Endometrial Cytology in Diagnosis of Endometrial Cancer: A Systematic Review and Meta-Analysis of Diagnostic Accuracy
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Quality Control
2.3. Statistical Analysis
3. Results
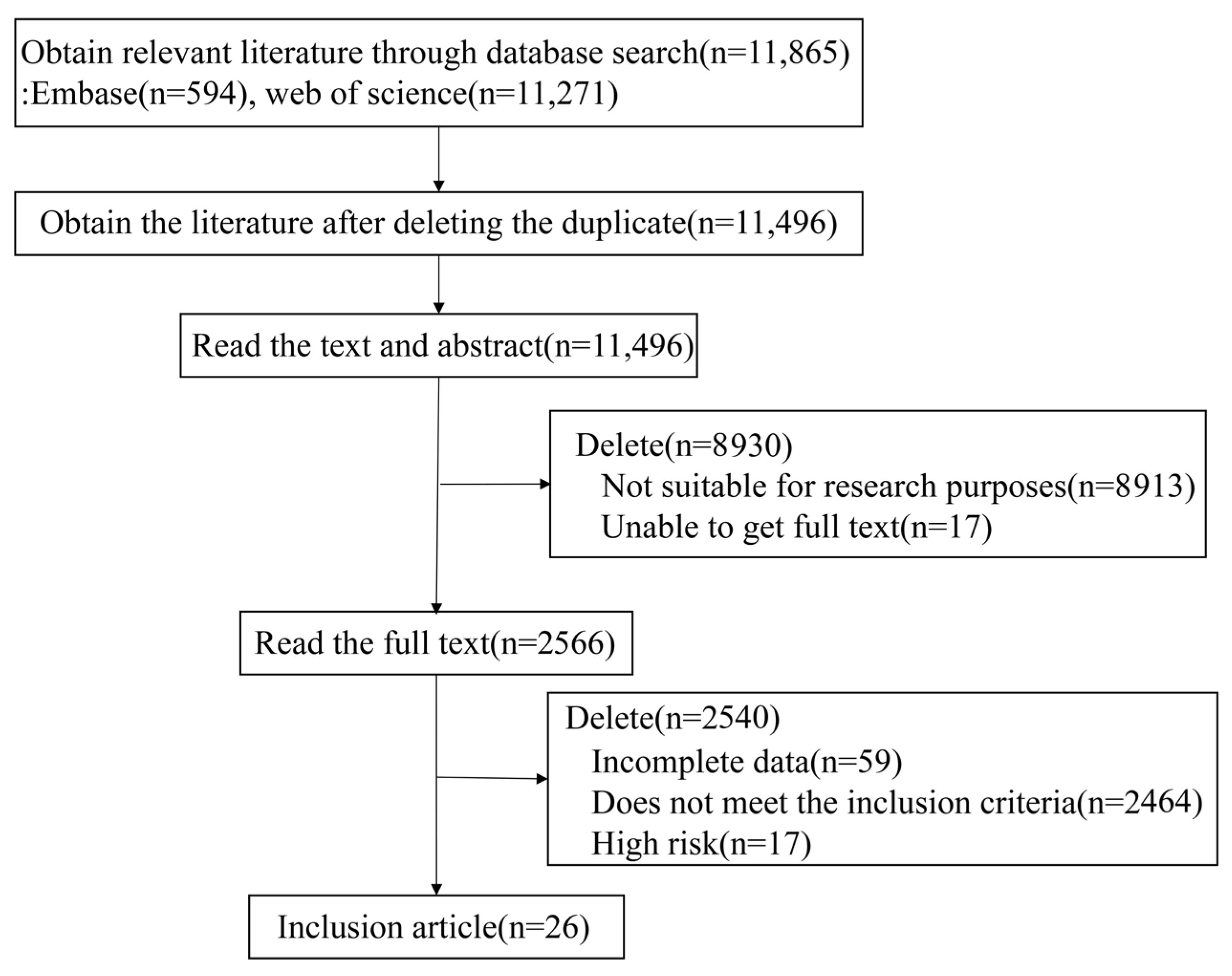
3.1. Study Selection
3.2. Basic Characteristics and Quality of Selected Literature
3.3. Diagnostic Accuracy of ECT
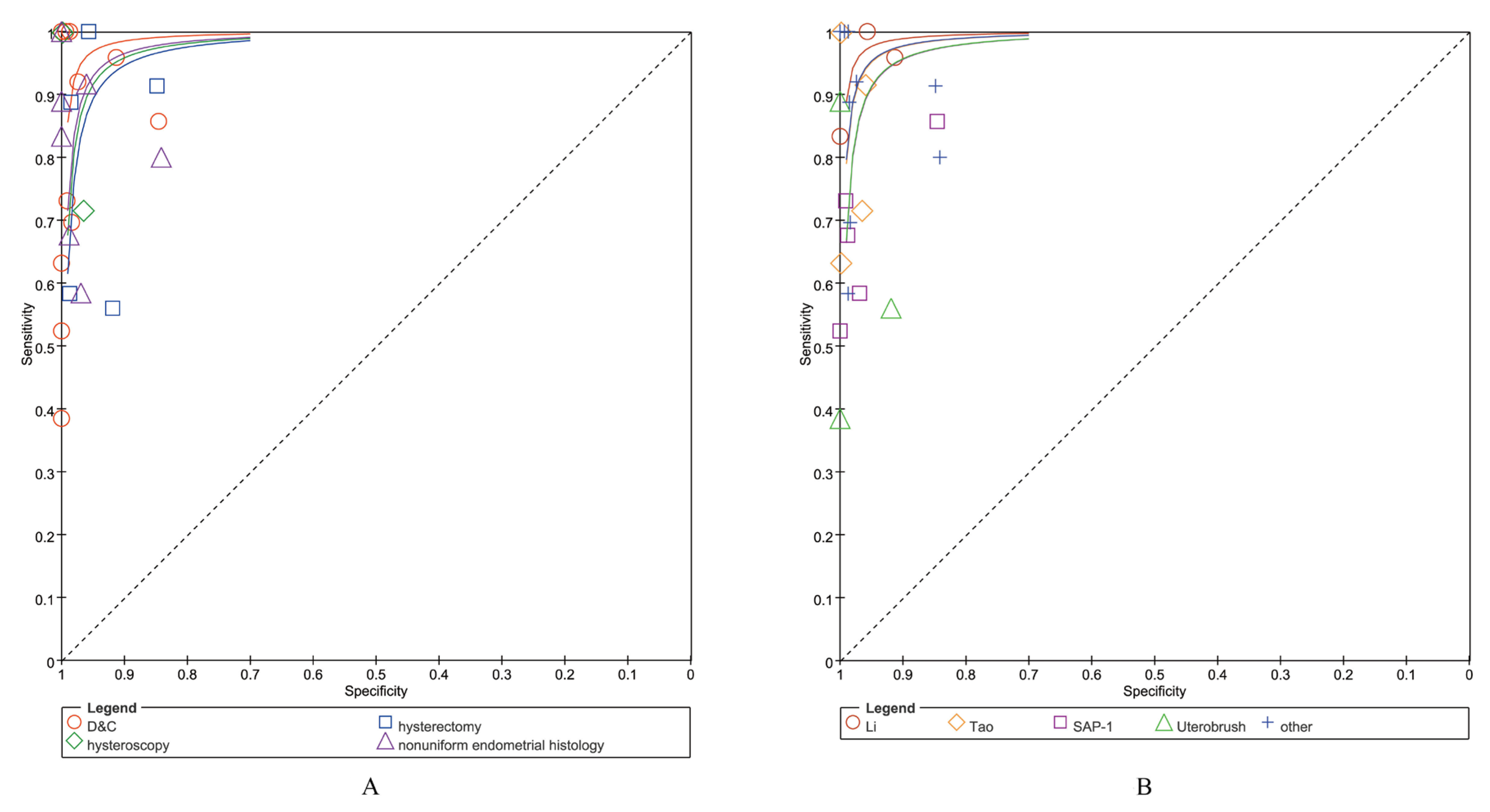
3.3.1. Diagnostic Accuracy of ECT Using Different Reference Standards
3.3.2. Diagnostic Accuracy of ECT with Different Cytological Sampling Methods
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Long, B.J.; Del, M.M.A.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of endometrial cancer risk with postmenopausal bleeding in women: A systematic review and meta-analysis. JAMA Intern. Med. 2018, 178, 1210–1222. [Google Scholar] [CrossRef]
- Pennant, M.E.; Mehta, R.; Moody, P.; Hackett, G.; Prentice, A.; Sharp, S.J.; Lakshman, R. Premenopausal abnormal uterine bleeding and risk of endometrial cancer. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 404–411. [Google Scholar] [CrossRef]
- Morrison, J.; Balega, J.; Buckley, L.; Clamp, A.; Crosbie, E.; Drew, Y.; Durrant, L.; Forrest, J.; Fotopoulou, C.; Gajjar, K.; et al. British gynaecological cancer society (bgcs) uterine cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, 50–89. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Sanli, S.; Bakir, V.L.; Ayas, S.; Yildiz, S.O.; Iyibozkurt, A.C.; Kartal, M.G.; Yavuz, E. Role of diffusion weighted mri in the differential diagnosis of endometrial cancer, polyp, hyperplasia, and physiological thickening. Clin. Imaging 2017, 41, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Gostout, B.S.; Dowdy, S.C.; Multinu, F.; Casarin, J.; Cliby, W.A.; Frigerio, L.; Kim, B.; Weaver, A.L.; Glaser, G.E.; et al. Clinical utility of preoperative computed tomography in patients with endometrial cancer. Int. J. Gynecol. Cancer 2017, 27, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, K.; Chen, R.; Zhao, J.; Wu, C.; Zhang, N.Y.; Ma, X.H.; Dong, Y.; Zhu, S.N.; Liao, Q.P. Liquid-based endometrial cytology associated with curettage in the investigation of endometrial carcinoma in a population of 1987 women. Arch. Gynecol. Obstet. 2017, 296, 99–105. [Google Scholar] [CrossRef]
- Munakata, S. Diagnostic value of endometrial cytology and related technology. Diagn. Cytopathol. 2022, 50, 363–366. [Google Scholar] [CrossRef]
- Norimatsu, Y.; Yanoh, K.; Hirai, Y.; Kurokawa, T.; Kobayashi, T.K.; Fulciniti, F. A diagnostic approach to endometrial cytology by means of liquid-based preparations. Acta Cytol. 2020, 64, 195–207. [Google Scholar] [CrossRef]
- Yanoh, K.; Norimatsu, Y.; Munakata, S.; Yamamoto, T.; Nakamura, Y.; Murata, T.; Kobayashi, T.K.; Hirai, Y. Evaluation of endometrial cytology prepared with the becton dickinson surepath method: A pilot study by the osaki study group. Acta Cytol. 2014, 58, 153–161. [Google Scholar] [CrossRef]
