Isolated Subclinical Right Ventricle Systolic Dysfunction in Patients after Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Battista, S.; Bar, F.; Mengozzi, G.; Zanon, E.; Grosso, M.; Molino, G. Hyperdynamic circulation in patients with cirrhosis: Direct measurement of nitric oxide levels in hepatic and portal veins. J. Hepatol. 1997, 26, 75–80. [Google Scholar] [CrossRef]
- Savale, L.; Watherald, J.; Sitbon, O. Portopulmonary Hypertension. Semin. Respir. Crit. Care Med. 2017, 38, 651–661. [Google Scholar] [PubMed]
- Liberal, R.; Grant, C.R.; Baptista, R.; Macedo, G. Porto-pulmonary hypertension: A comprehensive review. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Herve, P.; Lebrec, D.; Brenot, F.; Simonneau, G.; Humbert, M.; Sitbon, O.; Duroux, P. Pulmonary vascular disorders in portal hypertension. Eur. Respir. J. 1998, 11, 1153–1166. [Google Scholar] [CrossRef]
- Rodríguez-Vilarrupla, A.; Fernández, M.; Bosch, J.; García-Pagán, J.C. Current concepts on the pathophysiology of portal hypertension. Ann. Hepatol. 2007, 6, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Herve, P.; Le Pavec, J.; Sztrymf, B.; Decante, B.; Savale, L.; Sitbon, O. Pulmonary vascular abnormalities in cirrhosis. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Liu, P.; Lilly, L.; Bomzon, A.; Blendis, L. Role of cardiac structural and functional abnormalities in the pathogenesis of hyperdynamic circulation and renal sodium retention in cirrhosis. Clin. Sci. 1999, 97, 259–267. [Google Scholar] [CrossRef]
- Kia, L.; Shah, S.J.; Wang, E.; Sharma, D.; Selvaraj, S.; Medina, C.; Cahan, J.; Mahon, H.; Levitsky, J. Role of pretransplant echocardiographic evaluation in predicting outcomes following liver transplantation. Am. J. Transplant. 2013, 13, 2395–2401. [Google Scholar] [CrossRef]
- Jakab, S.S.; Garcia-Tsao, G. Evaluation and management of esophageal and gastric varices in patients with cirrhosis. Clin. Liver. Dis. 2020, 24, 335–350. [Google Scholar] [CrossRef]
- Poynard, T.; Cales, P.; Pasta, L.; Ideo, G.; Pascal, J.P.; Pagliaro, L.; Lebrec, D.; Franco—Italian Multicenter Study Group. Beta-adrenergic-antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices: An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. N. Engl. J. Med. 1991, 324, 1532–1538. [Google Scholar]
