Delirium Risk Score in Elderly Patients with Cervical Spinal Cord Injury and/or Cervical Fracture
Abstract
1. Introduction
2. Materials and Methods
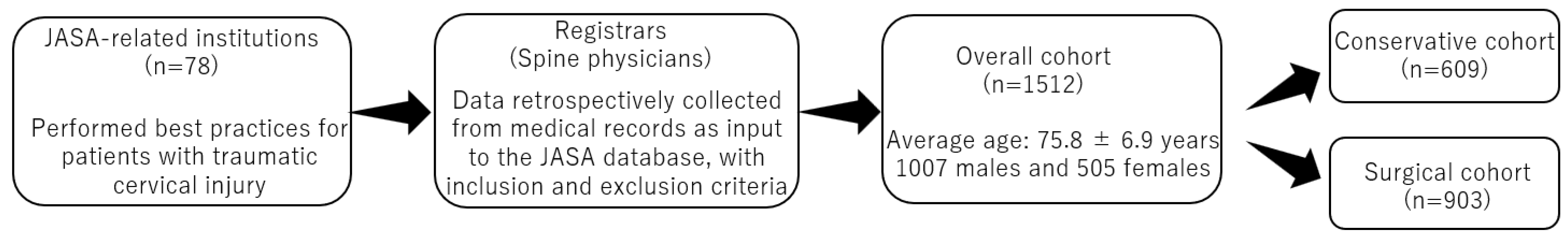
2.1. Patient Population
- Inclusion criteria: patients aged ≥65 years with traumatic cervical SCI and/or traumatic cervical fracture; patients treated conservatively or surgically between 2010 and 2020 at an institution registered in the JASA and those who were followed for at least three months after the injury;
- Exclusion criteria: patients with cervical metastasis; and those with any missing data;
- Registrars did not exclude patients on the basis of specific medications, surgical procedures, surgical instruments, and/or reasons other than the inclusion/exclusion criteria indicated above.
2.2. Collected Data
2.2.1. Patient Background Data
2.2.2. Delirium Data
2.2.3. Radiographic Data
2.2.4. Neurological Impairment Scale
2.2.5. Therapeutic Data
2.3. Statistical Analysis
2.3.1. Risk Factors for Delirium
2.3.2. Delirium Risk Score
3. Results
3.1. Patient Characteristics
3.2. Risk Factors of Delirium in the Conservative Cohort
3.3. Risk Factors of Delirium in the Surgical Cohort
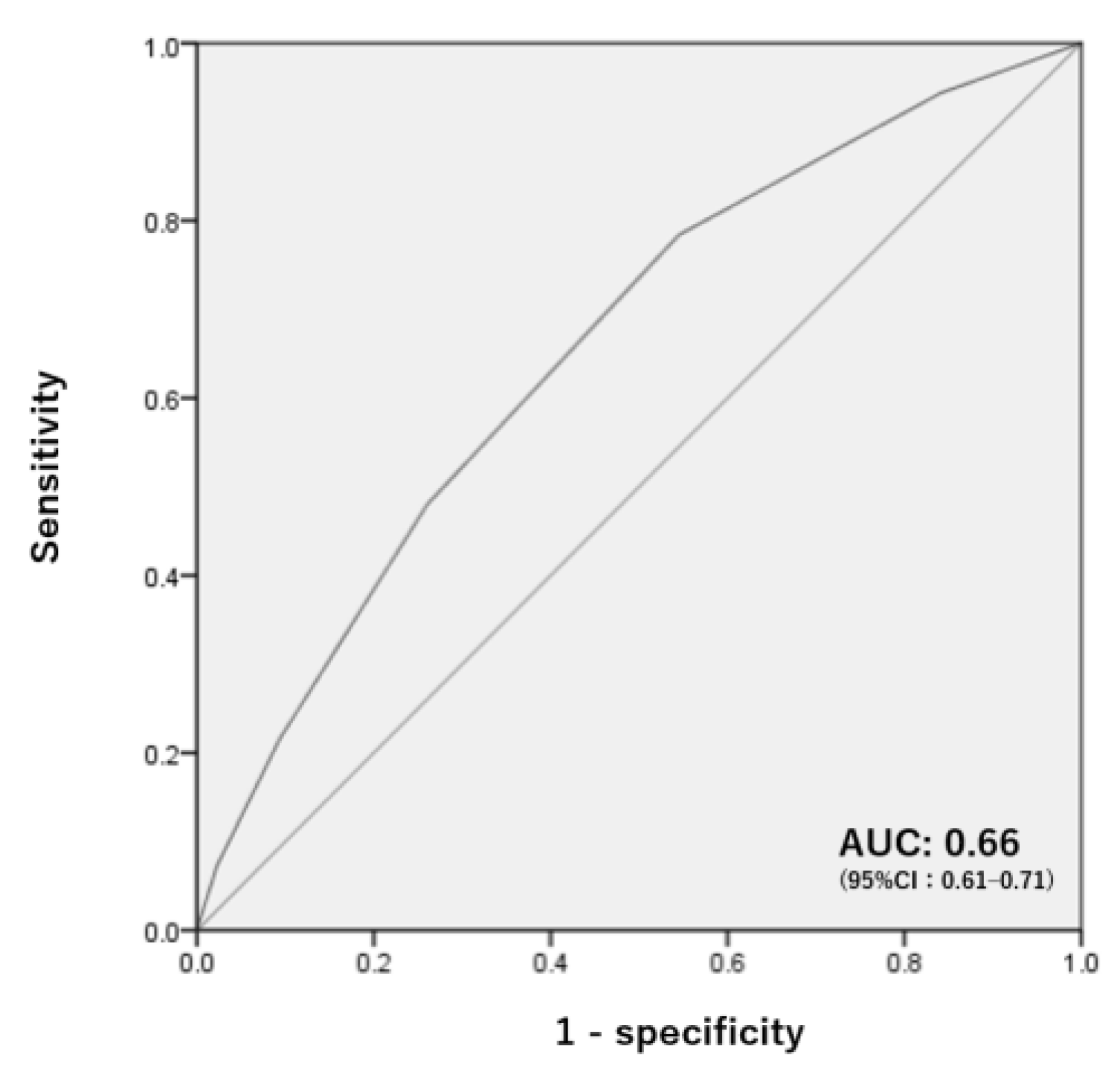
3.4. Establishment of a Delirium Risk Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pisani, M.A.; Araujo, K.L.; Van Ness, P.H.; Zhang, Y.; Ely, E.W.; Inouye, S.K. A research algorithm to improve detection of delirium in the intensive care unit. Crit. Care 2006, 10, R121. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Cosway, S.; Carver, D.; Jarrett, P.; Stadnyk, K.; Fisk, J. The risk of dementia and death after delirium. Age Ageing 1999, 28, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Leslie, D.L.; Zhang, Y.; Holford, T.R.; Bogardus, S.T.; Leo-Summers, L.S.; Inouye, S.K. Premature death associated with delirium at 1-year follow-up. Arch. Intern. Med. 2005, 165, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics; World Health Organization: Geneva, Switzerland, 2016; Volume 2016, pp. 7–11. [Google Scholar]
- AlEissa, S.I.; Tamai, K.; Konbaz, F.; Alturkistany, A.; Blattert, T.R.; Chhabra, H.S.; Costanzo, G.; Dohring, E.J.; Kandziora, F.; Kothe, R.; et al. SPINE20 A global advocacy group promoting evidence-based spine care of value. Eur. Spine J. 2021, 30, 2091–2101. [Google Scholar] [CrossRef]
- Kannus, P.; Sievanen, H.; Palvanen, M.; Jarvinen, T.; Parkkari, J. Prevention of falls and consequent injuries in elderly people. Lancet 2005, 366, 1885–1893. [Google Scholar] [CrossRef]
