A Scoping Review on the Incidence, Risk Factors, and Outcomes of Proximal Neck Dilatation after Standard and Complex Endovascular Repair for Abdominal Aortic Aneurysms
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Extraction
3. Results
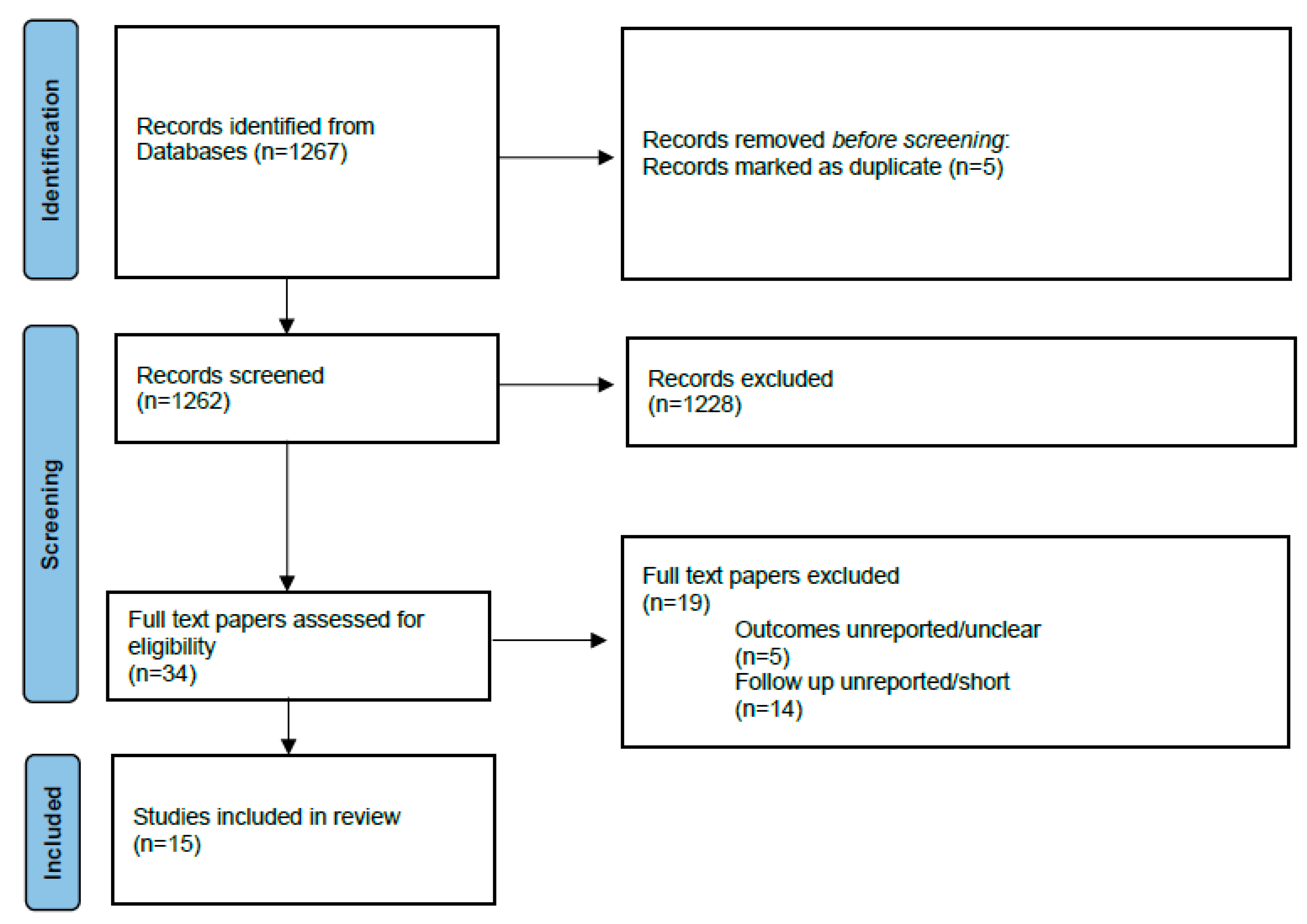
3.1. Literature Search
3.2. Definition of PND
3.3. Incidence of PND and Risk Factors
3.3.1. EVAR Group
3.3.2. FEVAR Group
3.4. Impact of PND on Outcomes (Survival, Reinterventions, Endoleaks, Sac Increase)
3.4.1. EVAR Group
3.4.2. FEVAR Group
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patel, R.; Powell, J.T.; Sweeting, M.J.; Epstein, D.M.; Barrett, J.K.; Greenhalgh, R.M. The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: Long-term follow-up and cost-effectiveness analysis. Health Technol. Assess. 2018, 22, 1–132. [Google Scholar] [CrossRef]
- Patel, R.; Sweeting, M.J.; Powell, J.T.; Greenhalgh, R.M. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): A randomised controlled trial. Lancet 2016, 388, 2366–2374. [Google Scholar] [CrossRef] [PubMed]
- Litwinski, R.A.; Donayre, C.E.; Chow, S.L.; Song, T.K.; Kopchok, G.; Walot, I.; White, R.A. The role of aortic neck dilation and elongation in the etiology of stent graft migration after endovascular abdominal aortic aneurysm repair with a passive fixation device. J. Vasc. Surg. 2006, 44, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Dayama, A.; Ricotta, J.J. Remodeling of aortic aneurysm and aortic neck on follow-up after endovascular repair with suprarenal fixation. J. Vasc. Surg. 2015, 61, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, E.; Czerny, M.; Beyersdorf, F.; Wolkewitz, M.; Berezowski, M.; Siepe, M.; Blanke, P.; Rylski, B. Abdominal aortic aneurysm neck remodeling after Anaconda stent graft implantation. J. Vasc. Surg. 2018, 68, 1354–1359.e2. [Google Scholar] [CrossRef]
- Kret, M.R.; Tran, K.; Lee, J.T. Change in Aortic Neck Diameter after Endovascular Aortic Aneurysm Repair. Ann. Vasc. Surg. 2017, 43, 115–120. [Google Scholar] [CrossRef]
- Oliveira, N.F.; Oliveira-Pinto, J.; van Rijn, M.J.; Baart, S.; Raa, S.T.; Hoeks, S.E.; Gonçalves, F.B.; Verhagen, H.J. Risk Factors, Dynamics, and Clinical Consequences of Aortic Neck Dilatation after Standard Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 26–35. [Google Scholar] [CrossRef]
- Deltomme, M.; Berge, S.V.D.; Mufty, H.; Laenen, A.; Houthoofd, S.; Fourneau, I.; Maleux, G. A Five-Year Computed Tomography Follow-up Study of Proximal Aortic Neck Dilatation after Endovascular Aortic Repair Using Four Contemporary Types of Endograft. Cardiovasc. Interv. Radiol. 2021, 44, 1384–1393. [Google Scholar] [CrossRef]
- de Donato, G.; Setacci, F.; Bresadola, L.; Castelli, P.; Chiesa, R.; Mangialardi, N.; Nano, G.; Setacci, C.; Ricci, C.; Gasparini, D.; et al. Aortic neck evolution after endovascular repair with TriVascular Ovation stent graft. J. Vasc. Surg. 2016, 63, 8–15. [Google Scholar] [CrossRef]