- Pascal, J.P.; Cales, P. Propranolol in the prevention of first upper gastrointestinal tract hemorrhage in patients with cirrhosis of the liver and esophageal varices. N. Engl. J. Med. 1987, 317, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Bosch, J. Management of varices and variceal hemorrhage in cirrhosis. N. Engl. J. Med. 2010, 362, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Lebrec, D.; Corbic, M.; Nouel, O.; Benhamou, J.P. Propranolol—A medical treatment for portal hypertension? Lancet 1980, 316, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Senzolo, M.; Cholongitas, E.; Burra, P.; Leandro, G.; Thalheimer, U.; Patch, D.; Burroughs, A.K. β-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: A meta-analysis. Liver Int. 2009, 29, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.-H.; Chen, W.-C.; Lin, C.-K.; Tsai, W.-L.; Chan, H.-H.; Chen, T.-A.; Yu, H.-C.; Hsu, P.-I.; Lai, K.-H. Improved survival in patients receiving medical therapy as compared with banding ligation for the prevention of esophageal variceal rebleeding. Hepatology 2008, 48, 580–587. [Google Scholar] [CrossRef]
- Bhutta, A.Q.; Garcia-Tsao, G.; Reddy, K.R.; Tandon, P.; Wong, F.; O’Leary, J.G.; Acharya, C.; Banerjee, D.; Abraldes, J.G.; Jones, T.M.; et al. Beta-blockers in hospitalised patients with cirrhosis and ascites: Mortality and factors determining discontinuation and reinitiation. Aliment. Pharmacol. Ther. 2018, 47, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Sersté, T.; Melot, C.; Francoz, C.; Durand, F.; Rautou, P.E.; Valla, D.; Moreau, R.; Lebrec, D. Deleterious efects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010, 52, 1017–1022. [Google Scholar] [CrossRef]
- Krag, A.; Wiest, R.; Albillos, A.; Gluud, L.L. The window hypothesis: Haemodynamic and non-haemodynamic efects of β-blockers improve survival of patients with cirrhosis during a window in the disease. Gut 2012, 61, 967–969. [Google Scholar] [CrossRef]
- Hillon, P.; Lebrec, D.; Muńoz, C.; Jungers, M.; Goldfarb, G.; Benhamou, J.P. Comparison of the effects of a cardioselective and a nonselective bblocker on portal hypertension in patients with cirrhosis. Hepatology 1982, 2, 528–531. [Google Scholar] [CrossRef]
- Westaby, D.; Melia, W.M.; Macdougall, B.R.; Hegarty, J.E.; Gimson, A.E.; Williams, R. B1 selective adrenoreceptor blockade for the long-term management of variceal bleeding. A prospective randomised trial to compare oral metoprolol with injection sclerotherapy in cirrhosis. Gut 1985, 26, 421–425. [Google Scholar] [CrossRef]
- Torregrosa, M.; Genesca, J.; Gonzalez, A.; Evangelista, A.; Mora, A.; Margarit, C.; Esteban, R.; Guardia, J. Role of Doppler echocardiography in the assessment of porto-pulmonary hypertension in liver transplantation candidates. J. Hepatol. 2005, 42, 68–74. [Google Scholar] [CrossRef] [PubMed]
