Proof-of-Concept Double-Blind Placebo-Controlled Trial Measuring Cartilage Composition in Early Rheumatoid Arthritis under TNF-α-Inhibitor Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MR Imaging
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Descriptive Statistics
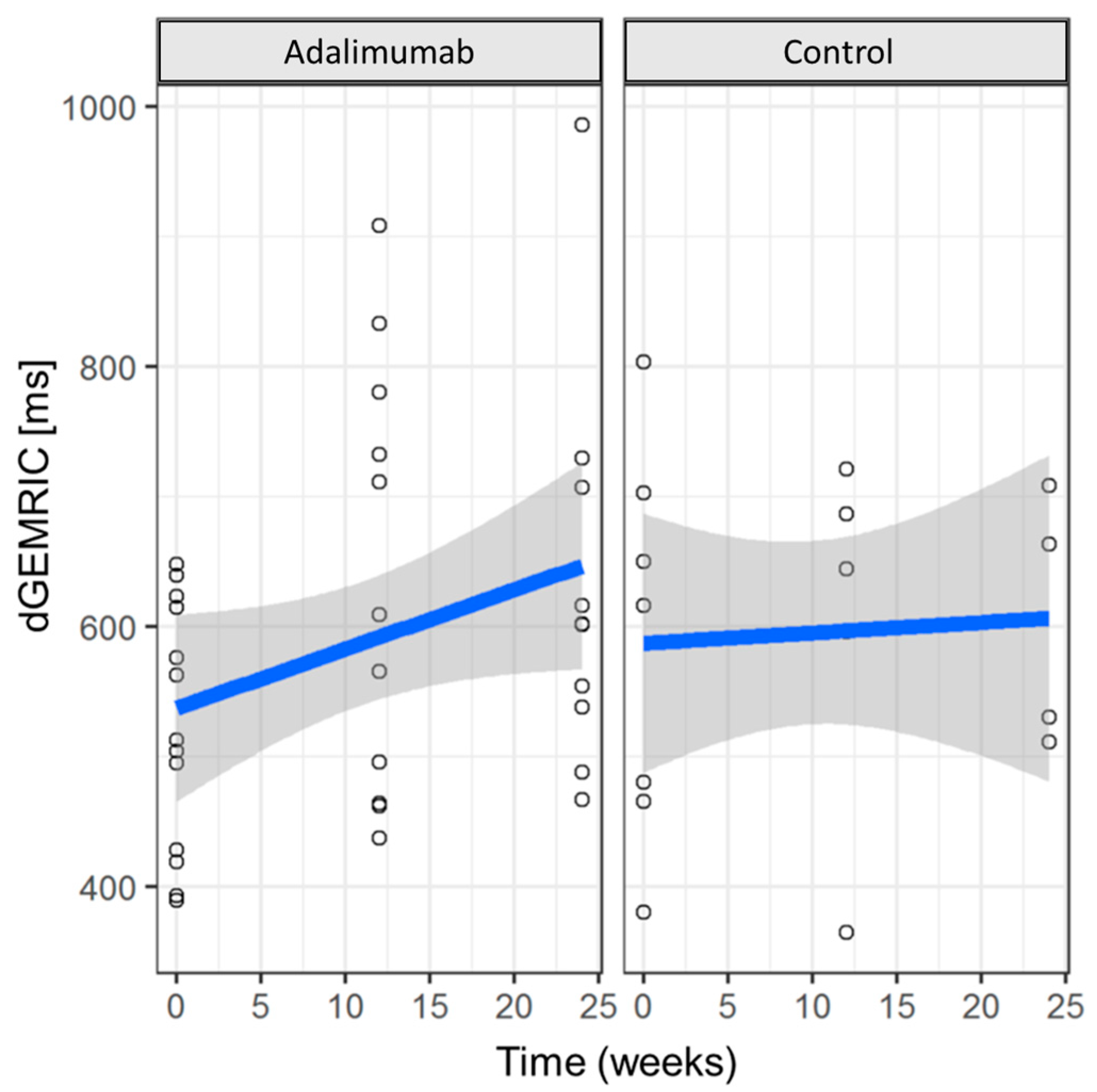
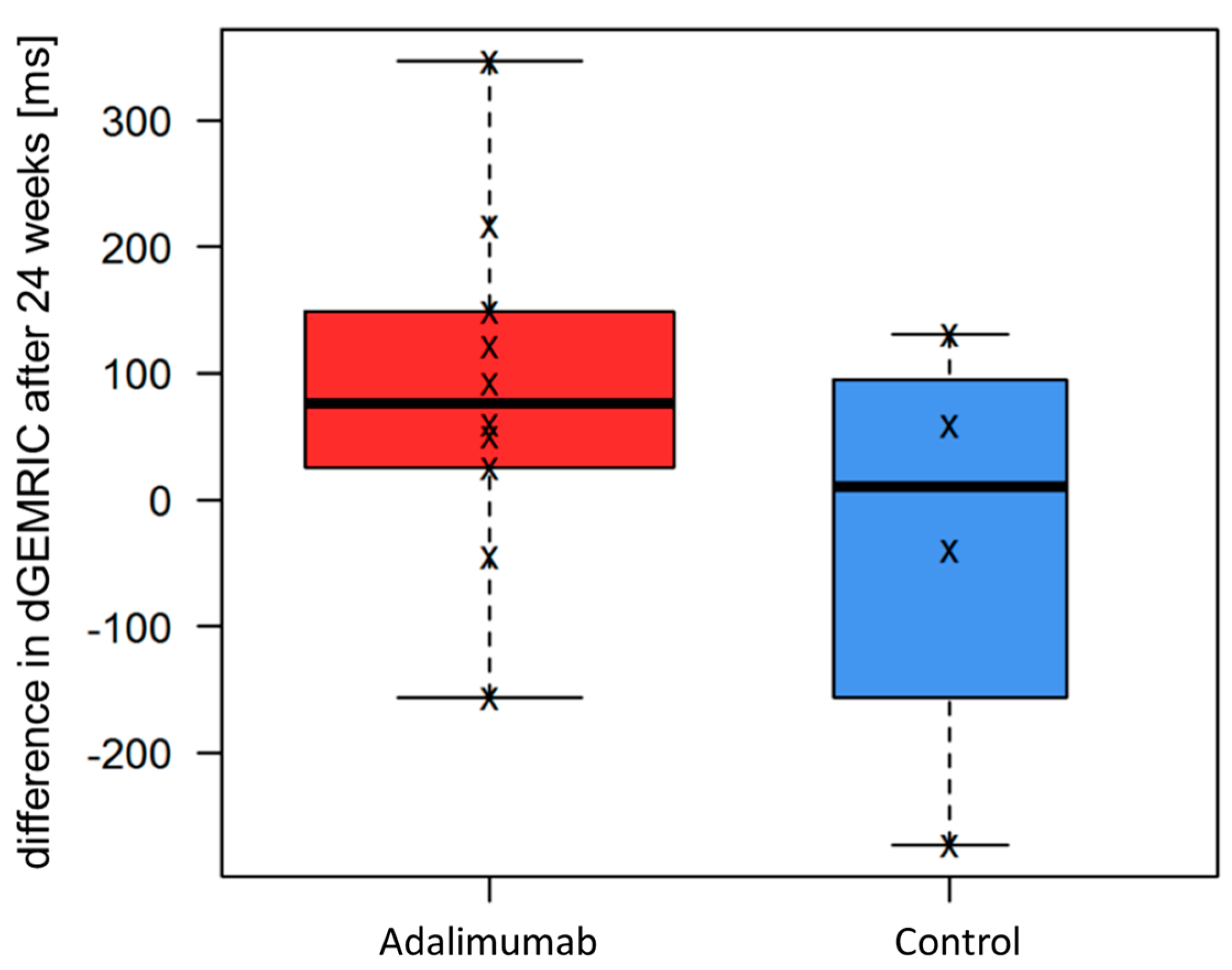
3.3. Cartilage Integrity (dGEMRIC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.M.; Weinblatt, M.E. Rheumatoid arthritis. Lancet 2001, 358, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Tobon, G.J.; Youinou, P.; Saraux, A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J. Autoimmun. 2010, 35, 10–14. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef]
- Combe, B.; Landewe, R.; Daien, C.I.; Hua, C.; Aletaha, D.; Alvaro-Gracia, J.M.; Bakkers, M.; Brodin, N.; Burmester, G.R.; Codreanu, C.; et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann. Rheum. Dis. 2017, 76, 948–959. [Google Scholar] [CrossRef]
- den Broeder, A.; van de Putte, L.; Rau, R.; Schattenkirchner, M.; Van Riel, P.; Sander, O.; Binder, C.; Fenner, H.; Bankmann, Y.; Velagapudi, R.; et al. A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alpha antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J. Rheumatol. 2002, 29, 2288–2298. [Google Scholar]
- Huang, F.; Zhang, F.C.; Bao, C.D.; Tao, Y.; Gu, J.R.; Xu, J.H.; Zhu, P.; Xu, H.J.; Zhang, Z.Y.; Zhao, D.B.; et al. [Adalimumab plus methotrexate for the treatment of rheumatoid arthritis: A multi-center randomized, double-blind, placebo-controlled clinical study.]. Zhonghua Nei Ke Za Zhi 2009, 48, 916–921. [Google Scholar] [PubMed]
- Takeuchi, T.; Yamanaka, H.; Ishiguro, N.; Miyasaka, N.; Mukai, M.; Matsubara, T.; Uchida, S.; Akama, H.; Kupper, H.; Arora, V.; et al. Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: The HOPEFUL 1 study. Ann. Rheum. Dis. 2014, 73, 536–543. [Google Scholar] [CrossRef]
