Feasibility of Non-Invasive Coronary Artery Disease Screening with Coronary CT Angiography before Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
2. Materials and Methods
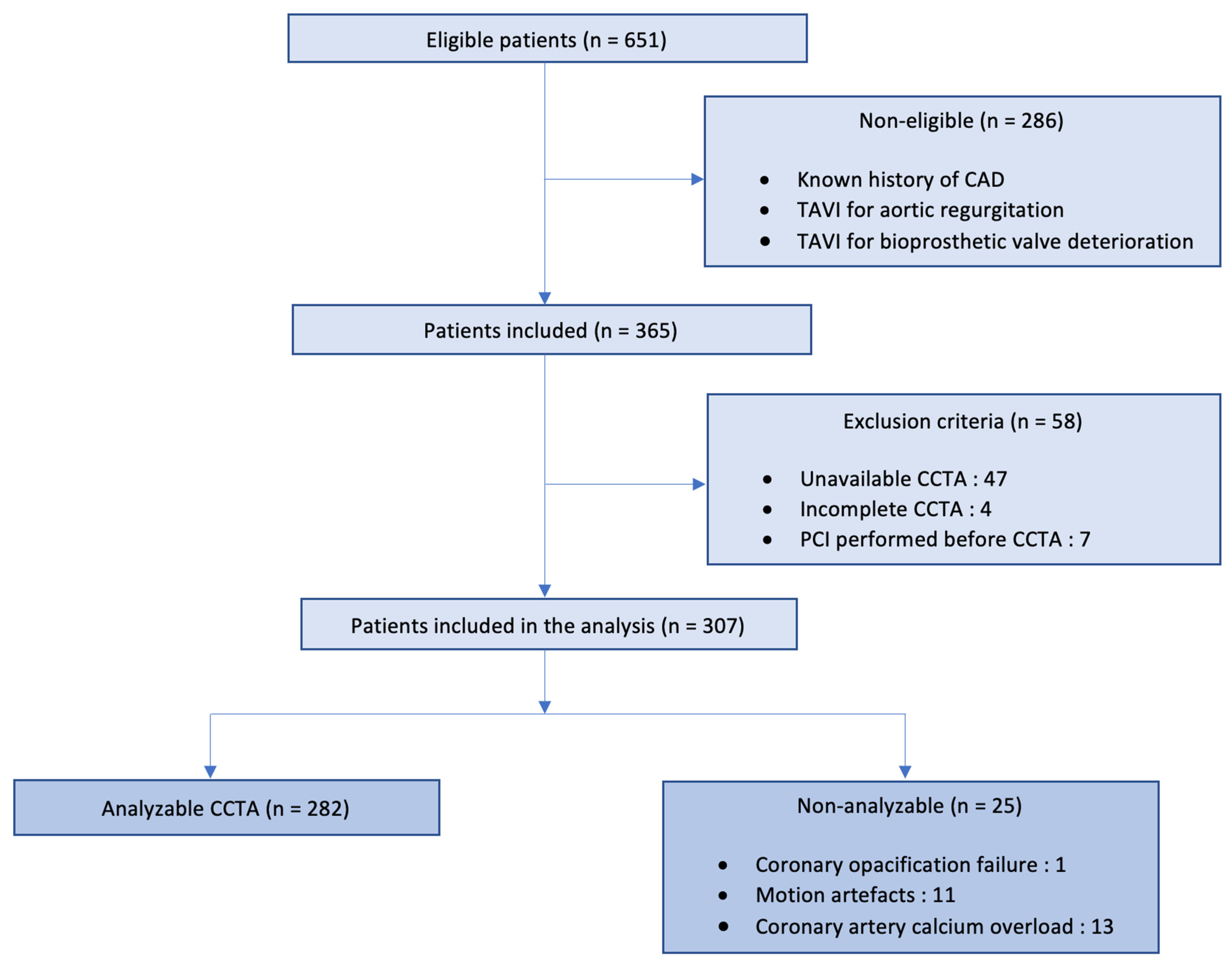
2.1. Study Design and Population
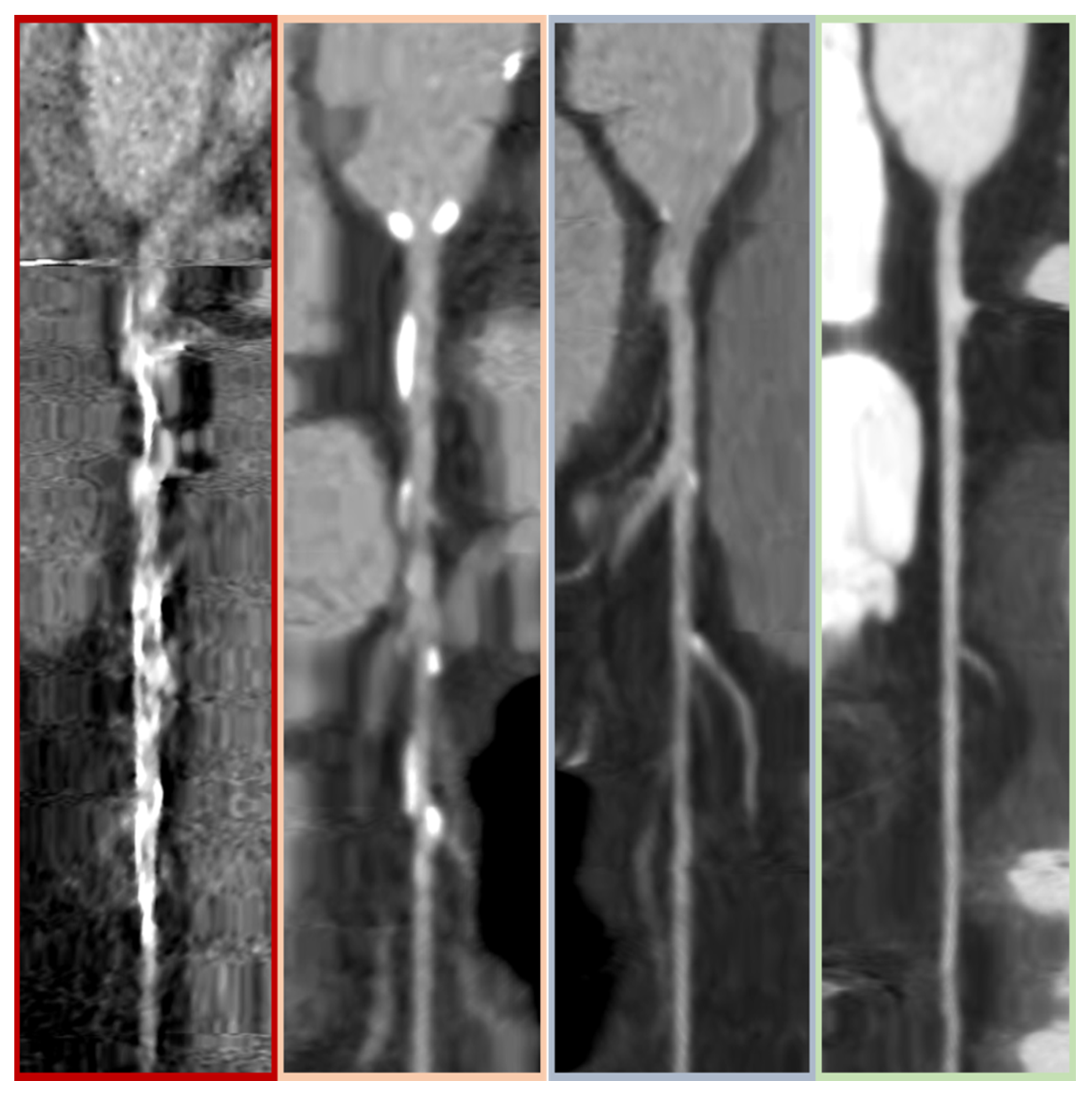
2.2. CCTA Parameters and Analysis
2.3. Invasive Coronary Angiography Analysis
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
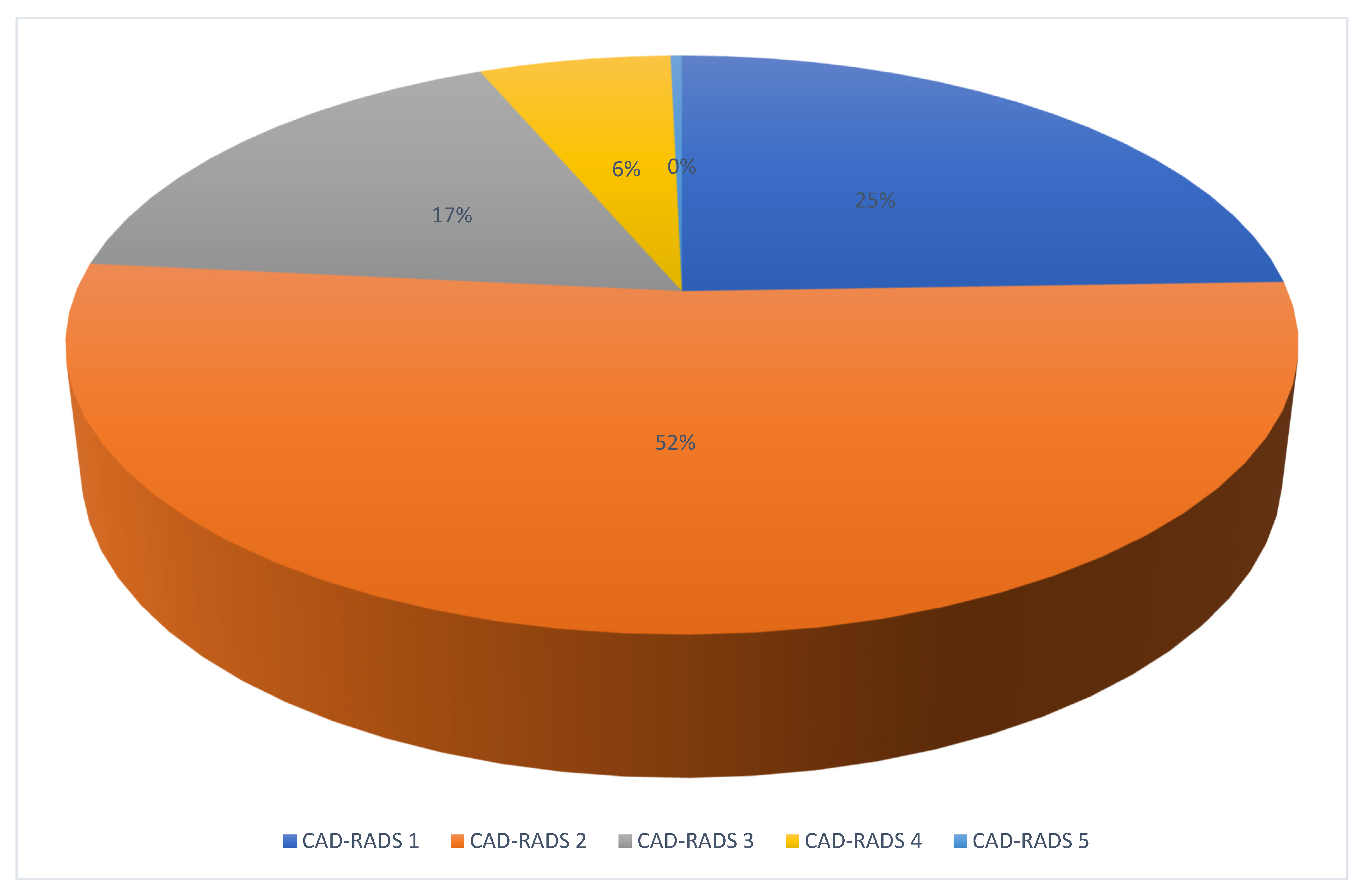
3.2. CCTA and ICA Analysis
3.3. Diagnostic Performance of CCTA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Jüni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic Performance of 64-Multidetector Row Coronary Computed Tomographic Angiography for Evaluation of Coronary Artery Stenosis in Individuals Without Known Coronary Artery Disease: Results From the Prospective Multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) Trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [PubMed]
- Mowatt, G. Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol. Assess. 2008, 12. [Google Scholar] [CrossRef]
- Strong, C.; Ferreira, A.; Teles, R.C.; Mendes, G.; Abecasis, J.; Cardoso, G.; Guerreiro, S.; Freitas, P.; Santos, A.C.; Saraiva, C.; et al. Diagnostic accuracy of computed tomography angiography for the exclusion of coronary artery disease in candidates for transcatheter aortic valve implantation. Sci. Rep. 2019, 9, 19942. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D.; Pontone, G.; Mushtaq, S.; Bartorelli, A.L.; Ballerini, G.; Bertella, E.; Segurini, C.; Conte, E.; Annoni, A.; Baggiano, A.; et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in 325 consecutive patients referred for transcatheter aortic valve replacement. Am. Heart J. 2014, 168, 332–339. [Google Scholar] [CrossRef]