- Akahane, T.; Kitazono, I.; Kobayashi, Y.; Nishida-Kirita, Y.; Yamaguchi, T.; Yanazume, S.; Tabata, K.; Kobayashi, H.; Tanimoto, A. Direct next-generation sequencing analysis using endometrial liquid-based cytology specimens for rapid cancer genomic profiling. Diagn. Cytopathol. 2021, 49, 1078–1085. [Google Scholar] [CrossRef]
- Akahane, T.; Kitazono, I.; Yanazume, S.; Kamio, M.; Togami, S.; Sakamoto, I.; Nohara, S.; Yokoyama, S.; Kobayashi, H.; Hiraki, T.; et al. Next-generation sequencing analysis of endometrial screening liquid-based cytology specimens: A comparative study to tissue specimens. BMC Med. Genom. 2020, 13, 101. [Google Scholar] [CrossRef]
- Di Lorito, A.; Zappacosta, R.; Capanna, S.; Gatta, D.M.; Rosini, S.; Schmitt, F.C. Expression of pten in endometrial liquid-based cytology. Acta Cytol. 2014, 58, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Munakata, S.; Yamamoto, T. Application of immunocytochemical and molecular analysis of six genes in liquid-based endometrial cytology. Diagn. Cytopathol. 2022, 50, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Norimatsu, Y.; Miyamoto, M.; Kobayashi, T.K.; Moriya, T.; Shimizu, K.; Yanoh, K.; Tsukayama, C.; Miyake, Y.; Ohno, E. Diagnostic utility of phosphatase and tensin homolog, beta-catenin, and p53 for endometrial carcinoma by thin-layer endometrial preparations. Cancer—Am. Cancer Soc. 2008, 114, 155–164. [Google Scholar]
- Norimatsu, Y.; Miyamoto, T.; Kobayashi, T.K.; Oda, T.; Moriya, T.; Yanoh, K.; Miyake, Y.; Ohno, E. Utility of thin-layer preparations in endometrial cytology: Immunocytochemical expression of pten, beta-catenin and p53 for benign endometrial lesions. Diagn. Cytopathol. 2008, 36, 216–223. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Douville, C.; Cohen, J.D.; Yen, T.T.; Kinde, I.; Sundfelt, K.; Kjaer, S.K.; Hruban, R.H.; Shih, I.M.; et al. Evaluation of liquid from the papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci. Transl. Med. 2018, 10, eaap8793. [Google Scholar] [CrossRef]
- Mcinnes, M.; Moher, D.; Thombs, B.D.; Mcgrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The prisma-dta statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.X.; Huang, X.W. Quadas-2 tool for quality assessment in diagnostic meta-analysis. Ann. Palliat. Med. 2022, 11, 1844–1845. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Abdelazim, I.A.; Abdelrazak, K.M.; Elbiaa, A.A.M.; Al-Kadi, M.; Yehia, A.H. Accuracy of endometrial sampling compared to conventional dilatation and curettage in women with abnormal uterine bleeding. Arch. Gynecol. Obstet. 2015, 291, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.B.; Monahan, P.B.; Warrell, D.W. Kuper brush in the diagnosis of endometrial lesions. Lancet 1971, 298, 1390–1392. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yunong, G.; Xiuhua, M.; Wanli, G.; Xi, Y.; Jian, Z.; Yue, C.; Naiyi, Z.; Nan, Z.; Qinping, L. Analysis of endometrial carcinoma screening method in 445 postmenopausal women. Chin. J. Pract. Gynecol. Obstetr. 2014, 30, 870–873. [Google Scholar]
