | 12 (75%) | 13 (81.3) | 1.000 |
| Femoral access | 4 (25%) | 3 (18.8%) | 1.000 |
| Thrombus aspiration | 15 (93.8%) | 10 (62.5%) | 0.083 |
| Direct stenting after thrombus aspiration | 5 (31.3%) | 3 (18.8%) | 0.414 |
| Number of implanted stents in the IRA | |||
| 0 | 1 (6.3%) | 1 (6.3%) | 1.000 |
| 1 | 11(68.8%) | 9 (56.3%) | 0.716 |
| 2 | 4 (25.0%) | 6 (37.5%) | 0.704 |
| Length of the stented segment, mm | 24 [IQR 18–41] | 30 [IQR 22–48] | 0.202 |
| Diameter of the stented segment, mm | 3 [IQR 3–3.5] | 3 [IQR 2.5–3.5] | 0.651 |
| Postdilatation | 11 (68.8%) | 10 (62.5%) | 1.000 |
| Intracoronary administration | |||
| Adenosine | 11 (68.8%) | 12 (75.0%) | 1.000 |
| Nitrates | 0 | 16 (100.0%) | - |
| Abciximab (0.25 mg/kg) | 0 | 2 (12.5%) | 0.913 |
| SALINE (n = 16) | SCT (n = 16) | p-Value | |
|---|---|---|---|
| Transient AV-block not requiring pacing | 4 (25.0%) | 2 (12.5%) | 0. 654 |
| AV-block requiring pacing for drug infusion | 0 | 0 | - |
| Transient hypotension not requiring IABP | 2 | 2 | 1.000 |
| Hypotension requiring IABP | 3 (18.8%) | 2 (12.5%) | 1.000 |
| Ventricular tachycardia | 0 (0%) | 1 (6.3%) | 1.000 |
| SALINE (n = 16) | SCT (n = 16) | p-Value | |
|---|---|---|---|
| STR > 70% | 12 (75.0%) | 3 (18.8%) | 0.004 |
| Final TIMI flow 3 with MBG ˃ 1 | 14 (87.5%) | 7 (43.8%) | 0.023 |
| Final TIMI flow 3 with MBG ≤ 1 or TIMI ˂ 3 | 2 (12.6%) | 9 (56.3%) | - |
| Combined STR and angiographic MVO resolution | 10 (62.5%) | 1 (6.3%) | <0.001 |
| LVEF at discharge, % | 52.9 ± 8.8 | 55.8 ± 11.4 | 0.478 |
| Hs-TnI (ng/L) peak | 76.821 ± 69.402 [IQR 29544, 86250] | 79.989 ± 78.261 [IQR 29932, 133549] | 0.720 |
| MACCE at 3 years | 1 (6.3%) | 8 (50.0%) | 0.047 |
| Cardiovascular death | 0 | 0 | |
| MI | 0 | 1 (6.3%) | |
| TLR | 0 | 2 (12.5%) | |
| HF hospitalization | 1 (6.3%) | 3 (18.8%) | |
| CVE | 0 | 2 (12.5%) | |
| LVEF Δ* at 1 year | +6.8 ± 8.5 | +5.6 ± 11.4 | 0.728 |
| Any Bleeding | 0 | 1 (6.3%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grancini, L.; Diana, D.; Centola, A.; Monizzi, G.; Mastrangelo, A.; Olivares, P.; Montorsi, P.; Alushi, B.; Bartorelli, A.L.; Galassi, A.R. The SALINE Technique for the Treatment of the No-Reflow Phenomenon during Percutaneous Coronary Intervention in STEMI. J. Clin. Med. 2023, 12, 2405. https://doi.org/10.3390/jcm12062405
Grancini L, Diana D, Centola A, Monizzi G, Mastrangelo A, Olivares P, Montorsi P, Alushi B, Bartorelli AL, Galassi AR. The SALINE Technique for the Treatment of the No-Reflow Phenomenon during Percutaneous Coronary Intervention in STEMI. Journal of Clinical Medicine. 2023; 12(6):2405. https://doi.org/10.3390/jcm12062405
Chicago/Turabian StyleGrancini, Luca, Davide Diana, Alice Centola, Giovanni Monizzi, Angelo Mastrangelo, Paolo Olivares, Piero Montorsi, Brunilda Alushi, Antonio L. Bartorelli, and Alfredo R. Galassi. 2023. "The SALINE Technique for the Treatment of the No-Reflow Phenomenon during Percutaneous Coronary Intervention in STEMI" Journal of Clinical Medicine 12, no. 6: 2405. https://doi.org/10.3390/jcm12062405
APA StyleGrancini, L., Diana, D., Centola, A., Monizzi, G., Mastrangelo, A., Olivares, P., Montorsi, P., Alushi, B., Bartorelli, A. L., & Galassi, A. R. (2023). The SALINE Technique for the Treatment of the No-Reflow Phenomenon during Percutaneous Coronary Intervention in STEMI. Journal of Clinical Medicine, 12(6), 2405. https://doi.org/10.3390/jcm12062405






