Infrared Thermography in Symptomatic Knee Osteoarthritis: Joint Temperature Differs Based on Patient and Pain Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Infrared Imaging Procedure and Analysis
2.2. Statistical Analysis
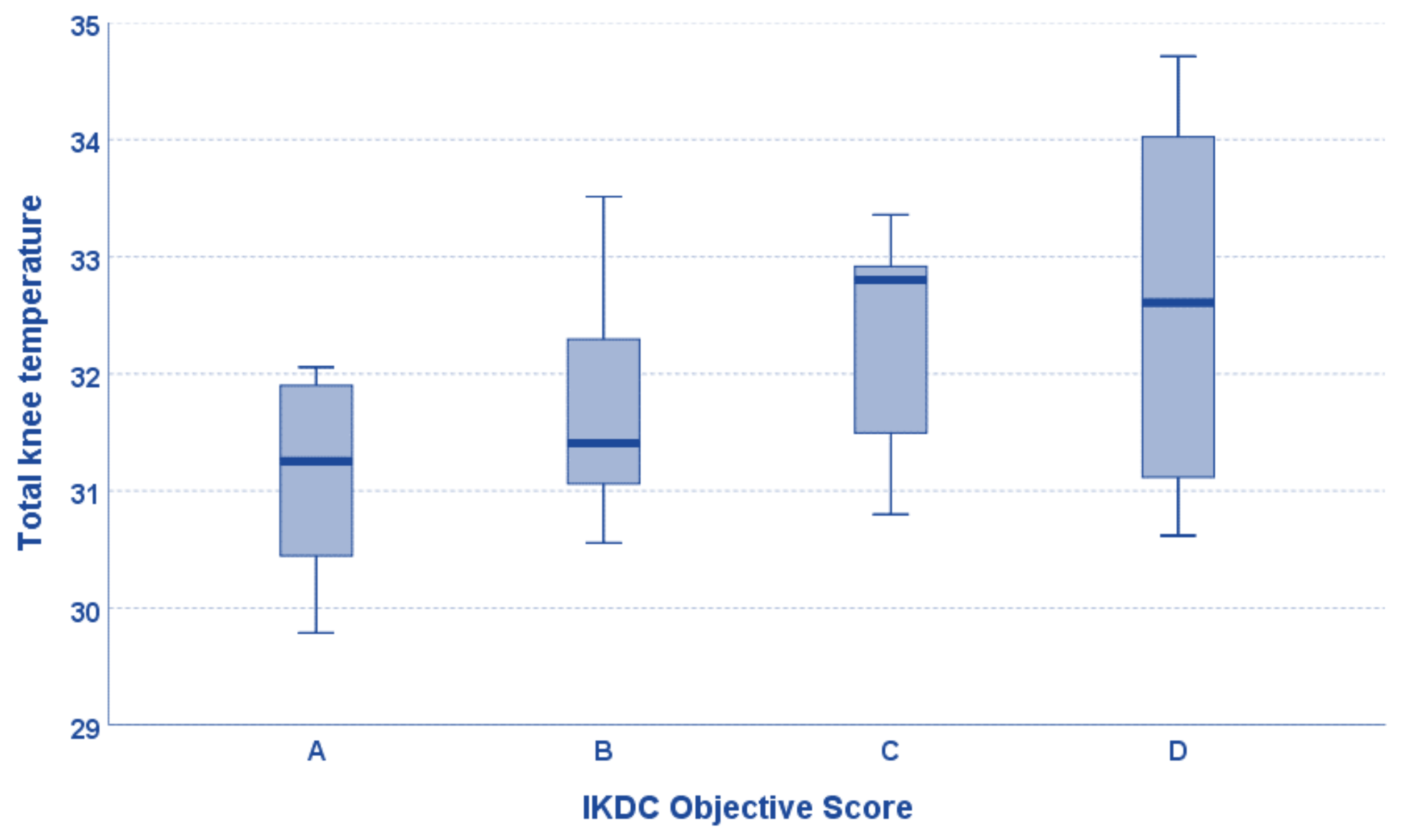
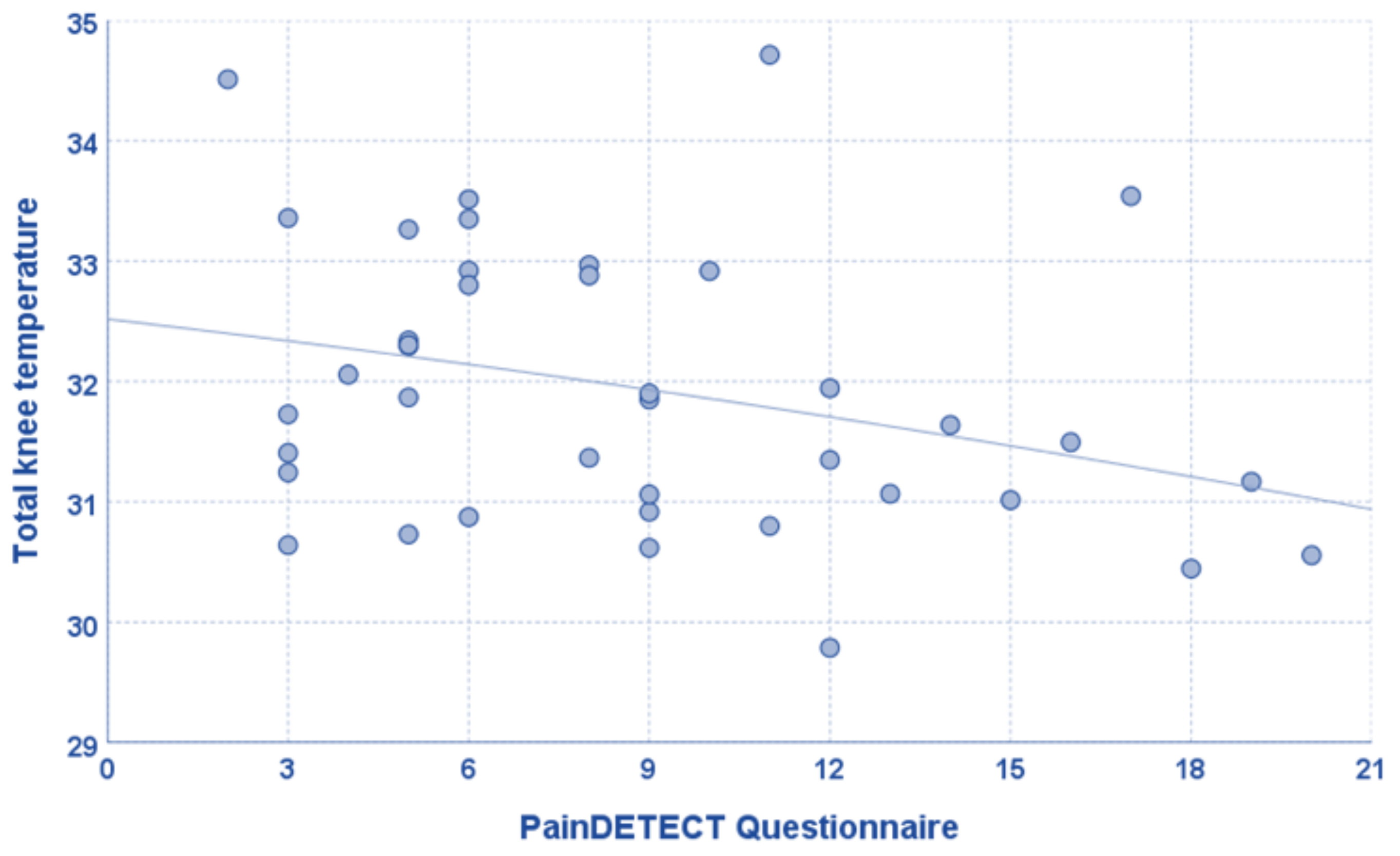
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, D.; Peleteiro, B.; Araújo, J.; Branco, J.; Santos, R.A.; Ramos, E. The effect of osteoarthritis definition on prevalence and incidence estimates: A systematic review. Osteoarthr. Cartil. 2011, 19, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Bierma-Zeinstra, S.M.; Verhagen, A.P. Osteoarthritis subpopulations and implications for clinical trial design. Arthritis Res. Ther. 2011, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. Identifying different osteoarthritis phenotypes through epidemiology. Osteoarthr. Cartil. 2010, 18, 601–604. [Google Scholar] [CrossRef]
- Deveza, L.A.; Nelson, A.E.; Loeser, R.F. Phenotypes of osteoarthritis: Current state and future implications. Clin. Exp. Rheumatol. 2019, 37, 64–72. [Google Scholar]
- De Girolamo, L.; Kon, E.; Filardo, G.; Marmotti, A.G.; Soler, F.; Peretti, G.M.; Vannini, F.; Madry, H.; Chubinskaya, S. Regenerative approaches for the treatment of early OA. Knee Surg. Sport. Traumatol. Arthrosc. 2016, 24, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Previtali, D.; Andriolo, L.; Frattura, G.D.L.; Boffa, A.; Candrian, C.; Zaffagnini, S.; Filardo, G. Pain Trajectories in Knee Osteoarthritis—A Systematic Review and Best Evidence Synthesis on Pain Predictors. J. Clin. Med. 2020, 9, 2828. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Di Rosa, M.; Ravalli, S.; Castorina, A.; Guglielmino, C.; Imbesi, R.; Vecchio, M.; Drago, F.; Szychlinska, M.A.; Musumeci, G. Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key. Int. J. Mol. Sci. 2019, 20, 511. [Google Scholar] [CrossRef]