- López-Candales, A.; Menendez, F.L.; Shah, S.A.; Friedrich, A. Measures of right ventricular systolic function in end stage liver disease patients awaiting transplant. Int. J. Cardiol. 2014, 171, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chan, A.C.; Chan, S.C.; Chok, S.H.; Sharr, W.; Fung, J.; Liu, J.H.; Zhen, Z.; Sin, W.C.; Lo, C.M.; et al. A detailed evaluation of cardiac function in cirrhotic patients and its alteration with or without liver transplantation. J. Cardiol. 2016, 67, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Sussman, N.L.; Kochar, R.; Fallon, M.B. Pulmonary complications in cirrhosis. Curr. Opin. Organ. Transplant. 2011, 16, 281–288. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right ventricular function in cardiovascular disease, partII: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008, 117, 1717–1731. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Quaife, R.A.; Leinwand, L.A.; Barst, R.J.; McGoon, M.D.; Meldrum, D.R.; Dupuis, J.; Long, C.S.; Rubin, L.J.; Smart, F.W.; et al. Right ventricular function and failure: Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006, 114, 1883–1891. [Google Scholar] [CrossRef]
- Matthews, J.C.; Dardas, T.F.; Dorsch, M.P.; Aaronson, K.D. Right-sided heart failure: Diagnosis and treatment strategies. Curr. Treat. Opt. Cardiovasc. Med. 2008, 10, 329–341. [Google Scholar] [CrossRef]
- Hernandez-Suarez, D.; López-Candales, A. Subclinical Right Ventricular Dysfunction in Patients with Severe Aortic Stenosis: A Retrospective Case Series. Cardiol. Ther. 2017, 6, 151–155. [Google Scholar] [CrossRef]
- Towheed, A.; Sabbagh, E.; Gupta, R.; Assiri, S.; Chowdhury, M.A.; Moukarbel, G.V.; Khuder, S.A.; Schwann, T.A.; Bonnell, M.R.; Cooper, C.J.; et al. Right ventricular dysfunction and short-term outcomes following left-sided valvular surgery: An echocardiographic study. J. Am. Heart Assoc. 2021, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş Inci, S.; Sade, L.E.; Altın, C.; Pirat, B.; Erken Pamukcu, H.; Yılmaz, S.; Müderrisoğlu, H. Subclinical myocardial dysfunction in liver transplant candidates determined using speckle tracking imaging. Turk. Kardiyol. Dern. Ars. 2019, 47, 638–645. [Google Scholar] [CrossRef]
- Acosta, F.; Sansano, T.; Palenciano, C.G.; Roqués, V.; Clavel, N.; González, P.; Robles, R.; Bueno, F.S.; Ramirez, P.; Parrilla, P. Relationship Between Cardiovascular State and Degree of Hepatic Dysfunction in Patients Treated with Liver Transplantation. Transplant Proc. 2016, 8, 231–262. [Google Scholar] [CrossRef]