- American College of Rheumatology Rheumatoid Arthritis Clinical Trials Task Force Imaging, G.; Outcome Measures in Rheumatology Magnetic Resonance Imaging Inflammatory Arthritis Working, G. Review: The utility of magnetic resonance imaging for assessing structural damage in randomized controlled trials in rheumatoid arthritis. Arthritis Rheumatol. 2013, 65, 2513–2523. [Google Scholar] [CrossRef]
- Ostergaard, M.; Peterfy, C.; Conaghan, P.; McQueen, F.; Bird, P.; Ejbjerg, B.; Shnier, R.; O'Connor, P.; Klarlund, M.; Emery, P.; et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J. Rheumatol. 2003, 30, 1385–1386. [Google Scholar]
- Aletaha, D.; Funovits, J.; Smolen, J.S. Physical disability in rheumatoid arthritis is associated with cartilage damage rather than bone destruction. Ann. Rheum. Dis. 2011, 70, 733–739. [Google Scholar] [CrossRef]
- Ostergaard, M.; Peterfy, C.G.; Bird, P.; Gandjbakhch, F.; Glinatsi, D.; Eshed, I.; Haavardsholm, E.A.; Lillegraven, S.; Boyesen, P.; Ejbjerg, B.; et al. The OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging (MRI) Scoring System: Updated Recommendations by the OMERACT MRI in Arthritis Working Group. J. Rheumatol. 2017, 44, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Miese, F.; Buchbender, C.; Scherer, A.; Wittsack, H.J.; Specker, C.; Schneider, M.; Antoch, G.; Ostendorf, B. Molecular imaging of cartilage damage of finger joints in early rheumatoid arthritis with delayed gadolinium-enhanced magnetic resonance imaging. Arthritis Rheumatol. 2012, 64, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Owman, H.; Ericsson, Y.B.; Englund, M.; Tiderius, C.J.; Tjornstrand, J.; Roos, E.M.; Dahlberg, L.E. Association between delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and joint space narrowing and osteophytes: A cohort study in patients with partial meniscectomy with 11 years of follow-up. Osteoarthr. Cartil. 