- Devivo, M.J. Epidemiology of traumatic spinal cord injury: Trends and future implications. Spinal Cord 2012, 50, 365–372. [Google Scholar] [CrossRef]
- Lenehan, B.; Street, J.; Kwon, B.K.; Noonan, V.; Zhang, H.; Fisher, C.G.; Dvorak, M.F. The epidemiology of traumatic spinal cord injury in British Columbia, Canada. Spine 2012, 37, 321–329. [Google Scholar] [CrossRef]
- DeVivo, M.J.; Chen, Y. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch. Phys. Med. Rehabil. 2011, 92, 332–338. [Google Scholar] [CrossRef]
- Miyakoshi, N.; Suda, K.; Kudo, D.; Sakai, H.; Nakagawa, Y.; Mikami, Y.; Suzuki, S.; Tokioka, T.; Tokuhiro, A.; Takei, H.; et al. A nationwide survey on the incidence and characteristics of traumatic spinal cord injury in Japan in 2018. Spinal Cord 2021, 59, 626–634. [Google Scholar] [CrossRef]
- Street, J.T.; Thorogood, N.P.; Cheung, A.; Noonan, V.K.; Chen, J.; Fisher, C.G.; Dvorak, M.F. Use of the Spine Adverse Events Severity System (SAVES) in patients with traumatic spinal cord injury. A comparison with institutional ICD-10 coding for the identification of acute care adverse events. Spinal Cord 2013, 51, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Thorogood, N.P.; Noonan, V.K.; Zhong, Y.; Fisher, C.G.; Dvorak, M.F.; Street, J. Onset, risk factors, and impact of delirium in patients with traumatic spinal cord injury. J. Neurotrauma 2013, 30, 1824–1829. [Google Scholar] [CrossRef]
- LaMantia, M.A.; Messina, F.C.; Hobgood, C.D.; Miller, D.K. Screening for delirium in the emergency department: A systematic review. Ann. Emerg. Med. 2014, 63, 551–560.e2. [Google Scholar] [CrossRef]
- Hendry, K.; Quinn, T.J.; Evans, J.; Scortichini, V.; Miller, H.; Burns, J.; Cunnington, A.; Stott, D.J. Evaluation of delirium screening tools in geriatric medical inpatients: A diagnostic test accuracy study. Age Ageing 2016, 45, 832–837. [Google Scholar] [CrossRef]
- Nakajima, H.; Yokogawa, N.; Sasagawa, T.; Ando, K.; Segi, N.; Watanabe, K.; Nori, S.; Watanabe, S.; Honjoh, K.; Funayama, T.; et al. Prognostic Factors for Cervical Spinal Cord Injury without Major Bone Injury in Elderly Patients. J. Neurotrauma 2022, 39, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Haberman, S.J. The Analysis of Residuals in Cross-Classified Tables. Biometrics 1973, 29, 205–220. [Google Scholar] [CrossRef]
- Tamai, K.; Terai, H.; Suzuki, A.; Nakamura, H.; Yamashita, M.; Eguchi, Y.; Imagama, S.; Ando, K.; Kobayashi, K.; Matsumoto, M.; et al. Risk factors of cervical surgery related complications in patients older than 80 years. Spine Surg. Relat. Res. 2017, 1, 179–184. [Google Scholar] [CrossRef]