- Ferenczy, A.; Gelfand, M.M. Outpatient endometrial sampling with endocyte—Comparative-study of its effectiveness with endometrial biopsy. Obstet. Gynecol. 1984, 63, 295–302. [Google Scholar]
- Fujiwara, H.; Takahashi, Y.; Takano, M.; Miyamoto, M.; Nakamura, K.; Kaneta, Y.; Hanaoka, T.; Ohwada, M.; Sakamoto, T.; Hirakawa, T.; et al. Evaluation of endometrial cytology: Cytohistological correlations in 1441 cancer patients. Oncology 2015, 88, 86–94. [Google Scholar] [CrossRef]
- Han, L.; Ma, S.; Zhao, L.; Liu, Y.; Wang, Y.; Feng, X.; Zhang, K.; Wang, L.; Wang, L.; Yin, P.; et al. Clinical evaluation of li brush endometrial samplers for diagnosing endometrial lesions in women with intrauterine devices. Front. Med. 2020, 7, 598689. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Du, J.; Zhao, L.; Sun, C.; Wang, Q.; Tuo, X.; Hou, H.; Liu, Y.; Wang, Q.; Ulain, Q.; et al. An efficacious endometrial sampler for screening endometrial cancer. Front. Oncol. 2019, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Hongzhen, Y.; Zhihao, W.; Qian, S.; Shan, H.; Xingjie, D.; Qiang, W. To analyze the feasibility of endometrial cytology in screening endometrial cancer from the perspective of cytopathology. J. Clin. Exp. Pathol. 2021, 37, 23–28. [Google Scholar]
- Iavazzo, C.; Vorgias, G.; Mastorakos, G.; Stefanatou, G.; Panoussi, A.; Alexiadou, A.; Plyta, S.; Lekka, C.; Kalinoglou, N.; Dertimas, V.; et al. Uterobrush method in the detection of endometrial pathology. Anticancer Res. 2011, 31, 3469–3474. [Google Scholar]
- Indraccolo, U.; Bracalenti, C.; Di Iorio, R.; Indraccolo, S.R. Could endometrial cytology be helpful in detecting endometrial malignancies? Eur. J. Gynaecol. Oncol. 2012, 33, 60–61. [Google Scholar]
- Kipp, B.R.; Medeiros, F.; Campion, M.B.; Distad, T.J.; Peterson, L.M.; Keeney, G.L.; Halling, K.C.; Clayton, A.C. Direct uterine sampling with the tao brush sampler using a liquid-based preparation method for the detection of endometrial cancer and atypical hyperplasia: A feasibility study. Cancer—Am. Cancer Soc. 2008, 114, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Klemi, P.J.; Alanen, K.A.; Salmi, T. Detection of malignancy in endometrium by brush sampling in 1042 symptomatic patients. Int. J. Gynecol. Cancer 1995, 5, 222–225. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Q.; Li, Y.; Zhao, L.; Wang, Y.; Feng, X.; Han, L.; Zhang, K.; Yin, P.; Hou, H.; et al. A clinical comparative study of two different endometrial cell samplers for evaluation of endometrial lesions by cytopathological diagnosis. Cancer Manag. Res. 2020, 12, 10551–10557. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yang, X.; Chen, R.; Zhao, J.; Dong, Y.; Zhang, N.; Ma, X.; Liao, Q. Liquid-based endometrial cytology associated with curettage in the investigation of endometrial carcinoma in postmenopausal women. Taiwan. J. Obstet. Gynecol. 2016, 55, 777–781. [Google Scholar] [CrossRef]