2014, 22, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Gray, M.L.; Burstein, D. Gd-DTPA2- as a measure of cartilage degradation. Magn. Reson. Med. 1996, 36, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Burstein, D.; Gold, G.E.; Eckstein, F.; Baum, T.; Mosher, T.J.; Carrino, J.A.; Guermazi, A. Articular cartilage in the knee: Current MR imaging techniques and applications in clinical practice and research. Radiographics 2011, 31, 37–61. [Google Scholar] [CrossRef]
- Schleich, C.; Muller-Lutz, A.; Sewerin, P.; Ostendorf, B.; Buchbender, C.; Schneider, M.; Antoch, G.; Miese, F. Intra-individual assessment of inflammatory severity and cartilage composition of finger joints in rheumatoid arthritis. Skelet. Radiol. 2015, 44, 513–518. [Google Scholar] [CrossRef]
- Muller-Lutz, A.; Schleich, C.; Sewerin, P.; Gross, J.; Pentang, G.; Wittsack, H.J.; Antoch, G.; Schneider, M.; Ostendorf, B.; Miese, F. Comparison of quantitative and semiquantitative dynamic contrast-enhanced MRI with respect to their correlation to delayed gadolinium-enhanced MRI of the cartilage in patients with early rheumatoid arthritis. J. Comput. Assist. Tomogr. 2015, 39, 64–69. [Google Scholar] [CrossRef]
- Sewerin, P.; Muller-Lutz, A.; Abrar, D.B.; Odendahl, S.; Eichner, M.; Schneider, M.; Ostendorf, B.; Schleich, C. Prevention of the progressive biochemical cartilage destruction under methotrexate therapy in early rheumatoid arthritis. Clin. Exp. Rheumatol. 2019, 37, 179–185. [Google Scholar]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Frize, M.; Ogungbemile, A. Estimating rheumatoid arthritis activity with infrared image analysis. Stud. Health Technol. Inform. 2012, 180, 594–598. [Google Scholar]
- Miese, F.R.; Ostendorf, B.; Wittsack, H.J.; Reichelt, D.C.; Mamisch, T.C.; Zilkens, C.; Lanzman, R.S.; Schneider, M.; Scherer, A. Metacarpophalangeal joints in rheumatoid arthritis: Delayed gadolinium-enhanced MR imaging of cartilage—A feasibility study. Radiology 2010, 257, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Miese, F.; Kropil, P.; Ostendorf, B.; Scherer, A.; Buchbender, C.; Quentin, M.; Lanzman, R.S.; Blondin, D.; Schneider, M.; Bittersohl, B.; et al. Motion correction improves image quality of dGEMRIC in finger joints. Eur. J. Radiol. 2011, 80, e427–e431. [Google Scholar] [CrossRef] [PubMed]