- Gargiulo, M.; Gallitto, E.; Wattez, H.; Verzini, F.; Massoni, C.B.; Loschi, D.; Freyrie, A.; Haulon, S. Outcomes of endovascular aneurysm repair performed in abdominal aortic aneurysms with large infrarenal necks. J. Vasc. Surg. 2017, 66, 1065–1072. [Google Scholar] [CrossRef]
- Kaladji, A.; Cardon, A.; Laviolle, B.; Heautot, J.-F.; Pinel, G.; Lucas, A. Evolution of the upper and lower landing site after endovascular aortic aneurysm repair. J. Vasc. Surg. 2012, 55, 24–32. [Google Scholar] [CrossRef]
- Mathlouthi, A.; Yei, K.; Barleben, A.; Al-Nouri, O.; Malas, M.B. Polymer based endografts have improved rates of proximal aortic neck dilatation and migration. Ann. Vasc. Surg. 2021, 77, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Savlovskis, J.; Krievins, D.; de Vries, J.-P.P.; Holden, A.; Kisis, K.; Gedins, M.; Ezite, N.; Zarins, C.K. Aortic neck enlargement after endovascular aneurysm repair using balloon-expandable versus self-expanding endografts. J. Vasc. Surg. 2015, 62, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Torsello, G.; Pratesi, G.; van der Meulen, S.; Ouriel, K. Aortoiliac remodeling and 5-year outcome of an ultralow-profile endograft. J. Vasc. Surg. 2019, 69, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Marcaccio, C.L.; Kim, N.H.; Patel, P.B.; Anjorin, A.C.; Zettervall, S.L.; Patel, V.I.; de Bruin, J.L.; Verhagen, H.J.; Schermerhorn, M.L. The effect of supraceliac versus infraceliac landing zone on outcomes following fenestrated endovascular repair of juxta-/pararenal aortic aneurysms. J. Vasc. Surg. 2022, 77, 9–19.e2. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.; Deslarzes-Dubuis, C.; Lee, J.T. Quantification of suprarenal aortic neck dilation after fenestrated endovascular aneurysm repair. J. Vasc. Surg. 2021, 73, 31–38. [Google Scholar] [CrossRef]
- Zettervall, S.L.; Dansey, K.; Kline, B.; Singh, N.; Starnes, B.W. Significant aortic neck dilation occurs after repair of juxtarenal aneurysms with fenestrated endovascular aneurysm repair. J. Vasc. Surg. 2021, 74, 1090–1097.e2. [Google Scholar] [CrossRef]
- Teter, K.; Li, C.; Ferreira, L.M.; Ferrer, M.; Rockman, C.; Jacobowitz, G.; Cayne, N.; Garg, K.; Maldonado, T.S. Fenestrated endovascular aortic aneurysm repair promotes positive infrarenal neck remodeling and greater sac shrinkage compared with endovascular aortic aneurysm repair. J. Vasc. Surg. 2022, 76, 344–351.e1. [Google Scholar] [CrossRef]
- Chatzelas, D.A.; Loutradis, C.N.; Pitoulias, A.G.; Kalogirou, T.E.; Pitoulias, G.A. A systematic review and meta-analysis of proximal aortic neck dilatation after endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2022, 77, 941–956.e1. [Google Scholar] [CrossRef]
- Kouvelos, G.N.; Oikonomou, K.; Antoniou, G.A.; Verhoeven, E.L.G.; Katsargyris, A. A Systematic Review of Proximal Neck Dilatation after Endovascular Repair for Abdominal Aortic Aneurysm. J. Endovasc. Ther. 2017, 24, 59–67. [Google Scholar] [CrossRef]