- Fokam, D.; Lehmann, C. Clinical assessment of arthritic knee pain by infrared thermography. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 20170218. [Google Scholar] [CrossRef]
- Ren, G.; Lutz, I.; Railton, P.; Wiley, J.P.; McAllister, J.; Powell, J.; Krawetz, R.J. Serum and synovial fluid cytokine profiling in hip osteoarthritis: Distinct from knee osteoarthritis and correlated with pain. BMC Musculoskelet. Disord. 2018, 19, 129. [Google Scholar] [CrossRef]
- Siqueira, M.B.P.; Frangiamore, S.; Klika, A.K.; Gajewski, N.; Barsoum, W.K.; Higuera, C.A. Comparison of Synovial Fluid Cytokine Levels between Traumatic Knee Injury and End-Stage Osteoarthritis. J. Knee Surg. 2017, 30, 128–133. [Google Scholar] [CrossRef]
- Salisbury, R.S.; Parr, G.; De Silva, M.; Hazleman, B.L.; Page-Thomas, D.P. Heat distribution over normal and abnormal joints: Thermal pattern and quantification. Ann. Rheum. Dis. 1983, 42, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Marins, J.C.B.; Moreira, D.G.; Cano, S.P.; Quintana, M.S.; Soares, D.D.; de Andrade Fernandes, A.; Da Silva, F.S.; Costa, C.M.A.; dos Santos Amorim, P.R. Time required to stabilize thermographic images at rest. Infrared Phys. Technol. 2014, 65, 30–35. [Google Scholar] [CrossRef]
- Denoble, A.E.; Hall, N.; Pieper, C.F.; Kraus, V.B. Patellar Skin Surface Temperature by Thermography Reflects Knee Osteoarthritis Severity. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2010, 3, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.; Costa, C.M.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I.; et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consensus statement on the measurement of human skin temperature. J. Therm. Biol. 2017, 69, 155–162. [Google Scholar] [CrossRef]
- Hildebrandt, C.; Raschner, C.; Ammer, K. An Overview of Recent Application of Medical Infrared Thermography in Sports Medicine in Austria. Sensors 2010, 10, 4700–4715. [Google Scholar] [CrossRef] [PubMed]
- Varjú, G.; Pieper, C.F.; Renner, J.B.; Kraus, V.B. Assessment of hand osteoarthritis: Correlation between thermographic and radiographic methods. Rheumatology 2004, 43, 915–919. [Google Scholar] [CrossRef]