- van den Boogert, T.P.W.; Vendrik, J.; Claessen, B.E.P.M.; Baan, J.; Beijk, M.A.; Limpens, J.; Boekholdt, S.A.M.; Hoek, R.; Planken, R.N.; Henriques, J.P. CTCA for detection of significant coronary artery disease in routine TAVI work-up: A systematic review and meta-analysis. Neth. Heart J. 2018, 26, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Gallone, G.; Poggi, V.; Bruno, F.; Serafini, A.; Depaoli, A.; De Filippo, O.; Conrotto, F.; Darvizeh, F.; Faletti, R.; et al. Diagnostic accuracy of coronary computed tomography angiography for the evaluation of obstructive coronary artery disease in patients referred for transcatheter aortic valve implantation: A systematic review and meta-analysis. Eur. Radiol. 2022, 32, 5189–5200. [Google Scholar] [CrossRef]
- Balloon-Expandable versus Self-Expanding Transcatheter Aortic Valve Replacement. Available online: https://www.ahajournals.org/doi/epdf/10.1161/CIRCULATIONAHA.119.043785 (accessed on 2 December 2022).
- Scholtz, J.-E.; Ghoshhajra, B. Advances in cardiac CT contrast injection and acquisition protocols. Cardiovasc. Diagn. Ther. 2017, 7, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.C.; Leipsic, J.; Abbara, S.; Achenbach, S.; Berman, D.; Bittencourt, M.; Budoff, M.; Chinnaiyan, K.; Choi, A.D.; Ghoshhajra, B.; et al. CAD-RADSTM 2.0—2022 Coronary Artery Disease-Reporting and Data System. JACC Cardiovasc. Imaging 2022, 15, 1974–2001. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Russ. J. Cardiol. 2020, 25, 119–180. [Google Scholar] [CrossRef]
- Chavarria, J.; Sibbald, M.; Velianou, J.; Natarajan, M.; Jaffer, I.; Smith, A.; Sheth, T. A Computed Tomography Protocol to Evaluate Coronary Artery Disease Before Transcatheter Aortic Valve Replacement. Can. J. Cardiol. 2022, 38, 23–30. [Google Scholar] [CrossRef]