- Filis, K.; Galyfos, G.; Sigala, F.; Tsioufis, K.; Tsagos, I.; Karantzikos, G.; Bakoyiannis, C.; Zografos, G. Proximal Aortic Neck Progression: Before and after Abdominal Aortic Aneurysm Treatment. Front. Surg. 2017, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Verzini, F.; Parlani, G.; De Rango, P.; Parente, B.; Giordano, G.; Mosca, S.; Maselli, A. Predictive factors and clinical consequences of proximal aortic neck dilatation in 230 patients undergoing abdominal aorta aneurysm repair with self-expandable stent-grafts. J. Vasc. Surg. 2003, 37, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Sternbergh, W.; Money, S.R.; Greenberg, R.K.; Chuter, T.A. Influence of endograft oversizing on device migration, endoleak, aneurysm shrinkage, and aortic neck dilation: Results from the zenith multicenter trial. J. Vasc. Surg. 2004, 39, 20–26. [Google Scholar] [CrossRef]
- Malach, L.; Tehrani, N.; Kolachina, S.; Krawczyk, K.; Wozniak, A.; Soult, M.; Aulivola, B.; Bechara, C.F. Effect of Stent-Graft Active Fixation and Oversizing on Aortic Neck Dilation after Endovascular Aneurysm Exclusion for Infrarenal Aortic Aneurysm. Ann. Vasc. Surg. 2022, 79, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Conners, M.S.; Sternbergh, W.; Carter, G.; Tonnessen, B.H.; Yoselevitz, M.; Money, S.R. Endograft migration one to four years after endovascular abdominal aortic aneurysm repair with the AneuRx device: A cautionary note. J. Vasc. Surg. 2002, 36, 476–484. [Google Scholar] [CrossRef]
- Oberhuber, A.; Buecken, M.; Hoffmann, M.; Orend, K.-H.; Mühling, B.M. Comparison of aortic neck dilatation after open and endovascular repair of abdominal aortic aneurysm. J. Vasc. Surg. 2012, 55, 929–934. [Google Scholar] [CrossRef]
- Pintoux, D.; Chaillou, P.; Azema, L.; Bizouarn, P.; Costargent, A.; Patra, P.; Gouëffic, Y. Long-Term Influence of Suprarenal or Infrarenal Fixation on Proximal Neck Dilatation and Stentgraft Migration after EVAR. Ann. Vasc. Surg. 2011, 25, 1012–1019. [Google Scholar] [CrossRef]
- D’Oria, M.; Di Girolamo, F.G.; Calvagna, C.; Gorgatti, F.; Altamura, N.; Lepidi, S.; Biolo, G.; Fiotti, N. Remodeling of abdominal aortic aneurysm sac following endovascular aortic repair: Association with clinical, surgical, and genetic factors. Cardiovasc. Pathol. 2022, 58, 107405. [Google Scholar] [CrossRef]
- van Rijswijk, R.E.; Jebbink, E.G.; Zeebregts, C.J.; Reijnen, M.M. A systematic review of anatomic predictors of abdominal aortic aneurysm remodeling after endovascular repair. J. Vasc. Surg. 2022, 75, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Nana, P.; Spanos, K.; Kouvelos, G.; Arnaoutoglou, E.; Giannoukas, A.; Matsagkas, M. Conical Aortic Neck as a Predictor of Outcome after Endovascular Aneurysm Exclusion: Midterm Results. Ann. Vasc. Surg. 2022, 90, 77–84. [Google Scholar] [CrossRef]