- Mori, V.; Sawicki, L.M.; Sewerin, P.; Eichner, M.; Schaarschmidt, B.M.; Oezel, L.; Gehrmann, S.; Bittersohl, B.; Antoch, G.; Schleich, C. Differences of radiocarpal cartilage alterations in arthritis and osteoarthritis using morphological and biochemical magnetic resonance imaging without gadolinium-based contrast agent administration. Eur. Radiol. 2018, 29, 2581–2588. [Google Scholar] [CrossRef]
- Miese, F.R.; Ostendorf, B.; Wittsack, H.J.; Reichelt, D.C.; Kropil, P.; Lanzman, R.S.; Mamisch, T.C.; Zilkens, C.; Jellus, V.; Quentin, M.; et al. Cartilage quality in finger joints: Delayed Gd(DTPA)(2)-enhanced MRI of the cartilage (dGEMRIC) at 3T. Rofo 2010, 182, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Beals, C.; Baumgartner, R.; Peterfy, C.; Balanescu, A.; Mirea, G.; Harabagiu, A.; Popa, S.; Cheng, A.; Feng, D.; Ashton, E.; et al. Magnetic resonance imaging of the hand and wrist in a randomized, double-blind, multicenter, placebo-controlled trial of infliximab for rheumatoid arthritis: Comparison of dynamic contrast enhanced assessments with semi-quantitative scoring. PLoS ONE 2017, 12, e0187397. [Google Scholar] [CrossRef]
- Tiderius, C.J.; Sandin, J.; Svensson, J.; Dahlberg, L.E.; Jacobsson, L. Knee cartilage quality assessed with dGEMRIC in rheumatoid arthritis patients before and after treatment with a TNF inhibitor. Acta Radiol. 2010, 51, 1034–1037. [Google Scholar] [CrossRef]
- Kuettner, K.E.; Cole, A.A. Cartilage degeneration in different human joints. Osteoarthr. Cartil. 2005, 13, 93–103. [Google Scholar] [CrossRef]