- Hamdan, A.; Wellnhofer, E.; Konen, E.; Kelle, S.; Goitein, O.; Andrada, B.; Raanani, E.; Segev, A.; Barbash, I.; Klempfner, R.; et al. Coronary CT angiography for the detection of coronary artery stenosis in patients referred for transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2015, 9, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Xie, J.X.; Cury, R.C.; Leipsic, J.; Crim, M.T.; Berman, D.S.; Gransar, H.; Budoff, M.J.; Achenbach, B.; Hartaigh, B.Ó.; Callister, T.Q.; et al. The Coronary Artery Disease-Reporting and Data System (CAD-RADS): Prognostic and Clinical Implications Associated with standardized coronary computed tomography angiography reporting. J. Am. Coll. Cardiol. Img. 2018, 11, 78–89. [Google Scholar] [CrossRef]
- Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; MacLean, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction (SCOT-HEART). N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar]
- Gohmann, R.F.; Lauten, P.; Seitz, P.; Krieghoff, C.; Lücke, C.; Gottschling, S.; Mende, M.; Weiß, S.; Wilde, J.; Kiefer, P.; et al. Combined Coronary CT-Angiography and TAVI-Planning: A Contrast-Neutral Routine Approach for Ruling-Out Significant Coronary Artery Disease. J. Clin. Med. 2020, 9, 1623. [Google Scholar] [CrossRef]
- Meier, D.; Depierre, A.; Topolsky, A.; Roguelov, C.; Dupré, M.; Rubimbura, V.; Eeckhout, E.; Qanadli, S.D.; Muller, O.; Mahendiran, T.; et al. Computed Tomography Angiography for the Diagnosis of Coronary Artery Disease Among Patients Undergoing Transcatheter Aortic Valve Implantation. J. Cardiovasc. Trans. Res. 2021, 14, 894–901. [Google Scholar] [CrossRef]