- Maksem, J.A.; Knesel, E. Liquid fixation of endometrial brush cytology ensures a well-preserved, representative cell sample with frequent tissue correlation. Diagn. Cytopathol. 1996, 14, 367–373. [Google Scholar] [CrossRef]
- Mardi, K.; Rao, M.; Bhardwaj, M.; Sharma, M. Utility of endometrial aspiration cytology for screening postmenopausal women for endometrial malignancies. Clin. Cancer Investig. J. 2017, 6, 35. [Google Scholar] [CrossRef]
- Mathelin, C.; Youssef, C.; Annane, K.; Brettes, J.; Bellocq, J.; Walter, P. Endometrial brush cytology in the surveillance of post-menopausal patients under tamoxifen: A prospective longitudinal study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 132, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Milojkovic, M.; Sijanovic, S. Assessment of reliability endometrial brush cytology in detection etiology of late postmenopausal bleedings. Arch. Gynecol. Obstet. 2004, 269, 259–262. [Google Scholar] [CrossRef]
- O’Flynn, H.; Ryan, N.A.J.; Narine, N.; Shelton, D.; Rana, D.; Crosbie, E.J. Diagnostic accuracy of cytology for the detection of endometrial cancer in urine and vaginal samples. Nat. Commun. 2021, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Polson, D.W.; Morse, A.; Beard, R.W. An alternative to the diagnostic dilatation and curettage—endometrial cytology. Br. Med. J. 1984, 288, 981–983. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Vandendael, A.; Wranz, P.A.; Lombard, C.J. Endopap-versus pipelle-sampling in the diagnosis of postmenopausal endometrial disease. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 64, 91–94. [Google Scholar] [CrossRef]
- Wen, J.; Chen, R.; Zhao, J.; Dong, Y.; Yang, X.; Liao, Q. Combining endometrium sampling device and surepath preparation to screen for endometrial carcinoma. Chin. Med. J. 2015, 128, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Harshbarger, K.E.; Berner, H.W.; Elsheikh, T.M. Endometrial brush biopsy (tao brush). Histologic diagnosis of 200 cases with complementary cytology: An accurate sampling technique for the detection of endometrial abnormalities. Am. J. Clin. Pathol. 2000, 114, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Qinping, L.; Cheng, W.; Naiyi, Z.; Jiang, Z.; Yue, C. Accuracy of the endometial cytology test for the screening of endometrial cancer. Chin. J. Obstet. Gynecol. 2013, 48, 884–890. [Google Scholar]