- Zuidema, R.; van der Riet, C.; El Moumni, M.; Schuurmann, R.C.; Ünlü, Ç.; de Vries, J.-P.P. Pre-operative Aortic Neck Characteristics and Post-operative Sealing Zone as Predictors of Type 1a Endoleak and Migration after Endovascular Aneurysm Repair: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Marone, E.M.; Freyrie, A.; Ruotolo, C.; Michelagnoli, S.; Antonello, M.; Speziale, F.; Veroux, P.; Gargiulo, M.; Gaggiano, A. Expert Opinion on Hostile Neck Definition in Endovascular Treatment of Abdominal Aortic Aneurysms (a Delphi Consensus). Ann. Vasc. Surg. 2020, 62, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Grando, B.; Taglialavoro, J.; Gorgatti, F.; Calvagna, C.; Bassini, S.; Riccitelli, F.; Griselli, F.; D’Andrea, A.; Lepidi, S. Association between Psoas Muscle Sarcopenia and Long-Term Survival following Elective Endovascular Aortic Repair. J. Surg. Res. 2022, 280, 459–468. [Google Scholar] [CrossRef]
- Mezzetto, L.; D’Oria, M.; Mani, K.; Scali, S.; Gonçalves, F.B.; Trimarchi, S.; Budtz-Lilly, J.; DeMartino, R.; Veraldi, G.; Mastrorilli, D.; et al. Scoping review of radiologic assessment and prognostic impact of skeletal muscle sarcopenia in patients undergoing endovascular repair for aortic disease. J. Vasc. Surg. 2022, 76, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Veraldi, G.F.; Mezzetto, L.; Vaccher, F.; Scorsone, L.; Bonvini, S.; Raunig, I.; Wassermann, V.; Tasselli, S. Technical Success and Long-Term Results with Excluder/C3 Endoprosthesis in Narrow Aortic Bifurcations: First Italian Multicentre Experience. Ann. Vasc. Surg. 2018, 52, 57–66. [Google Scholar] [CrossRef]
- Laczynski, D.J.; Caputo, F.J. Systematic review and meta-analysis of endovascular abdominal aortic repair in large diameter infrarenal necks. J. Vasc. Surg. 2021, 74, 309–315.e2. [Google Scholar] [CrossRef]
- Xodo, A.; D’Oria, M.; Mendes, B.; Bertoglio, L.; Mani, K.; Gargiulo, M.; Budtz-Lilly, J.; Antonello, M.; Veraldi, G.F.; Pilon, F.; et al. Peri-Operative Management of Patients Undergoing Fenestrated-Branched Endovascular Repair for Juxtarenal, Pararenal and Thoracoabdominal Aortic Aneurysms: Preventing, Recognizing and Treating Complications to Improve Clinical Outcomes. J. Pers. Med. 2022, 12, 1018. [Google Scholar] [CrossRef]
- D’Oria, M.; Budtz-Lilly, J.; Lindstrom, D.; Lundberg, G.; Jonsson, M.; Wanhainen, A.; Mani, K.; Unosson, J. Comparison of Early and Mid-Term Outcomes after Fenestrated-Branched Endovascular Aortic Repair in Patients with or without Prior Infrarenal Repair. J. Endovasc. Ther. 2022, 29, 544–554. [Google Scholar] [CrossRef]
- Budtz-Lilly, J.M.; D’Oria, M.; Gallitto, E.M.; Bertoglio, L.; Kölbel, T.M.; Lindström, D.M.; Dias, N.M.; Lundberg, G.M.; Böckler, D.M.; Parlani, G.; et al. European Multicentric Experience with Fenestrated-Branched ENDOvascular Stent-grafting after Previous FAILed Infrarenal Aortic Repair: The EU-FBENDO-FAIL Registry. Ann. Surg. 2022. publish ahead of print. [Google Scholar] [CrossRef]
- Valdivia, A.R.; Oikonomou, K.; Milner, R.; Kasprzak, P.; Reijnen, M.M.P.J.; Pitoulias, G.; Torsello, G.B.; Pfister, K.; de Vries, J.-P.P.M.; Chaudhuri, A. The Effect of EndoAnchors on Aneurysm Sac Regression for Patients Treated with Infrarenal Endovascular Repair with Hostile Neck Anatomies: A Propensity Scored Analysis. J. Endovasc. Ther. 2022. [Google Scholar] [CrossRef] [PubMed]