- Chieffo, A.; Giustino, G.; Spagnolo, P.; Panoulas, V.F.; Montorfano, M.; Latib, A.; Figini, F.; Agricola, E.; Gerli, C.; Franco, A.; et al. Routine Screening of Coronary Artery Disease With Computed Tomographic Coronary Angiography in Place of Invasive Coronary Angiography in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2015, 8, e002025. [Google Scholar] [CrossRef] [PubMed]
- Snow, T.M.; Ludman, P.; Banya, W.; DeBelder, M.; MacCarthy, P.M.; Davies, S.W.; Di Mario, C.; Moat, N.E. Management of concomitant coronary artery disease in patients undergoing transcatheter aortic valve implantation: The United Kingdom TAVI Registry. Int. J. Cardiol. 2015, 199, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, T.; Sharma, R.; Abramowitz, Y.; Kapadia, S.; Latib, A.; Jilaihawi, H.; Poddar, K.L.; Giustino, G.; Ribeiro, H.B.; Tchetche, D.; et al. Outcomes in Patients with Transcatheter Aortic Valve Replacement and Left Main Stenting. J. Am. Coll. Cardiol. 2016, 67, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Millan-Iturbe, O.; Sawaya, F.J.; Lønborg, J.; Chow, D.H.; Bieliauskas, G.; Engstrøm, T.; Søndergaard, S.; De Backer, O. Coronary artery disease, revascularization, and clinical outcomes in transcatheter aortic valve replacement: Real-world results from the East Denmark Heart Registry. Catheter. Cardiovasc. Interv. 2018, 92, 818–826. [Google Scholar] [CrossRef]
- Patterson, T.; Clayton, T.; Dodd, M.; Khawaja, Z.; Morice, M.C.; Wilson, K.; Kim, W.-K.; Meneveau, N.; Hambrecht, R.; Byrne, J.; et al. ACTIVATION (PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION): A Randomized Clinical Trial. JACC Cardiovasc. Interv. 2021, 14, 1965–1974. [Google Scholar] [CrossRef]