- Yanaki, F.; Hirai, Y.; Hanada, A.; Ishitani, K.; Matsui, H. Liquid-based endometrial cytology using surepath is not inferior to suction endometrial tissue biopsy in clinical performance for detecting endometrial cancer including atypical endometrial hyperplasia. Acta Cytol. 2017, 61, 133–139. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Kerlikowske, K.; Feldstein, V.A.; Subak, L.; Scheidler, J.; Segal, M.; Brand, R.; Grady, D. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA 1998, 280, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- De Iaco, P.; Marabini, A.; Stefanetti, M.; Del, V.C.; Bovicelli, L. Acceptability and pain of outpatient hysteroscopy. J. Am. Assoc. Gynecol. Laparosc. 2000, 7, 71–75. [Google Scholar] [PubMed]
- Visser, N.; Reijnen, C.; Massuger, L.; Nagtegaal, I.D.; Bulten, J.; Pijnenborg, J. Accuracy of endometrial sampling in endometrial carcinoma: A systematic review and meta-analysis. Obstet. Gynecol. 2017, 130, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, Y.; Lv, S.; Wang, Q.; Sun, C.; Dong, X.; He, M.; Ulain, Q.; Yuan, Y.; Tuo, X.; et al. Endometrial sampling devices for early diagnosis of endometrial lesions. J. Cancer Res. Clin. Oncol. 2016, 142, 2515–2522. [Google Scholar] [CrossRef]
- Clark, T.J.; Voit, D.; Gupta, J.K.; Hyde, C.; Song, F.; Khan, K.S. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: A systematic quantitative review. JAMA 2002, 288, 1610–1621. [Google Scholar] [CrossRef]
- Jones, E.R.; Carter, S.; O’Flynn, H.; Njoku, K.; Barr, C.E.; Narine, N.; Shelton, D.; Rana, D.; Crosbie, E.J. Developing tests for endometrial cancer detection (detect): Protocol for a diagnostic accuracy study of urine and vaginal samples for the detection of endometrial cancer by cytology in women with postmenopausal bleeding. BMJ Open 2021, 11, e50755. [Google Scholar] [CrossRef]
- Davey, E.; Barratt, A.; Irwig, L.; Chan, S.F.; Macaskill, P.; Mannes, P.; Saville, A.M. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: A systematic review. Lancet 2006, 367, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Dey, P. The emerging role of deep learning in cytology. Cytopathology 2021, 32, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Rao, J. Computational technology with artificial intelligence and machine learning: What should a cytologist do with it? Acta Cytol. 2021, 65, 283–285. [Google Scholar] [CrossRef] [PubMed]
- William, W.; Ware, A.; Basaza-Ejiri, A.H.; Obungoloch, J. A review of image analysis and machine learning techniques for automated cervical cancer screening from pap-smear images. Comput. Methods Programs Biomed. 2018, 164, 15–22. [Google Scholar] [CrossRef] [PubMed]

| Country | Study Design | ECT Sampling | Reference Standard | Menopausal Status | Effective Sample Number | |
|---|---|---|---|---|---|---|
| Alex Ferenczy, 1984 [25] | Canada | prospectively | Endocyte | D&C | pre-&post-menopause | 180 |
| Benjamin R. Kipp, 2008 [32] | USA | prospectively | Tao brush | Hysterectomy | pre-&post-menopause | 139 |
| C. Iavazzo, 2011 [30] | Greece | prospectively | Uterobrush | Histopathologic findings | pre-&post-menopause | 100 |