- Seixas, A.; Ammer, K. Systematic Reviews and Meta-analysis about Infrared Thermography in Muscoloskeletal Research: Trends and critical appraisal. Thermol. Int. 2022, 32, 65–73. [Google Scholar]
- Ammer, K. Temperature of the human knee-a review. Thermol. Int. 2012, 22, 137–151. [Google Scholar]
- Schiavon, G.; Capone, G.; Frize, M.; Zaffagnini, S.; Candrian, C.; Filardo, G. Infrared Thermography for the Evaluation of Inflammatory and Degenerative Joint Diseases: A Systematic Review. Cartilage 2021, 13, 1790S–1801S. [Google Scholar] [CrossRef]
- Güneş, Ü.Y.; Zaybak, A. Does the body temperature change in older people? J. Clin. Nurs. 2008, 17, 2284–2287. [Google Scholar] [CrossRef] [PubMed]
- Júnior, P.R.H.; Sardeli, A.V. The Effect of Aging on Body Temperature: A Systematic Review and Meta- Analysis. Curr. Aging Sci. 2021, 14, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-H.; Leasure, A.-R.; Dai, Y.-T. A systematic review of body temperature variations in older people. J. Clin. Nurs. 2010, 19, 4–16. [Google Scholar] [CrossRef]
- Blatteis, C.M. Age-Dependent Changes in Temperature Regulation–A Mini Review. Gerontology 2012, 58, 289–295. [Google Scholar] [CrossRef]
- Petrofsky, J.S.; Lohman, E., III; Suh, H.J.; Garcia, J.; Anders, A.; Sutterfield, C.; Khandge, C. The effect of aging on conductive heat exchange in the skin at two environmental temperatures. Med. Sci. Monit. 2006, 12, CR400–CR408. [Google Scholar] [PubMed]
- Ferreira, J.J.A.; Mendonça, L.C.S.; Nunes, L.A.O.; Andrade Filho, A.C.C.; Rebelatto, J.R.; Salvini, T.F. Exercise-Associated Thermographic Changes in Young and Elderly Subjects. Ann. Biomed. Eng. 2008, 36, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Vujcic, M.; Nedeljkovic, R. Thermography in the detection and follow up of chondromalacia patellae. Ann. Rheum. Dis. 1991, 50, 921–925. [Google Scholar] [CrossRef]