| Analyzable CCTA (n = 282) | Non-Analyzable CCTA (n = 25) | p | |
|---|---|---|---|
| Male sex, n (%) | 122 (43.3) | 12 (48) | 0.647 |
| Age (yrs), mean ± SD | 82.1 ± 7.2 | 82.3 ± 7.3 | |
| BMI (kg/m2), mean ± SD | 26.6 ± 5.05 | 27.5 ± 5.4 | 0.250 |
| EuroSCORE II, mean ± SD | 3.52 ± 2.65 | 3.54 ± 2.25 | 1 |
| STS score, mean ± SD | 3.56 ± 3.85 | 4.16 ± 0.92 | 0.019 |
| Previous medical history | |||
| Diabetes, n (%) | 81 (28.7) | 9 (36) | 0.444 |
| Hypertension, n (%) | 200 (70.9) | 20 (80) | 0.334 |
| Dyslipidemia, n (%) | 110 (39) | 13 (52) | 0.204 |
| Current smokers, n (%) | 18 (6.4) | 1 (4) | 1 |
| Atrial fibrillation, n (%) | 80 (28.4) | 4 (16) | 0.462 |
| Chronic kidney disease, n (%) § | 23 (8.2) | 3 (12) | 0.456 |
| Peripheral vessel disease, n (%) §§ | 15 (5.32) | 3 (12) | 0.172 |
| Stroke, n (%) | 20 (7.09) | 5 (20) | 0.048 |
| Amyloidosis, n (%) | 25 (8.80) | 4 (16) | 0.275 |
| Active cancer, n (%) | 104 (36.9) | 6 (24) | 0.198 |
| Echocardiographic characteristics | |||
| Indexed aortic valve area (cm2/m), mean ± SD | 0.42 ± 0.11 | 0.41 ± 0.10 | 0.853 |
| Mean gradient (mmHg), mean ± SD | 53.2 ± 15.9 | 51.6 ± 11.7 | 0.770 |
| Peak velocity (m/s), mean ± SD | 4.5 ± 0.7 | 4.6 ± 0.4 | 0.616 |
| LVEF (%), mean ± SD | 59.9 ± 11.8 | 60.8 ± 9 | 0.905 |
| Tomography characteristics | |||
| Aortic valve calcium score, mean ± SD | 3136.1 ± 1987.3 | 3494.9 ± 1575.6 | 0.112 |
| Iodine contrast product (mL), mean ± SD | 84.7 ± 9.9 | 85 ± 10.1 | 0.790 |
| Dose length product (mGy/cm), mean ± SD | 1377.1 ± 553 | 1756.4 ± 1079.3 | 0.172 |
| TN | TP | FP | FN | Se | Sp | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| LMS | 280 | 0 | 1 | 1 | 0 | 99.6 | 0 | 99.6% |
| LAD | 224 | 22 | 28 | 8 | 73.3% | 88.9% | 44% | 96.6% |