| Carole Mathelin, 2007 [38] | France | prospectively | Endobrush | D&C | not clear | 150 |
| D. W. Polson, 1984 [41] | UK | prospectively | Aspiration | Curettage | postmenopause | 51 |
| E. Blanche Butler, 1971 [23] | UK | prospectively | Kuper brush | D&C or hysterectomy | pre-&post-menopause | 41 |
| Fumiko Yanaki, 2017 [46] | Japan | retrospective | Honest Super brush | D&C | not clear | 1116 |
| Helena O’Flynn, 2021 [40] | UK | prospectively | Evalyn brush | Hysterectomy | pre-&post-menopause | 216 |
| Hiroyuki Fujiwara, 2015 [26] | Japan | prospectively | endometrial cytology | Hysterectomy | pre-&post-menopause | 2802 |
| Howard Her-Juing Wu, 2003 [44] | USA | prospectively | Tao brush | D&C | pre-&post-menopause | 156 |
| Ibrahim A. Abdelazim, 2014 [22] | Egypt | prospectively | Tao brush | D&C | pre-&post-menopause | 217 |
| Jia Wen, 2015 [43] | China | prospectively | SAP-1 | D&C | pre-&post-menopause | 254 |
| John A. Maksem, 1995 [36] | USA | prospectively | MedScand cytobrush | Hysterectomy | not clear | 639 |
| Kavita Mardi, 2017 [37] | India | prospectively | aspiration cytology | D&C | postmenopause | 86 |
| Ke Ma, 2016 [35] | China | prospectively | SAP-1 | D&C or hysterectomy | postmenopause | 567 |
| Kenji Yanoh, 2014 [11] | Japan | prospectively | SurePath | Endometrial biopsy or curettage | pre-/postmenopause | 102 |
| Lu Han, 2018 [28] | China | prospectively | Li brush | Hysterectomy | pre-/postmenopause | 299 |
| Lu Han, 2020 [27] | China | prospectively | Li brush | D&C | pre-/postmenopause | 212 |
| Miodrag Milojkovic, 2002 [39] | Croatia | prospectively | Uterobrush | Hysterectomy | postmenopause | 62 |
| P. J. Klemi, 1995 [33] | Finland | prospectively | Uterobrush | D&C | pre-/postmenopause | 313 |
| Shulan Lv, 2020 [34] | China | prospectively | Tao brush/Li brush | D&C or hysterectomy | pre-&post-menopause | 224 |
| Thierry Van den Bosch, 1996 [42] | South Africa | prospectively | Endopap | Hysteroscopy or hysterectomy | postmenopause | 87 |
| U. Indraccolo, 2011 [31] | Italy | prospectively | sterile brush | Hysteroscopy | not clear | 37 |
| Wu Cheng, 2014 [24] | China | prospectively | SAP-1 | D&C | postmenopause | 231 |
| Yang Xi, 2013 [45] | China | prospectively | SAP-1 | D&C or hysterectomy | pre-&post-menopause | 1520 |
| Yu Hongzhen, 2021 [29] | China | prospectively | SAP-1 | D&C | not clear | 1725 |
| Study and Reference Standard | Sens | Spec | LR- | LR+ |
|---|---|---|---|---|
| D&C | ||||
| D. W. Polson, 1984 [41] | 1.00 | 1.00 | 0.05 | 80.18 |
| Ibrahim A. Abdelazim, 2014 [22] | 1.00 | 1.00 | 0.17 | ∞ |
| Lu Han, 2020 [27] | 0.96 | 0.91 | 0.05 | 11.03 |
| Jia Wen, 2015 [43] | 0.73 | 0.99 | 0.27 | 83.31 |
| P. J. Klemi, 1995 [33] | 0.38 | 1.00 | 0.61 | ∞ |
| Howard Her-Juing Wu, 2003 [44] | 0.63 | 1.00 | 0.38 | ∞ |
| Carole Mathelin, 2007 [38] | 1.00 | 0.99 | 0.13 | 86.33 |