- Collins, A.J.; Ring, E.F.; Cosh, J.A.; Bacon, P.A. Quantitation of thermography in arthritis using multi-isothermal analysis. I. The thermographic index. Ann. Rheum. Dis. 1974, 33, 113–115. [Google Scholar] [CrossRef]
- Ramirez-GarciaLuna, J.L.; Bartlett, R.; Arriaga-Caballero, J.E.; Fraser, R.D.J.; Saiko, G. Infrared Thermography in Wound Care, Surgery, and Sports Medicine: A Review. Front. Physiol. 2022, 13, 838528. [Google Scholar] [CrossRef]
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–399. [Google Scholar]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Neves, E.B.; Salamunes, A.C.C.; de Oliveira, R.M.; Stadnik, A.M.W. Effect of body fat and gender on body temperature distribution. J. Therm. Biol. 2017, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaciuba-Uscilko, H.; Grucza, R. Gender differences in thermoregulation. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Chudecka, M.; Lubkowska, A. Thermal maps of young women and men. Infrared Phys. Technol. 2015, 69, 81–87. [Google Scholar] [CrossRef]
- Arciero, P.J.; Goran, M.I.; Poehlman, E.T. Resting metabolic rate is lower in women than in men. J. Appl. Physiol. 1993, 75, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, Y.; Liu, Z.; Long, H.; Ruan, Z.; Zhao, S. Causal associations of obesity related anthropometric indicators and body compositions with knee and hip arthritis: A large-scale genetic correlation study. Front. Endocrinol. 2022, 13, 1011896. [Google Scholar] [CrossRef]
- Boffa, A.; Merli, G.; Andriolo, L.; Lattermann, C.; Salzmann, G.M.; Filardo, G. Synovial Fluid Biomarkers in Knee Osteoarthritis: A Systematic Review and Quantitative Evaluation Using BIPEDs Criteria. Cartilage 2021, 13, 82S–103S. [Google Scholar] [CrossRef]
- Kanthawang, T.; Bodden, J.; Joseph, G.B.; Lane, N.E.; Nevitt, M.; McCulloch, C.E.; Link, T.M. Obese and overweight individuals have greater knee synovial inflammation and associated structural and cartilage compositional degeneration: Data from the osteoarthritis initiative. Skelet. Radiol. 2021, 50, 217–229. [Google Scholar] [CrossRef]