- Xu, H.; Li, W.; Xu, Z.; Shi, X. Evaluation of the right ventricular ejection fraction during classic orthotopic liver transplantation without venovenous bypass. Clin. Transplant. 2012, 26, E485–E491. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Yang, S.-M.; Cho, H.; Ju, J.-W.; Jang, H.S.; Lee, H.-J.; Kim, W.H. The Prognostic Role of Right Ventricular Stroke Work Index during Liver Transplantation. J. Clin. Med. 2021, 10, 4022. [Google Scholar] [CrossRef] [PubMed]
- Gjeorgjievski, M.; Cappell, M.S. Portal hypertensive gastropathy: A systematic review of the pathophysiology, clinical presentation, natural history and therapy. World J. Hepatol. 2016, 8, 231–262. [Google Scholar] [CrossRef]
- Li, T.; Ke, W.; Sun, P.; Chen, X.; Belgaumkar, A.; Huang, Y.; Xian, W.; Li, J.; Zheng, Q. Carvedilol for portal hypertension in cirrhosis: Systematic review with meta-analysis. BMJ Open. 2016, 6, e010902. [Google Scholar] [CrossRef]
- La Mura, V.; Abraldes, J.G.; Raffa, S.; Retto, O.; Berzigotti, A.; García-Pagán, J.C.; Bosch, J. Prognostic value ofacute hemodynamic response to i.v. propranolol in patients with cirrhosis and portal hypertension. J. Hepatol. 2009, 51, 279–287. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W.; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef]
- Villanueva, C.; Albillos, A.; Genescà, J.; Garcia-Pagan, J.C.; Calleja, J.L.; Aracil, C.; Bañares, R.; Morillas, R.M.; Poca, M.; Peñas, B.; et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2019, 393, 1597–1608. [Google Scholar] [CrossRef]
- Brito-Azevedo, A.; Perez, R.M.; Coelho, H.S.; Fernandes, E.S.; Castiglione, R.C.; Villela-Nogueira, C.A.; Bouskela, E. The anti-inflammatory role of propranolol in cirrhosis: Preventing the inflammatory exhaustion? J. Hepatol. 2017, 66, 240–241. [Google Scholar] [CrossRef]
- Reiberger, T.; Ferlitsch, A.; Payer, B.A.; Mandorfer, M.; Heinisch, B.B.; Hayden, H.; Lammert, F.; Trauner, M.; Peck-Radosavljevic, M.; Vogelsang, H. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J. Hepatol. 2013, 58, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Brito-Azevedo, A.; Perez Rde, M.; Coelho, H.S.; Fernandes Ede, S.; Castiglione, R.C.; Villela-Nogueira, C.A.; Bouskela, E. Propranolol improves endothelial dysfunction in advanced cirrhosis: The ‘endothelial exhaustion’ hypothesis. Gut 2016, 65, 1391–1392. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, W.W.; Ribeiro-Oliveira, A., Jr.; Ribeiro Rda, C.; Vieira, R.L.; Almeida, J.; Nadu, A.P.; Simões e Silva, A.C.; Santos, R.A. Effect of propranolol on the splanchnic and peripheral renin angiotensin system in cirrhotic patients. World J. Gastroenterol. 2008, 14, 6824–6830. [Google Scholar] [CrossRef] [PubMed]