- Moller-Bisgaard, S.; Horslev-Petersen, K.; Ejbjerg, B.; Hetland, M.L.; Ornbjerg, L.M.; Glinatsi, D.; Moller, J.; Boesen, M.; Christensen, R.; Stengaard-Pedersen, K.; et al. Effect of Magnetic Resonance Imaging vs Conventional Treat-to-Target Strategies on Disease Activity Remission and Radiographic Progression in Rheumatoid Arthritis: The IMAGINE-RA Randomized Clinical Trial. JAMA 2019, 321, 461–472. [Google Scholar] [CrossRef]
- Peterfy, C.; Emery, P.; Tak, P.P.; Ostergaard, M.; DiCarlo, J.; Otsa, K.; Navarro Sarabia, F.; Pavelka, K.; Bagnard, M.A.; Gylvin, L.H.; et al. MRI assessment of suppression of structural damage in patients with rheumatoid arthritis receiving rituximab: Results from the randomised, placebo-controlled, double-blind RA-SCORE study. Ann. Rheum. Dis. 2016, 75, 170–177. [Google Scholar] [CrossRef]

| Sequence/Parameter | STIR | T1 TSE | T1 VIBE | T1 FLIP | T1 VIBE | T1 TSE | T1 TSE fs | T1 |
|---|---|---|---|---|---|---|---|---|
| Contrast agent | no | no | no | no | yes | yes | yes | yes |
| Orientation | coronal | coronal | coronal | coronal | coronal | coronal | transversal | sagittal |
| TE/TR [ms/ms] | 31/ 5560 | 27/ 862 | 5.8/ 1.9 | 5.8/ 1.9 | 5.8/ 1.9 | 27/ 862 | 16/ 702 | 4.56/ 15 |
| Flip angle [°] | 120 | 150 | 8 | 8 + 26 | 8 | 150 | 90 | 5 + 26 |
| Slice thickness [mm] | 2.5 | 2.5 | 3 | 3 | 3 | 2.5 | 2.5 | 2 |
| FoV [mm × mm] | 130 × 130 | 140 × 140 | 140 × 140 | 140 × 140 | 140 × 140 | 140 × 140 | 120 × 120 | 90 × 53.5 |
| Number of images | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Basic resolution | 448 | 512 | 128 | 128 | 128 | 512 | 384 | 384 |
| Number of acquired slices | 17 | 17 | 10 | 10 | 10 | 17 | 20 | 10 |
| Adalimumab | Control Group | |||
|---|---|---|---|---|
| Pooled MCP2 and MCP3 dGEMRIC Values | t0 | Δ(t2 − t0) | t0 | Δ(t2 − t0) |
| Min. | 390 | −156.20 | 380.2 | −273.00 |
| 1st Qu. | 428.5 | 31.62 | 473.2 | −97.88 |
| Median | 513.0 | 75.88 | 616.0 | 9.50 |
| Mean | 523.7 | 85.78 | 585.5 | −30.75 |
| 3rd Qu. | 614.8 | 141.60 | 676.2 | 76.62 |
| Max. | 647.8 | 346.50 | 803.2 | 131.00 |