- Hung, A.; Sayre, E.C.; Guermazi, A.; Esdaile, J.M.; Kopec, J.A.; Thorne, A.; Singer, J.; Wong, H.; Nicolaou, S.; Cibere, J. Association of Body Mass Index with Incidence and Progression of Knee Effusion on Magnetic Resonance Imaging and on Knee Examination. Arthritis Care Res. 2016, 68, 511–516. [Google Scholar] [CrossRef]
- Distel, E.; Cadoudal, T.; Durant, S.; Poignard, A.; Chevalier, X.; Benelli, C. The infrapatellar fat pad in knee osteoarthritis: An important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 2009, 60, 3374–3377. [Google Scholar] [CrossRef]
- Fujisaka, S.; Usui, I.; Ikutani, M.; Aminuddin, A.; Takikawa, A.; Tsuneyama, K.; Mahmood, A.; Goda, N.; Nagai, Y.; Takatsu, K.; et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia 2013, 56, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.; van Caam, A.; Van Der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef]
- Fang, T.; Zhou, X.; Jin, M.; Nie, J.; Li, X. Molecular mechanisms of mechanical load-induced osteoarthritis. Int. Orthop. 2021, 45, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, J.J.; Ho, H.; Harner, C.D.; Fu, F.H. Use of the International Knee Documentation Committee guidelines to assess outcome following anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 1998, 6, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mendieta, E.D.M.; Ibáñez, T.C.; Jaeger, J.U.; Hernán, G.B.; Mola, E.M. Clinical and ultrasonographic findings related to knee pain in osteoarthritis. Osteoarthr. Cartil. 2006, 14, 540–544. [Google Scholar] [CrossRef]
- Ammer, K.; Engelbert, B.; Hamerle, S.; Kern, E.; Solar, S.; Kuchar, K. Correlation between the WOMAC index and temperature in patients with knee pain. Eur. J. Thermol. 1998, 8, 50–56. [Google Scholar]