- Bistrian, B.R.; Blackburn, G.L.; Vitale, J.; Cochran, D.; Naylor, J. Prevalence of malnutrition in general medical patients. JAMA 1976, 235, 1567–1570. [Google Scholar] [CrossRef]
- Katsumi, A.; Abe, A.; Tamura, S.; Matsushita, T. Anemia in older adults as a geriatric syndrome: A review. Geriatr. Gerontol. Int. 2021, 21, 549–554. [Google Scholar] [CrossRef]
- Le, E.; Aarabi, B.; Hersh, D.S.; Shanmuganathan, K.; Diaz, C.; Massetti, J.; Akhtar-Danesh, N. Predictors of intramedullary lesion expansion rate on MR images of patients with subaxial spinal cord injury. J. Neurosurg. Spine 2015, 22, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Hoshino, M.; Yasuda, H.; Hori, Y.; Ohyama, S.; Terai, H.; Hayashi, K.; Tsujio, T.; Kono, H.; Suzuki, A.; et al. Development of a scoring system for predicting adjacent vertebral fracture after balloon kyphoplasty. Spine J. 2019, 19, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, H.; Okada, R.; Takagi, T.; Takahashi, M.; Murata, H.; Harada, H.; Nishimura, T.; Asakura, H.; Ogawa, H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014, 3, 1359–1367. [Google Scholar] [CrossRef]
- Zhang, D.F.; Su, X.; Meng, Z.T.; Cui, F.; Li, H.L.; Wang, D.X.; Li, X.Y. Preoperative severe hypoalbuminemia is associated with an increased risk of postoperative delirium in elderly patients: Results of a secondary analysis. J. Crit. Care 2018, 44, 45–50. [Google Scholar] [CrossRef]
- Lachmann, G.; Feinkohl, I.; Borchers, F.; Ottens, T.H.; Nathoe, H.M.; Sauer, A.M.; Dieleman, J.M.; Radtke, F.M.; van Dijk, D.; Spies, C.; et al. Diabetes, but Not Hypertension and Obesity, Is Associated with Postoperative Cognitive Dysfunction. Dement. Geriatr. Cogn. Disord. 2018, 46, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Bryczkowski, S.B.; Lopreiato, M.C.; Yonclas, P.P.; Sacca, J.J.; Mosenthal, A.C. Risk factors for delirium in older trauma patients admitted to the surgical intensive care unit. J. Trauma. Acute Care Surg. 2014, 77, 944–951. [Google Scholar] [CrossRef]
- Burton, J.K.; Craig, L.E.; Yong, S.Q.; Siddiqi, N.; Teale, E.A.; Woodhouse, R.; Barugh, A.J.; Shepherd, A.M.; Brunton, A.; Freeman, S.C.; et al. Non-pharmacological interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst. Rev. 2021, 7, CD013307. [Google Scholar] [CrossRef]
- Park, S.K.; Lim, T.; Cho, H.; Yoon, H.K.; Lee, H.J.; Lee, J.H.; Yoo, S.; Kim, J.T.; Kim, W.H. Comparative effectiveness of pharmacological interventions to prevent postoperative delirium: A network meta-analysis. Sci. Rep. 2021, 11, 11922. [Google Scholar] [CrossRef]

| Delirium | Controls | p-Value | |
|---|---|---|---|