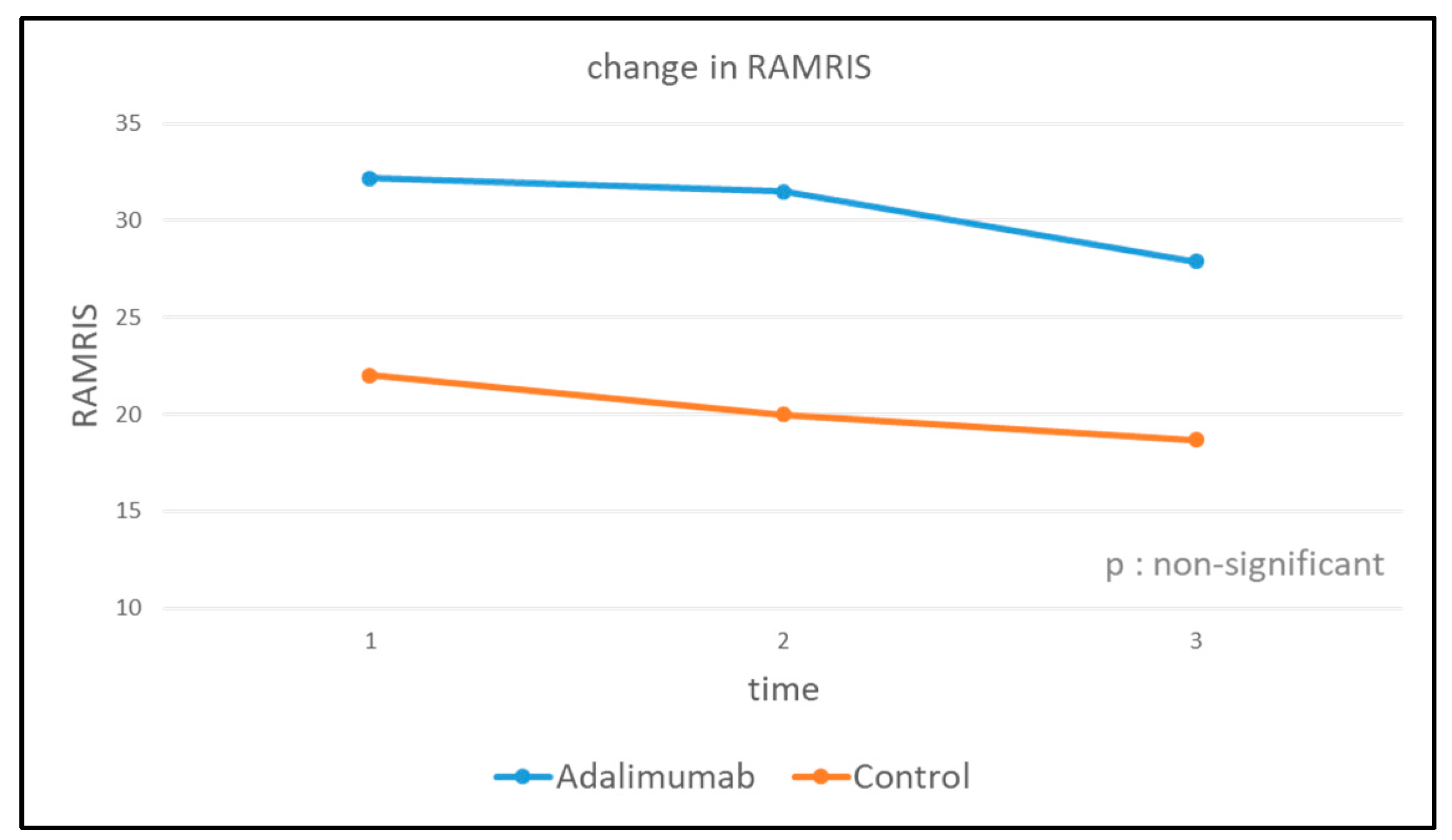
| RAMRIS | |||
|---|---|---|---|
| Adalimumab | t0 | t1 | t2 |
| Max. | 75 | 70 | 57 |
| Median | 32.18 | 31.50 | 27.90 |
| Min. | 10 | 8 | 8 |
| Control group | |||
| Max. | 29 | 28 | 28 |
| Median | 22.00 | 20.00 | 18.67 |
| Min. | 17 | 14 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frenken, M.; Ostendorf, B.; Brinks, R.; Schleich, C.; Wilms, L.M.; Vordenbäumen, S.; Müller-Lutz, A.; Richter, J.G.; Sander, O.; Antoch, G.; et al. Proof-of-Concept Double-Blind Placebo-Controlled Trial Measuring Cartilage Composition in Early Rheumatoid Arthritis under TNF-α-Inhibitor Therapy. J. Clin. Med. 2023, 12, 2306. https://doi.org/10.3390/jcm12062306
Frenken M, Ostendorf B, Brinks R, Schleich C, Wilms LM, Vordenbäumen S, Müller-Lutz A, Richter JG, Sander O, Antoch G, et al. Proof-of-Concept Double-Blind Placebo-Controlled Trial Measuring Cartilage Composition in Early Rheumatoid Arthritis under TNF-α-Inhibitor Therapy. Journal of Clinical Medicine. 2023; 12(6):2306. https://doi.org/10.3390/jcm12062306
Chicago/Turabian StyleFrenken, Miriam, Benedikt Ostendorf, Ralph Brinks, Christoph Schleich, Lena M. Wilms, Stefan Vordenbäumen, Anja Müller-Lutz, Jutta G. Richter, Oliver Sander, Gerald Antoch, and et al. 2023. "Proof-of-Concept Double-Blind Placebo-Controlled Trial Measuring Cartilage Composition in Early Rheumatoid Arthritis under TNF-α-Inhibitor Therapy" Journal of Clinical Medicine 12, no. 6: 2306. https://doi.org/10.3390/jcm12062306
APA StyleFrenken, M., Ostendorf, B., Brinks, R., Schleich, C., Wilms, L. M., Vordenbäumen, S., Müller-Lutz, A., Richter, J. G., Sander, O., Antoch, G., Schneider, M., Baraliakos, X., Abrar, D. B., & Sewerin, P. (2023). Proof-of-Concept Double-Blind Placebo-Controlled Trial Measuring Cartilage Composition in Early Rheumatoid Arthritis under TNF-α-Inhibitor Therapy. Journal of Clinical Medicine, 12(6), 2306. https://doi.org/10.3390/jcm12062306