- Willett, I.; Esler, M.; Burke, F.; Leonard, P.; Dudley, F. Total and renal sympathetic nervous system activity in alcoholic cirrhosis. J. Hepatol. 1985, 1, 639–648. [Google Scholar] [CrossRef]
- Murray, J.F.; Dawson, A.M.; Sherlock, S. Circulatory changes in chronic liverdisease. Am. J. Med. 1958, 24, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Rosoff, L., Jr.; Zia, P.; Reynolds, T.; Horton, R. Studies of renin and aldosterone in cirrhotic patients with ascites. Gastroenterology 1975, 69, 698–705. [Google Scholar] [CrossRef]
- Rosoff, L., Jr.; Williams, J.; Moult, P.; Williams, H.; Sherlock, S. Renal hemodynamics, and the renin–angiotensin system in cirrhosis: Relationship to sodium retention. Dig. Dis. Sci. 1979, 24, 25–32. [Google Scholar] [CrossRef]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Baveno VII Faculty. Baveno VII-Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef]
| Overall (n = 34) | RVD (−) (n = 27) | RVD (+) (n = 7) | p Value | |
|---|---|---|---|---|
| Age, years; Mean ± SD | 54.1 ± 8.7 | 53.6 ± 8.7 | 56.1 ± 9.1 | 0.65 |
| Gender, female; % (n) | 32.4 (11) | 29.6 (8) | 42.9 (3) | 0.51 |
| BMI, kg/m2; Median (Range) | 29.6 [11.5] | 29.4 [11.5] | 30.5 [7.4] | 0.40 |
| Diagnosis-to-transplantation time, years; Mean ± SD | 5.5 ± 5.3 | 5.8 ± 5.5 | 4.6 ± 4.3 | 0.71 |
| Etiology, viral; % (n) | 47.1 (16) | 48.1 (13) | 42.9 (3) | 0.80 |
| Child–Pugh score; Mean ± SD | 8.2 ± 1.6 | 8.3 ± 1.5 | 8.5 ± 2.6 | 0.43 |
| MELD score; Mean ± SD | 18.3 ± 6.3 | 19.3 ± 6.3 | 14.6 ± 5.1 | 0.06 |
| Esophageal varices grade; Mean ± SD | 1.4 ± 1.1 | 1.5 ± 1.1 | 1.3 ± 1.1 | 0.65 |
| History of GI Bleeding; % (n) | 17.6 (6) | 11.1 (3) | 42.9 (3) | 0.05 |
| Hypertension; % (n) | 38.2 (13) | 44.4 (12) | 14.3 (1) | 0.14 |
| Smoking history (>20 package years); % (n) | 41.2 (14) | 37 (10) | 57.1 (4) | 0.34 |
| Coronary artery disease; % (n) | 5.9 (2) | 0 (0) | 28.6 (2) | <0.01 |
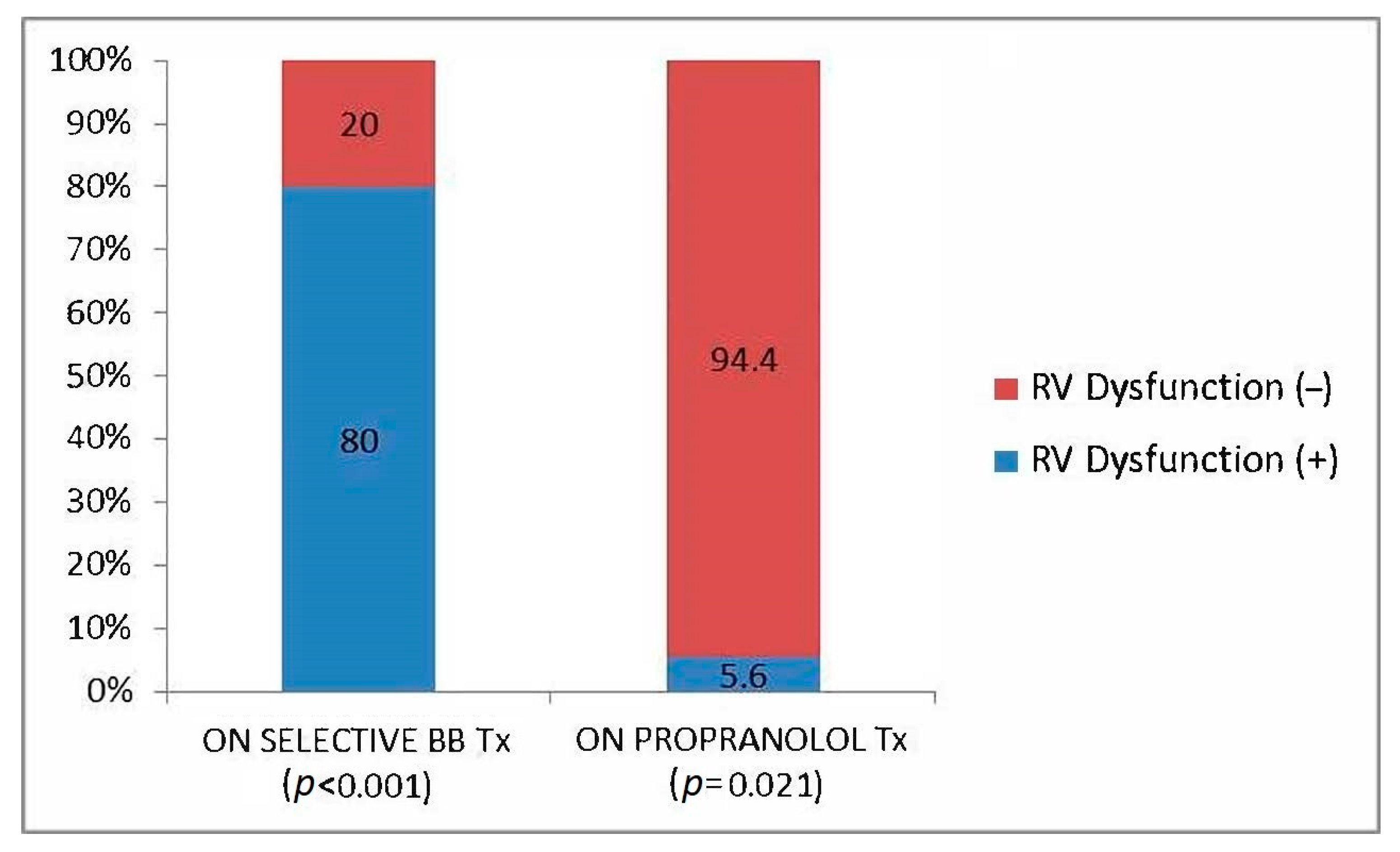
| Propranolol use; % (n) | 52.9 (18) | 63 (17) | 14.3 (1) | 0.02 |
| Spironolactone use; % (n) | 61.8 (21) | 63 (17) | 57.1 (4) | 0.78 |
| Other diuretic use; % (n) | 67.6 (23) | 66.7 (18) | 71.4 (5) | 0.81 |
| RAS-B use; % (n) | 14.7 (5) | 18.5 (5) | 0 (0) | 0.22 |
| CCB use; % (n) | 17.6 (6) | 18.5 (5) | 14.3 (1) | 0.79 |