- Moss, P.; Benson, H.A.E.; Will, R.; Wright, A. Patients with Knee Osteoarthritis Who Score Highly on the PainDETECT Questionnaire Present with Multimodality Hyperalgesia, Increased Pain, and Impaired Physical Function. Clin. J. Pain 2018, 34, 15–21. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- French, H.P.; Smart, K.M.; Doyle, F. Prevalence of neuropathic pain in knee or hip osteoarthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2017, 47, 1–8. [Google Scholar] [CrossRef]
- Moreton, B.J.; Tew, V.; das Nair, R.; Wheeler, M.; Walsh, D.A.; Lincoln, N.B. Pain Phenotype in Patients with Knee Osteoarthritis: Classification and Measurement Properties of painDETECT and Self-Report Leeds Assessment of Neuropathic Symptoms and Signs Scale in a Cross-Sectional Study. Arthritis Care Res. 2015, 67, 519–528. [Google Scholar] [CrossRef]
- Ohtori, S.; Orita, S.; Yamashita, M.; Ishikawa, T.; Ito, T.; Shigemura, T.; Nishiyama, H.; Konno, S.; Ohta, H.; Takaso, M.; et al. Existence of a Neuropathic Pain Component in Patients with Osteoarthritis of the Knee. Yonsei Med. J. 2012, 53, 801–805. [Google Scholar] [CrossRef] [PubMed]
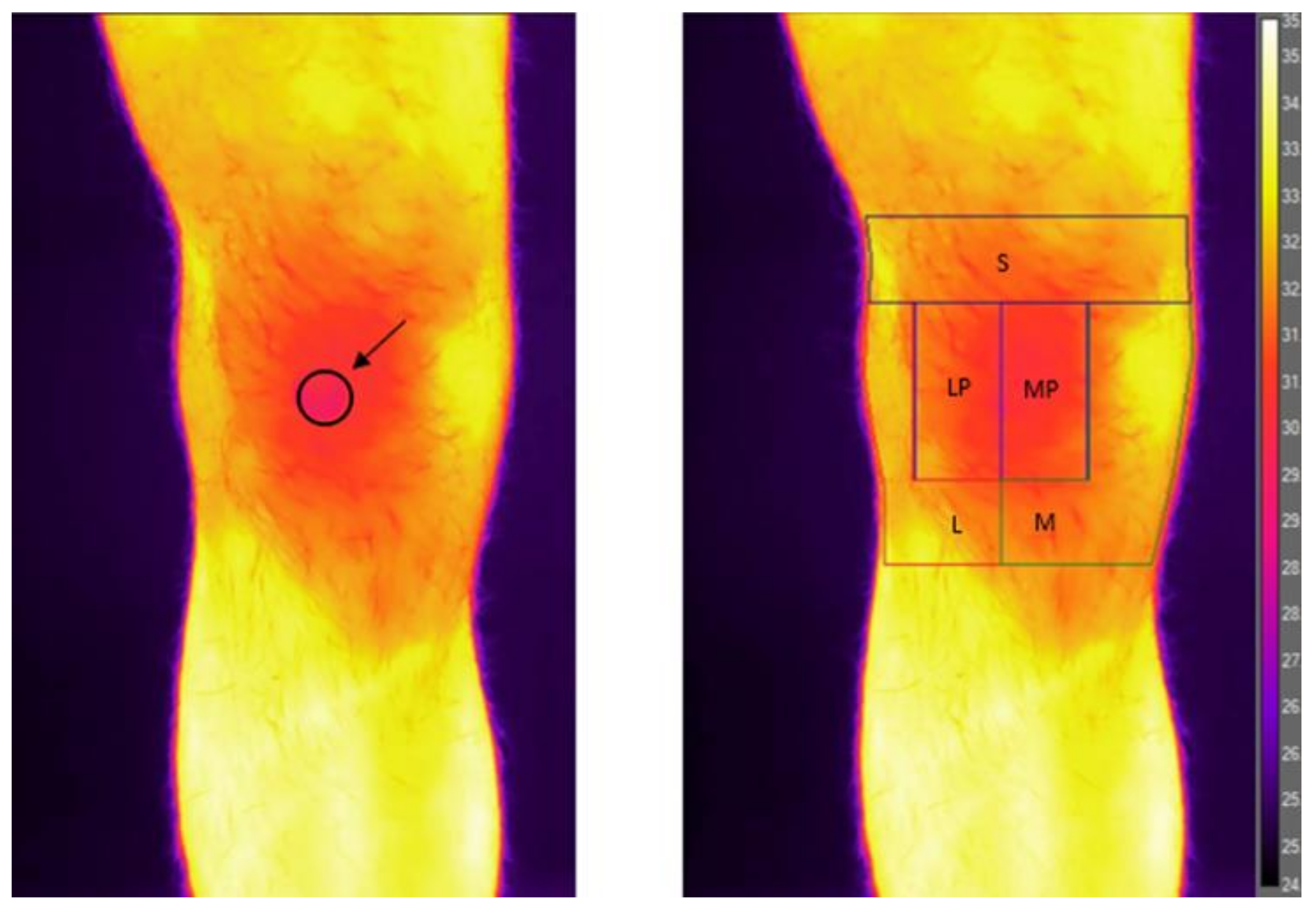
| Sex (M/W) | 26/14 |
| Age (years) | 61.3 ± 9.3 [43–75] |
| BMI (Kg/m2) | 25.2 ± 3.0 [19.9–31.1] |
| Side | Right: 21-Left: 19 |
| Symptoms duration (months) | 108.5 ± 91.4 [18–372] |
| Symptoms onset | Acute: 13-Chronic: 27 |
| Previous knee surgery (yes/no) | 22/18 |
| Smoke (yes/no) | 9/31 |
| Kellgren–Lawrence grade | Grade 2: 19 Grade 3: 18 Grade 4: 3 |
| VAS pain | 5.0 ± 2.4 [1–9] |
| IKDC Subjective score | 42.3 ± 15.1 [18.4–81.6] |
| IKDC Objective score | Grade 1: 6 Grade 2: 17 Grade 3: 9 Grade 4: 8 |
| KOOS Pain | 61.9 ± 19.1 [17–94] |
| KOOS Symptoms | 60.5 ± 20.5 [18–100] |
| KOOS ADL | 69.9 ± 18.6 [38–100] |
| KOOS QoL | 34.5 ± 16.9 [0–75] |
| KOOS Sport/Rec | 42.6 ± 17.7 [20–90] |
| Tegner score pre-treatment | 2.3 ± 1.2 [1–5] |
| PainDETECT Questionnaire | 8.8 ± 4.9 [2–20] |
| Neuropathic pain (yes/no) | 8/32 |
| Area | Mean Temperature | Minimum Temperature | Maximum Temperature |
|---|---|---|---|
| Total knee | 31.9 ± 1.6 | 30.3 ± 1.1 | 33.6 ± 1.2 |
| Medial | 32.1 ± 1.0 | 30.7 ± 1.0 | 33.4 ± 1.2 |