| Number of patients | 56 | 553 | |
| Age (years) ± SD | 81.0 ± 7.0 | 76.7 ± 7.5 | <0.001 * |
| Female/Male | 17/39 | 216/337 | 0.248 # |
| BMI ± SD | 21.7 ± 5.6 | 22.0 ± 3.8 | 0.747 * |
| Pre-injury mobility | 0.028 # | ||
| Independent | 43 | 488 | |
| Walk with assistance | 12 | 54 | |
| Wheelchair/bedridden | 1 | 8 | |
| Blood test data | |||
| TP (g/dL) ± SD | 6.5 ± 0.9 | 6.7 ± 0.7 | 0.165 * |
| Alb (g/dL) ± SD | 3.4 ± 1.0 | 3.8 ± 0.7 | 0.011 * |
| Hb (g/dL) ± SD | 12.0 ± 2.2 | 12.6 ± 1.9 | 0.038 * |
| Comorbidity | |||
| Dementia | 8 | 40 | 0.070 # |
| Diabetes | 11 | 122 | 0.738 # |
| Hypertension | 32 | 267 | 0.211 # |
| ASIA Impairment Scale | 0.022 # | ||
| A/B/C/D/No neurological deficits | 6/5/9/12/24 | 37/20/100/225/171 | |
| Radiographic findings | |||
| Cervical fracture | 36 | 267 | 0.025 # |
| Cervical OPLL | 10 | 97 | 1.000 # |
| Spinal signal change on MRI | 29 | 287 | 0.987 # |
| Comorbid major organs injury | 27 | 167 | 0.006 # |
| Conservative therapy | |||
| Steroid use | 11 | 95 | 0.711 # |
| Halo-traction | 9 | 57 | 0.173 # |
| Neck brace | 44 | 434 | 0.861 # |
| Explanatory Variables | Reference | aOR | p-Value | 95% CI | |
|---|---|---|---|---|---|
| Age | ≥80 years | >80 | 2.26 | 0.024 | 1.11–4.59 |
| Mobility | with assistance | Independent | 1.42 | 0.430 | 0.59–3.34 |
| Hypoalbuminemia | (3.5 g/dL > Alb) | 3.5≤ | 2.15 | 0.043 | 1.03–4.50 |
| Anemia | (12 g/dL > Hb) | 12≤ | 0.77 | 0.475 | 0.37–1.59 |
| ASIA scale | A, B | C, D, NoD | 1.77 | 0.204 | 0.73–4.27 |
| Cervical fracture | with | without | 2.33 | 0.020 | 1.14–4.76 |
| Major-organ injury | with | without | 2.01 | 0.045 | 1.01–3.99 |
| Delirium | Controls | p-Value | |
|---|---|---|---|
| Number of patients | 66 | 837 | |
| Age (years) ± SD | 77.2 ± 6.1 | 74.8 ± 6.3 | 0.003 * |
| Female/Male | 12/54 | 260/577 | <0.001 # |
| BMI ± SD | 22.5 ± 3.9 | 22.1 ± 3.4 | 0.459 * |
| Pre-injury mobility | 0.024 # | ||
| Independent | 53 | 757 | |
| Walk with assistance | 6 | 44 | |
| Wheelchair/bedridden | 7 | 36 | |
| Blood test data | |||
| TP (g/dL) ± SD | 6.6 ± 0.7 | 6.6 ± 0.7 | 0.825 * |
| Alb (g/dL) ± SD | 3.6 ± 0.6 | 3.7 ± 0.7 | 0.241 * |
| Hb (g/dL) ± SD | 12.8 ± 2.0 | 12.7 ± 1.9 | 0.712 * |
| Comorbidity | |||
| Dementia | 13 | 34 | <0.001 # |
| Diabetes | 21 | 176 | 0.045 # |
| Hypertension | 39 | 393 | 0.073 # |
| ASIA Impairment Scale | 0.008 # | ||
| A/B/C/D/No neurological deficits | 10/3/21/10/22 | 81/48/196/271/241 | |
| Radiographic findings | |||
| Cervical fracture | 48 | 483 | 0.019 # |
| Cervical OPLL | 16 | 109 | 0.015 # |
| Spinal signal change on MRI | 37 | 209 | <0.001 # |