| LCx | 266 | 5 | 11 | 1 | 83.3% | 96% | 31.3% | 99.6% |
| RCA | 250 | 16 | 11 | 6 | 72.7% | 95.8% | 59.2% | 97.7% |
| Overall | 211 | 43 | 23 | 5 | 89.59% | 90.17% | 65.15% | 97.69% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyer, J.; Bartoli, A.; Deharo, P.; Vaillier, A.; Ferrara, J.; Barral, P.-A.; Jaussaud, N.; Morera, P.; Porto, A.; Collart, F.; et al. Feasibility of Non-Invasive Coronary Artery Disease Screening with Coronary CT Angiography before Transcatheter Aortic Valve Implantation. J. Clin. Med. 2023, 12, 2285. https://doi.org/10.3390/jcm12062285
Boyer J, Bartoli A, Deharo P, Vaillier A, Ferrara J, Barral P-A, Jaussaud N, Morera P, Porto A, Collart F, et al. Feasibility of Non-Invasive Coronary Artery Disease Screening with Coronary CT Angiography before Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine. 2023; 12(6):2285. https://doi.org/10.3390/jcm12062285
Chicago/Turabian StyleBoyer, Jérémy, Axel Bartoli, Pierre Deharo, Antoine Vaillier, Jérôme Ferrara, Pierre-Antoine Barral, Nicolas Jaussaud, Pierre Morera, Alizée Porto, Frédéric Collart, and et al. 2023. "Feasibility of Non-Invasive Coronary Artery Disease Screening with Coronary CT Angiography before Transcatheter Aortic Valve Implantation" Journal of Clinical Medicine 12, no. 6: 2285. https://doi.org/10.3390/jcm12062285
APA StyleBoyer, J., Bartoli, A., Deharo, P., Vaillier, A., Ferrara, J., Barral, P.-A., Jaussaud, N., Morera, P., Porto, A., Collart, F., Jacquier, A., & Cuisset, T. (2023). Feasibility of Non-Invasive Coronary Artery Disease Screening with Coronary CT Angiography before Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine, 12(6), 2285. https://doi.org/10.3390/jcm12062285