| Kenji Yanoh, 2014 [11] | 0.92 | 0.97 | 0.08 | 35.42 |
| Fumiko Yanaki, 2017 [46] | 0.70 | 0.98 | 0.31 | 43.42 |
| Alex Ferenczy, 1984 [25] | 1.00 | 1.00 | 0.17 | ∞ |
| Kavita Mardi, 2017 [37] | 1.00 | 0.99 | 0.05 | 49.4 |
| Yu Hongzhen, 2021 [29] | 0.52 | 1.00 | 0.48 | ∞ |
| Wu Cheng, 2014 [24] | 0.86 | 0.85 | 0.17 | 5.56 |
| Pooled sens/spec/LR (range) | 0.81 (0.77–0.85) | 0.99 (0.98–0.99) | 0.22 (0.13–0.36) | 59.74 (25.17–∞) |
| I2 (%) | 80.6 | 93.3 | 77.4 | 86.6 |
| Hysteroscopy | ||||
| U. Indraccolo, 2011 [31] | 1.00 | 1.00 | 0.10 | 61.20 |
| Benjamin R. Kipp, 2008 [32] | 0.72 | 0.97 | 0.29 | 20.77 |
| Pooled sens/spec/LR (range) | 0.73 (0.62–0.82) | 0.98 (0.92–1.00) | 0.29 (0.20–0.41) | 25.68 (7.53–87.58) |
| I2 (%) | 61.5 | 45.3 | 0.0 | 0.0 |
| Hysterectomy | ||||
| Miodrag Milojkovic, 2002 [39] | 0.56 | 0.92 | 0.48 | 6.91 |
| Lu Han, 2018 [28] | 1.00 | 0.96 | 0.01 | 21.93 |
| Hiroyuki Fujiwara, 2015 [26] | 0.89 | 0.99 | 0.11 | 60.40 |
| John A. Maksem, 1995 [36] | 0.58 | 0.99 | 0.42 | 45.72 |
| Helena O’Flynn, 2021 [40] | 0.91 | 0.85 | 0.10 | 6.02 |
| Pooled sens/spec/LR (range) | 0.89 (0.87–0.90) | 0.98 (0.97–0.98) | 0.18 (0.08–0.42) | 19.40 (6.09–61.80) |
| I2 (%) | 88.8 | 92.4 | 92.5 | 94.7 |
| Nonuniform endometrial samples | ||||
| Shulan Lv, 2020 (1) [34] | 0.92 | 0.96 | 0.09 | 23.15 |
| Shulan Lv, 2020 (2) [34] | 0.83 | 1.00 | 0.19 | ∞ |
| Thierry Van den Bosch, 1996 [42] | 0.80 | 0.84 | 0.24 | 5.05 |
| E. Blanche Butler, 1971 [23] | 1.00 | 1.00 | 0.07 | 66.86 |
| Ke Ma, 2016 [35] | 0.58 | 0.97 | 0.43 | 18.94 |
| C. Iavazzo, 2011 [30] | 0.89 | 1.00 | 0.15 | ∞ |
| Yang Xi, 2013 [45] | 0.68 | 0.99 | 0.33 | 56.28 |
| Pooled sens/spec/LR (range) | 0.67 (0.61–0.73) | 0.98 (0.97–0.98) | 0.31 (0.22–0.44) | 28.63 (11.18–73.36) |
| I2 (%) | 64.7 | 88.2 | 42.7 | 84.8 |
| Total pooled sens/spec/LR (range) | 0.84 (0.83–0.86) | 0.98 (0.98–0.98) | 0.21 (0.15–0.30) | 34.65 (20.90–57.45) |
| Total I2 (%) | 86.8 | 91.2 | 88.8 | 87.8 |
| Study and Cytologic Sampling Method | Sens | Spec | LR- | LR+ |
|---|---|---|---|---|
| Li brush | ||||
| Shulan Lv, 2020 (2) [34] | 0.83 | 1.00 | 0.19 | ∞ |
| Lu Han, 2018 [28] | 1.00 | 0.96 | 0.01 | 21.93 |
| Lu Han, 2020 [27] | 0.96 | 0.91 | 0.05 | 11.03 |
| Pooled sens/spec/LR (range) | 0.96 (0.92–0.99) | 0.96 (0.93–0.97) | 0.06 (0.01–0.28) | 18.61 (8.25–42.01) |
| I2 (%) | 69.5 | 84.5 | 70.8 | 63.5 |
| Tao brush | ||||
| Shulan Lv, 2020 (1) [34] | 0.92 | 0.96 | 0.09 | 23.15 |
| Ibrahim A. Abdelazim, 2014 [22] | 1.00 | 1.00 | 0.17 | ∞ |
| Benjamin R. Kipp, 2008 [32] | 0.72 | 0.97 | 0.29 | 20.77 |
| Howard Her-Juing Wu, 2003 [44] | 0.63 | 1.00 | 0.38 | ∞ |
| Pooled sens/spec/LR (range) | 0.73 (0.64–0.81) | 0.99 (0.97–1.00) | 0.30 (0.23–0.41) | 40.71 (13.37–∞) |
| I2 (%) | 38.4 | 78.9 | 0.0 | 41.2 |
| SAP-1 | ||||
| Jia Wen, 2015 [43] | 1.00 | 0.99 | 0.27 | 83.31 |
| Ke Ma, 2016 [35] | 0.92 | 0.97 | 0.43 | 18.94 |