- Tassiopoulos, A.K.; Monastiriotis, S.; Jordan, W.D.; Muhs, B.E.; Ouriel, K.; De Vries, J.P. Predictors of early aortic neck dilatation after endovascular aneurysm repair with EndoAnchors. J. Vasc. Surg. 2017, 66, 45–52. [Google Scholar] [CrossRef] [PubMed]
| Author | Year of Publication | Country of Corresponding Author | Type of Study | Period of Enrollment | Type of Procedure | N. Patients | Type of Endograft |
|---|---|---|---|---|---|---|---|
| Kaladiji et al. [11] | 2012 | France | Retrospective, Single center | 2003–2007 | EVAR a | 61 | Talent, Medtronic (31) Zenith, Cook (23) Excluder, Gore (6) Anaconda, Vascutek (1) |
| Savlovskis et al. [13] | 2015 | USA | Retrospective, Multicenter | 2008–2010 | EVAR | 105 | Endurant Medtronic (56) Nellix, Endologix (49) |
| Tsilimparis et al. [4] | 2015 | Germany | Retrospective, Multicenter | Ongoing | EVAR | 736 | Zenith, Cook |
| de Donato et al. [9] | 2016 | Italy | Retrospective, Multicenter | 2010–2012 | EVAR | 161 | Ovation, Endologix |
| Kret et al. [6] | 2016 | USA | Retrospective, Single center | 2008–2014 | EVAR | 86 | Zenith, Cook (26) Excluder, Gore (26) Endurant, Medtronic (22) Powerlink, Endologix (10) Ovation, Trivascular (2) |
| Gargiulo et al. [10] | 2017 | Italy | Retrospective, Multicenter | 2009–2012 | EVAR | 118 | Zenith, Cook (74) Endurant, Medtronic (28) Anaconda, Vascutek (9) Excluder, Gore (6) Ovation, Trivascular (1) |
| Vukovic et al. [5] | 2018 | Canada | Retrospective, Single center | 2005–2014 | EVAR | 126 | Anaconda (Vascutek, Inchinnan, United Kingdom) |
| Torsello et al. [14] | 2019 | Germany | Prospective, Multicenter | 2010–2011 | EVAR | 60 | Incraft |
| Deltomme et al. [8] | 2021 | Belgium | Retrospective, Single center | 2007–2015 | EVAR | 120 | Zenith, Cook (30) Excluder, Gore (30) Endurant, Medtronic (30) Ovation, Trivascular (30) |
| Mathlouthi et al. [12] | 2021 | USA | Prospective, Multicenter | 2009–2012 | EVAR | 238 | Ovation, Trivascular |
| Oliveira et al. [7] | 2021 | Portugal | Retrospective, Single center | 2000–2015 | EVAR | 460 | Endurant, Medtronic (243) Excluder, Gore (181) Talent, Medtronic (13) Zenith, Cook (6) Others (17) |
| Tran et al. [16] | 2021 | USA | Retrospective, Single center | 2012–2018 | FEVAR b | 43 | ZFEN, Cook |
| Zettervall et al. [17] | 2021 | USA | Retrospective, Multicenter | ongoing | FEVAR | 56 | Zenith, Cook (23); Endurant, Medtronic (4); Treo, Bolton (1) |
| Teter et al. [18] | 2022 | USA | Retrospective, Multicenter | 2011–2019 | FEVAR | 120 | ZFEN, Cook (30); Endurant, Medtronic (30), Excluder, Gore (30), Zenith, Cook (30) |
| Rastogi et al. [15] | 2022 | Netherlands | Retrospective, Multicenter | 2008–2018 | FEVAR | 84 | ZFEN, Cook |
| Author | Age, Years | Male | Arterial Hypertension | Diabetes Mellitus | Respiratory Disease | Chronic Kidney Disease | Cardiac Disease | Aneurysm Diameter, mm |
|---|---|---|---|---|---|---|---|---|
| Kaladiji et al. [11] | 74.6 | 57 (93.4%) | NA | 5 (8.2%) | 2 (3.3%) | 1 (1.6%, end-stage) | 26 (42.6%) | NA |