| Comorbid major organs injury | 18 | 199 | 0.550 # |
| Surgical therapy | |||
| Early intervention (≤24 h) | 2 | 85 | 0.079 # |
| Surgical method | |||
| Posterior decomp | 14 | 261 | 0.094 # |
| Posterior fusion ± decomp | 42 | 497 | |
| Anterior fusion ± decomp | 9 | 57 | |
| Combined fusion ± decomp | 1 | 22 |
| Explanatory Variables | Reference | aOR | p-Value | 95% CI | |
|---|---|---|---|---|---|
| Age | ≥80 years | >80 | 2.75 | <0.001 | 1.58–4.80 |
| Sex | female | male | 0.54 | 0.070 | 0.28–1.05 |
| Mobility | with assistance | independent | 2.28 | 0.023 | 1.12–4.62 |
| Diabetes | with | without | 1.91 | 0.030 | 1.06–3.44 |
| Hypertension | with | without | 1.53 | 0.128 | 0.89–2.64 |
| ASIA scale | A, B | C, D, ND | 1.22 | 0.604 | 0.58–2.59 |
| Cervical fracture | with | without | 2.15 | 0.019 | 1.14–4.06 |
| Cervical OPLL | with | without | 0.84 | 0.567 | 0.43–1.59 |
| Signal change | with | without | 1.08 | 0.800 | 0.58–2.02 |
| Number of Factors | Sensitivity | Specificity |
|---|---|---|
| 1 | 0.944 | 0.159 |
| 2 | 0.784 | 0.455 |
| 3 | 0.480 | 0.740 |
| 4 | 0.216 | 0.905 |
| 5 | 0.008 | 0.992 |
| 6 | 0.001 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamai, K.; Terai, H.; Nakamura, H.; Yokogawa, N.; Sasagawa, T.; Nakashima, H.; Segi, N.; Ito, S.; Funayama, T.; Eto, F.; et al. Delirium Risk Score in Elderly Patients with Cervical Spinal Cord Injury and/or Cervical Fracture. J. Clin. Med. 2023, 12, 2387. https://doi.org/10.3390/jcm12062387
Tamai K, Terai H, Nakamura H, Yokogawa N, Sasagawa T, Nakashima H, Segi N, Ito S, Funayama T, Eto F, et al. Delirium Risk Score in Elderly Patients with Cervical Spinal Cord Injury and/or Cervical Fracture. Journal of Clinical Medicine. 2023; 12(6):2387. https://doi.org/10.3390/jcm12062387
Chicago/Turabian StyleTamai, Koji, Hidetomi Terai, Hiroaki Nakamura, Noriaki Yokogawa, Takeshi Sasagawa, Hiroaki Nakashima, Naoki Segi, Sadayuki Ito, Toru Funayama, Fumihiko Eto, and et al. 2023. "Delirium Risk Score in Elderly Patients with Cervical Spinal Cord Injury and/or Cervical Fracture" Journal of Clinical Medicine 12, no. 6: 2387. https://doi.org/10.3390/jcm12062387
APA StyleTamai, K., Terai, H., Nakamura, H., Yokogawa, N., Sasagawa, T., Nakashima, H., Segi, N., Ito, S., Funayama, T., Eto, F., Yamaji, A., Watanabe, K., Yamane, J., Takeda, K., Furuya, T., Yunde, A., Nakajima, H., Yamada, T., Hasegawa, T., ... Kato, S. (2023). Delirium Risk Score in Elderly Patients with Cervical Spinal Cord Injury and/or Cervical Fracture. Journal of Clinical Medicine, 12(6), 2387. https://doi.org/10.3390/jcm12062387