| Lateral | 31.9 ± 1.0 | 30.5 ± 1.1 | 33.1 ± 1.1 |
| Medial Patella | 31.6 ± 1.4 | 30.9 ± 1.3 | 32.5 ± 1.4 |
| Lateral Patella | 31.6 ± 1.5 | 30.9 ± 1.4 | 32.6 ± 1.5 |
| Suprapatellar | 32.1 ± 1.2 | 30.7 ± 1.1 | 33.3 ± 1.2 |
| Area | Age | BMI |
|---|---|---|
| Total knee | Rho = −0.380, p = 0.016 | Rho = 0.421, p = 0.007 |
| Medial | Rho = −0.450, p = 0.004 | Rho = 0.333, p = 0.036 |
| Lateral | Rho = −0.387, p = 0.014 | Rho = 0.365, p = 0.020 |
| Medial Patella | Rho = −0.265, p = 0.098 | Rho = 0.461, p = 0.003 |
| Lateral Patella | Rho = −0.329, p = 0.038 | Rho = 0.512, p = 0.001 |
| Suprapatellar | Rho = −0.377, p = 0.016 | Rho = 0.433, p = 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marziani, L.; Boffa, A.; Angelelli, L.; Andriolo, L.; Di Martino, A.; Zaffagnini, S.; Filardo, G. Infrared Thermography in Symptomatic Knee Osteoarthritis: Joint Temperature Differs Based on Patient and Pain Characteristics. J. Clin. Med. 2023, 12, 2319. https://doi.org/10.3390/jcm12062319
De Marziani L, Boffa A, Angelelli L, Andriolo L, Di Martino A, Zaffagnini S, Filardo G. Infrared Thermography in Symptomatic Knee Osteoarthritis: Joint Temperature Differs Based on Patient and Pain Characteristics. Journal of Clinical Medicine. 2023; 12(6):2319. https://doi.org/10.3390/jcm12062319
Chicago/Turabian StyleDe Marziani, Luca, Angelo Boffa, Lucia Angelelli, Luca Andriolo, Alessandro Di Martino, Stefano Zaffagnini, and Giuseppe Filardo. 2023. "Infrared Thermography in Symptomatic Knee Osteoarthritis: Joint Temperature Differs Based on Patient and Pain Characteristics" Journal of Clinical Medicine 12, no. 6: 2319. https://doi.org/10.3390/jcm12062319
APA StyleDe Marziani, L., Boffa, A., Angelelli, L., Andriolo, L., Di Martino, A., Zaffagnini, S., & Filardo, G. (2023). Infrared Thermography in Symptomatic Knee Osteoarthritis: Joint Temperature Differs Based on Patient and Pain Characteristics. Journal of Clinical Medicine, 12(6), 2319. https://doi.org/10.3390/jcm12062319