| Savlovskis et al. [13] | 71,9 | 84 (80%) | NA | NA | NA | NA | NA | 54.5 (50.5–60.0) |
| Tsilimparis et al. [4] | NA | NA | NA | NA | NA | NA | NA | 55.4 (54.8–56.0) |
| de Donato et al. [9] | 75.2 | 148 (92%) | 94 (62%) | 35 (22%) | NA | 3 (2%) | 57 (35%) | 57.7 |
| Kret et al. [6] | 75.6 | 74 (86.1%) | 73 (86,1%) | 15 (17.4%) | 18 (20.9%) | NA | 43 (50%) | 57.1 (42–82) |
| Gargiulo et al. [10] | 73.9 | NA (91%) | 101 (86%) | 22 (19%) | 43 (36%) | 30 (25%) | 47 (40%) | 60.8 (51–100) |
| Vukovic et al. [5] | 70 | 96 (76%) | 112 (89%) | 26 (21%) | 13 (10%) | 32 (25%) | 66 (52%) | NA |
| Torsello et al. [14] | 74 | 57 (95%) | NA | NA | NA | NA | NA | 52.6 |
| Deltomme et al. [8] | 72.7 | 112 (93%) | 84 (70%) | 16 (13.3%) | NA | 51 (42.5%) | 40 (33.3%) | 63.5 |
| Mathlouthi et al. [12] | 73.3 | 193 (81%) | 205 (86.1%) | 54 (22.6%) | 66 (27.7) | 32 (13.4%) | 108 (75.6%) | 54 ± 8 |
| Oliveira et al. [7] | 73.1 | 408 (88.6%) | 330 (71.7%) | 78 (16.9%) | 68 (14.7%) | 101 (21.9%) | 78 (16.9%) | 59.0 (54.0–67.0) |
| Tran et al. [16] | 72 | 37 (86%) | NA | NA | NA | NA | NA | 61.9 |
| Zettervall et al. [17] | 74.5 | 44 (78.5%) | NA | 12 (21.4%) | 35 (62.5%) | NA | 25 (44.6%) | 62 (58–64) |
| Teter et al. [18] | 73.5 | NA | 26 (86%) | 4 (13.3%) | 6 (20%) | NA | 12 (40%) | NA |
| Rastogi et al. [15] | 73 | 77 (91) | 65 (81) | 11 (13.4) | 29 (36.3) | 25 (29) | NA | 60.1 |
| Author | Level of Neck Measurement | Neck Dilation, Cut-Off | Neck Length, mm | Neck Diameter, mm | Graft Oversizing | Suprarenal Fixation | Infrarenal Fixation | Inside IFU |
|---|---|---|---|---|---|---|---|---|
| Kaladiji et al. [11] | Infrarenal aorta (D1a), 15 mm below the lowest RA a (D1b), the origin of the aneurysm (D1c) | ≥3 mm | NA | D1a: 23.9 ± 3.3 D1b: 24.3 ± 3.9 D1c: 25 ± 4 | 16% (± 9%) | 55 (90.1%) | 6 (9.9%) | 61 (100%) |
| Savlovskis et al. [13] | (1) below the lowermost RA (2) at the proximal end of the stent structure (3) 5 mm below the level of the proximal end of the stent | any variation | 25.6 (19.9–31.4) | Level 1: 25.5 (23.9–26.5) Level 2: 25.4 (24.2–26.8) Level 3: 25.5 (24.3–26.7) | 12.8% (±2.7%) | 56 (53.3%) | 49 (46.6%) | 105 (100%) |
| Tsilimparis et al. [4] | lowest RA | any variation | NA | 23.8 (23.6–24.1) | 19% (±8%) | 736 (100%) | 0 | 736 (100%) |
| de Donato et al. [9] | Zone A: from upper limit of suprarenal stent to a tangent horizontal plane at the lowermost RA Zone B: from the lowermost RA to the first ring; Zone C: the first polymer-filled ring. | ≥2 mm | NA | NA | 10–20% | 161 (100%) | 0 | NA |
| Kret et al. [6] | lowest RA and 10 mm below the lowest RA a. | any variation | NA | 24.5 (17.7–36.7) | 13.6% (± 11.5%) | 50 (58.1%) | 36 (41.8%) | 73 (84.8%) |
| Gargiulo et al. [10] | 1 cm below the lowest RA (D1), at the level of each RA (D2, D3), at the level of the SMA b (D4), and at the level of the celiac trunk (D5). | ≥3 mm | 23.5 (10–37) | 29.7 (28–36) | 17% (± 9%) | 102 (86.4%) | 16 (13.4%) | 94 (79.6%) |
| Vukovic et al. [5] | 1 cm above and at the level of RA | any variation | >15 | 22 (21–24) | 17% (9–26%) | 0 | 126 (100%) | NA |