| Yu Hongzhen, 2021 [29] | 0.83 | 1.00 | 0.48 | ∞ |
| Wu Cheng, 2014 [24] | 0.56 | 0.85 | 0.17 | 5.56 |
| Yang Xi, 2013 [45] | 1.00 | 0.99 | 0.33 | 56.28 |
| Pooled sens/spec/LR (range) | 0.84 (0.83–0.86) | 0.98 (0.98–0.99) | 0.34 (0.26–0.46) | 40.22 (11.09–∞) |
| I2 (%) | 86.8 | 97.1 | 64.2 | 94.7 |
| Uterobrush | ||||
| Miodrag Milojkovic, 2002 [39] | 0.56 | 0.92 | 0.48 | 6.91 |
| P. J. Klemi, 1995 [33] | 0.38 | 1.00 | 0.61 | ∞ |
| C. Iavazzo, 2011 [30] | 0.89 | 1.00 | 0.15 | ∞ |
| Pooled sens/spec/LR (range) | 0.57 (0.42–0.72) | 0.99 (0.98–1.00) | 0.48 (0.29–0.79) | 48.59 (3.67–∞) |
| I2 (%) | 67.8 | 86.6 | 53.3 | 75.4 |
| Other | ||||
| D. W. Polson, 1984 [41] | 1.00 | 1.00 | 0.05 | 80.18 |
| U. Indraccolo, 2011 [31] | 1.00 | 1.00 | 0.10 | 61.20 |
| Carole Mathelin, 2007 [38] | 1.00 | 0.99 | 0.13 | 86.33 |
| Thierry Van den Bosch, 1996 [42] | 0.80 | 0.84 | 0.24 | 5.05 |
| Hiroyuki Fujiwara, 2015 [26] | 0.89 | 0.99 | 0.11 | 60.40 |
| Kenji Yanoh, 2014 [11] | 0.92 | 0.97 | 0.08 | 35.42 |
| E. Blanche Butler, 1971 [23] | 1.00 | 1.00 | 0.07 | 66.86 |
| John A. Maksem, 1995 [36] | 0.58 | 0.99 | 0.42 | 45.72 |
| Fumiko Yanaki, 2017 [46] | 0.70 | 0.98 | 0.31 | 43.42 |
| Alex Ferenczy, 1984 [25] | 1.00 | 1.00 | 0.17 | ∞ |
| Kavita Mardi, 2017 [37] | 1.00 | 0.99 | 0.05 | 49.40 |
| Helena O’Flynn, 2021 [40] | 0.91 | 0.85 | 0.10 | 6.02 |
| Pooled sens/spec/LR (range) | 0.88 (0.87–0.90) | 0.98 (0.97–0.98) | 0.16 (0.10–0.26) | 35.69 (15.47–92.33) |
| I2 (%) | 64.8 | 88.2 | 69.6 | 89.9 |
| Total pooled sens/spec/LR (range) | 0.84 (0.83–0.86) | 0.98 (0.98–0.98) | 0.21 (0.15–0.30) | 34.65 (20.90–57.45) |
| Total I2 (%) | 86.8 | 91.2 | 88.8 | 87.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Jiang, R.; Yao, Y.; Wang, Y.; Liu, W.; Qian, L.; Li, J.; Weimer, J.; Huang, X. Endometrial Cytology in Diagnosis of Endometrial Cancer: A Systematic Review and Meta-Analysis of Diagnostic Accuracy. J. Clin. Med. 2023, 12, 2358. https://doi.org/10.3390/jcm12062358
Wang T, Jiang R, Yao Y, Wang Y, Liu W, Qian L, Li J, Weimer J, Huang X. Endometrial Cytology in Diagnosis of Endometrial Cancer: A Systematic Review and Meta-Analysis of Diagnostic Accuracy. Journal of Clinical Medicine. 2023; 12(6):2358. https://doi.org/10.3390/jcm12062358
Chicago/Turabian StyleWang, Ting, Ruoan Jiang, Yingsha Yao, Yaping Wang, Wu Liu, Linhua Qian, Juanqing Li, Joerg Weimer, and Xiufeng Huang. 2023. "Endometrial Cytology in Diagnosis of Endometrial Cancer: A Systematic Review and Meta-Analysis of Diagnostic Accuracy" Journal of Clinical Medicine 12, no. 6: 2358. https://doi.org/10.3390/jcm12062358
APA StyleWang, T., Jiang, R., Yao, Y., Wang, Y., Liu, W., Qian, L., Li, J., Weimer, J., & Huang, X. (2023). Endometrial Cytology in Diagnosis of Endometrial Cancer: A Systematic Review and Meta-Analysis of Diagnostic Accuracy. Journal of Clinical Medicine, 12(6), 2358. https://doi.org/10.3390/jcm12062358