| RVD (−) (n = 27) | RVD (+) (n = 7) | p Value | |
|---|---|---|---|
| eGFR, mL/min/1.73 m2; Median (Range) | 99.7 [143.5] | 99.8 [85.2] | 0.93 |
| Albumin, g/dL; Mean ± SD | 3.3 ± 0.6 | 3.3 ± 0.6 | 0.97 |
| INR, Median (Range) | 1.5 [2.1] | 1.5 [1] | 0.93 |
| ALT, U/L; Mean ± SD | 42 ± 31.1 | 39.5 ± 26.7 | 0.87 |
| GGT, U/L; Mean ± SD | 69 ± 62.1 | 131 ± 119.5 | 0.16 |
| Hemoglobin, g/dL; Median (Range) | 10.5 [9.2] | 12.6 [5] | 0.24 |
| Platelet count, ×1000/µL; Mean ± SD | 87.8 ± 55.8 | 148.1 ± 152.9 | 0.59 |
| CRP, mg/L; Mean ±SD | 16.3 ± 24.5 | 14 ± 18.1 | 0.90 |
| NT-ProBNP, pg/mL; Mean ± SD | 161 ± 166.5 | 257.9 ± 353.8 | 0.30 |
| Portal vein diameter, mm; Median (Range) | 10 [7.2] | 11 [3.8] | 0.59 |
| Portal vein velocity, cm/s; Mean ± SD | 113.4 ± 77.2 | 72.3 ± 45.4 | 0.18 |
| Portal vein output, mL/min; Mean ± SD | 2460 ± 1787 | 2122 ± 1125 | 0.87 |
| Hepatic vein velocity, cm/s; Mean ± SD | 59.7 ± 32.4 | 56 ± 36 | 0.62 |
| RVD (−) (n = 27) | RVD (+) (n = 7) | p Value | |
|---|---|---|---|
| Initial estimated sPAP, mmHg; Mean ± SD | 29.6 ± 6 | 28.3 ± 2.2 | 0.68 |
| Follow-up estimated sPAP, mmHg; Mean ± SD | 28 ± 7.5 | 27.6 ± 7 | 0.87 |
| LVEF, %; Mean ± SD | 64 ± 3.3 | 63.3 ± 2.6 | 0.29 |
| E/E’; Mean ± SD | 7.2 ± 1.9 | 7 ± 2.2 | 0.84 |
| RV:LV ratio; Median (Range) | 0.75 [0.28] | 0.77 [0.35] | 0.36 |
| RA area, cm2; Median (Range) | 12.5 [11.6] | 14.4 [7] | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celiker Guler, E.; Omaygenc, M.O.; Naki, D.D.; Yazar, A.; Karaca, I.O.; Korkut, E. Isolated Subclinical Right Ventricle Systolic Dysfunction in Patients after Liver Transplantation. J. Clin. Med. 2023, 12, 2289. https://doi.org/10.3390/jcm12062289
Celiker Guler E, Omaygenc MO, Naki DD, Yazar A, Karaca IO, Korkut E. Isolated Subclinical Right Ventricle Systolic Dysfunction in Patients after Liver Transplantation. Journal of Clinical Medicine. 2023; 12(6):2289. https://doi.org/10.3390/jcm12062289
Chicago/Turabian StyleCeliker Guler, Emel, Mehmet Onur Omaygenc, Deniz Dilan Naki, Arzu Yazar, Ibrahim Oguz Karaca, and Esin Korkut. 2023. "Isolated Subclinical Right Ventricle Systolic Dysfunction in Patients after Liver Transplantation" Journal of Clinical Medicine 12, no. 6: 2289. https://doi.org/10.3390/jcm12062289
APA StyleCeliker Guler, E., Omaygenc, M. O., Naki, D. D., Yazar, A., Karaca, I. O., & Korkut, E. (2023). Isolated Subclinical Right Ventricle Systolic Dysfunction in Patients after Liver Transplantation. Journal of Clinical Medicine, 12(6), 2289. https://doi.org/10.3390/jcm12062289