| Torsello et al. [14] | at lowest RA and 15 mm below the lowest RA | ≥15% from baseline | NA | Lowest RA: 22.3 (17–29.5) Below RA: 23.0 (18–29.1) | NA | 60 (100%) | 0 | 60 (100%) |
| Deltomme et al. [8] | outer diameter at SMA and lowest RA | any variation | NA | SMA level: 25.7 (18.9–34); RA level: 24.3 (17.5–33) | 10–20% | 90 (75%) | 30 (25%) | NA |
| Mathlouthi et al. [12] | lowest RA | ≥3 mm | 22 ± 12 | 22.4 ± 3 | NA | 238 (100%) | 0 | 238 (100%) |
| Oliveira et al. [7] | start of proximal covered stent and lowest RA | any variation | 28.0 (20.0–40.0) | 24.0 (22.0–26.0) | 20.0% (13.6–28.0%) | 278 (60.4%) | 182 (39.6%) | NA |
| Tran et al. [16] | A, top of the fixation struts; B, middle of the fixation struts; C, top of the graft fabric at the level of the gold marker; D, middle of the first seal stent; E, bottom of the first seal stent; F, middle of the second seal stent; and G, bottom of the second seal stent | ≥3 mm | NA | Suprarenal: 25.8 Infrarenal: 25.7 | 19.6% (±5.5%) | 43 (100%) | 0 | NA |
| Zettervall et al. [17] | at SMA and lowest RA | ≥3 mm | NA | SMA level: 25.5 RA level: 25.4 | SMA level: 16.5% (9.9–22%) RA level: 15% (11–20%) | 56 (100%) | 0 | NA |
| Teter et al. [18] | The aortic neck and visceral aorta were divided into 5 mm segments ranging from 20 mm above the lowest RA to 20 mm below the lowest RA. | Any variation | NA | FEVAR c: 26.6 (±4.0) EVAR d: 23.5 (± 2.9) | FEVAR: 14.4% EVAR: 18.3% | 90 (75%) | 30 (25%) | NA |
| Rastogi et al. [15] | at both the start of the covered stent and at the bottom of the scallop and averaged as well | ≥10% of baseline | 29.5 | 25.4 ± 2.7 | 20.1% (± 9.1%) | 84 (100%) | 0 | NA |
| Author | F-Up, Months | Neck Dilation | Graft Migration | TIaEL | AAA-Related Reintervention | Neck-Related Reintervention | Type of Reinterventions |
|---|---|---|---|---|---|---|---|
| Kaladiji et al. [11] | 39 (24–84) | Dilation: 3.7 mm ± 2.8 for D1a; 4.4 mm ± 2.5 mm for D1b; 4.4 mm ± 3.1 for D1c; Neck diameter > 20%: 11.5% for D1a, 13.1% for D1b, and 14.8% for D1c | 1 (1.6%) | 1 (1.6%) | NA | 1 (1.6%) | Proximal angioplasty (1) |
| Savlovskis et al. [13] | <36 | >6 mm vs. 0 (Endurant vs. Nellix) Level 1: 0.65 mm/y vs. 0.17 mm/y Level 2: 0.92 mm/y vs. 0.18 mm/y Level 3: 1.0 mm/y vs. 0.22 mm/y | +2.8 mm (Endurant); +2.6 mm (Nellix) | NA | NA | NA | NA |
| Tsilimparis et al. [4] | <60 | 0.47 mm/month in the early period 0.10 mm/month in the later period | NA | NA | NA | NA | NA |
| de Donato et al. [9] | 32 (24–50) | Zone A: +0.18 mm; Zone B: −0.32 mm; Zone C: −0.06 mm | 0 | 2 (1.2%) | 6 (3.7%) | 1 (0.6%) | Proximal angioplasty (1) Graft relining (1) |
| Kret et al. [6] | 21.9 (3.7–63.8) | Early period: +1.34 mm (+5.9%) Late Period: +5.36 mm (+21.7%) Dilation >10% in 55 (62.9%) | 0 | 2 (2.5%) | 2 (2.5%) | 2 (2.5%) | Surgical conversion (1) |
| Gargiulo et al. [10] | 37.9 (24–103) | All necks increased Significant dilation in D1: 42%, D2: 31%, D3: 32%; | NA | 14 (12%) | 19 (16%) | 8 (7%) | Surgical conversions (7) |
| Vukovic et al. [5] | <48 | 2–4 mm Oversize >20%: 0.5 mm/y | Continuous migration, average 6 mm; Oversize >20%: 1.9 mm/y | 8 (6.3%) | 17 (13.4%) | 7 (5.5%) | Surgical conversions (6) Fenestrated cuff (1) |
| Torsello et al. [14] | <60 | 25/60 patient (41%) Average: +4.5 mm (+19%) at 15 mm from the lowest RA No modification at renal level | 1.9–3.1 mm, | 2 (3%) | 3 (5%) | 2 (3%) | Proximal cuff (1) Chimney graft (1) |
| Deltomme et al. [8] | <60 | At SMA level: no changes only with Endurant At RA level: significant changes with all endografts | 0 | 7 (5.8%) | 18 (15%) | 7 (5.8%) | Proximal cuff (4), Fenestrated cuff (1), Embolization (2) |
| Mathlouthi et al. [12] | <60 | 10 patients (4.2%) Mean dilation: 1 mm | 0 | NA (3.7%) | 43 (18.1%) | 0 | 0 |
| Oliveira et al. [7] | <60 | Mean dilation: 3.0 mm (0.4–5.0) Dilation >10%: 241 (52.4%); Dilation >20%: 131 (28.5%); Dilation 1.5%/y | NA | NA | NA | NA | NA |
| Tran et al. [16] | 30.3 | 32 patients (74.4%); Mean dilation: 3.1 mm (0.99/y) | 0 | 1 (2.3) | 8 (18.6%) | 1 (2.3%) | NA |
| Zettervall et al. [17] | <36 | Dilation at SMA a: 28 patients (50%); Dilation at RA b: 36 patients (64%) | NA | 0 | NA | NA | NA |
| Teter et al. [18] | <52 | Dilation at SMA level: 15.5%; Dilation at RA level: + 8.5% | NA | NA | 5 (16.6%) | NA | NA |
| Rastogi et al. [15] | <42 | Dilation > 10%: 47.6%; Dilation > 20%: 20.2% | 0 | 2 (2.4%) | NA | 1 (1.2%) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzetto, L.; D’Oria, M.; Lepidi, S.; Mastrorilli, D.; Calvagna, C.; Bassini, S.; Taglialavoro, J.; Bruno, S.; Veraldi, G.F. A Scoping Review on the Incidence, Risk Factors, and Outcomes of Proximal Neck Dilatation after Standard and Complex Endovascular Repair for Abdominal Aortic Aneurysms. J. Clin. Med. 2023, 12, 2324. https://doi.org/10.3390/jcm12062324
Mezzetto L, D’Oria M, Lepidi S, Mastrorilli D, Calvagna C, Bassini S, Taglialavoro J, Bruno S, Veraldi GF. A Scoping Review on the Incidence, Risk Factors, and Outcomes of Proximal Neck Dilatation after Standard and Complex Endovascular Repair for Abdominal Aortic Aneurysms. Journal of Clinical Medicine. 2023; 12(6):2324. https://doi.org/10.3390/jcm12062324
Chicago/Turabian StyleMezzetto, Luca, Mario D’Oria, Sandro Lepidi, Davide Mastrorilli, Cristiano Calvagna, Silvia Bassini, Jacopo Taglialavoro, Salvatore Bruno, and Gian Franco Veraldi. 2023. "A Scoping Review on the Incidence, Risk Factors, and Outcomes of Proximal Neck Dilatation after Standard and Complex Endovascular Repair for Abdominal Aortic Aneurysms" Journal of Clinical Medicine 12, no. 6: 2324. https://doi.org/10.3390/jcm12062324
APA StyleMezzetto, L., D’Oria, M., Lepidi, S., Mastrorilli, D., Calvagna, C., Bassini, S., Taglialavoro, J., Bruno, S., & Veraldi, G. F. (2023). A Scoping Review on the Incidence, Risk Factors, and Outcomes of Proximal Neck Dilatation after Standard and Complex Endovascular Repair for Abdominal Aortic Aneurysms. Journal of Clinical Medicine, 12(6), 2324. https://doi.org/10.3390/jcm12062324






