Selected Serum Markers Associated with Pathogenesis and Clinical Course of Type 1 Diabetes in Pediatric Patients—The Effect of Disease Duration
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Methods
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
3.1. Patient Characteristics
3.2. Concentrations of the Selected Active Substances in T1D Patients and Healthy Controls
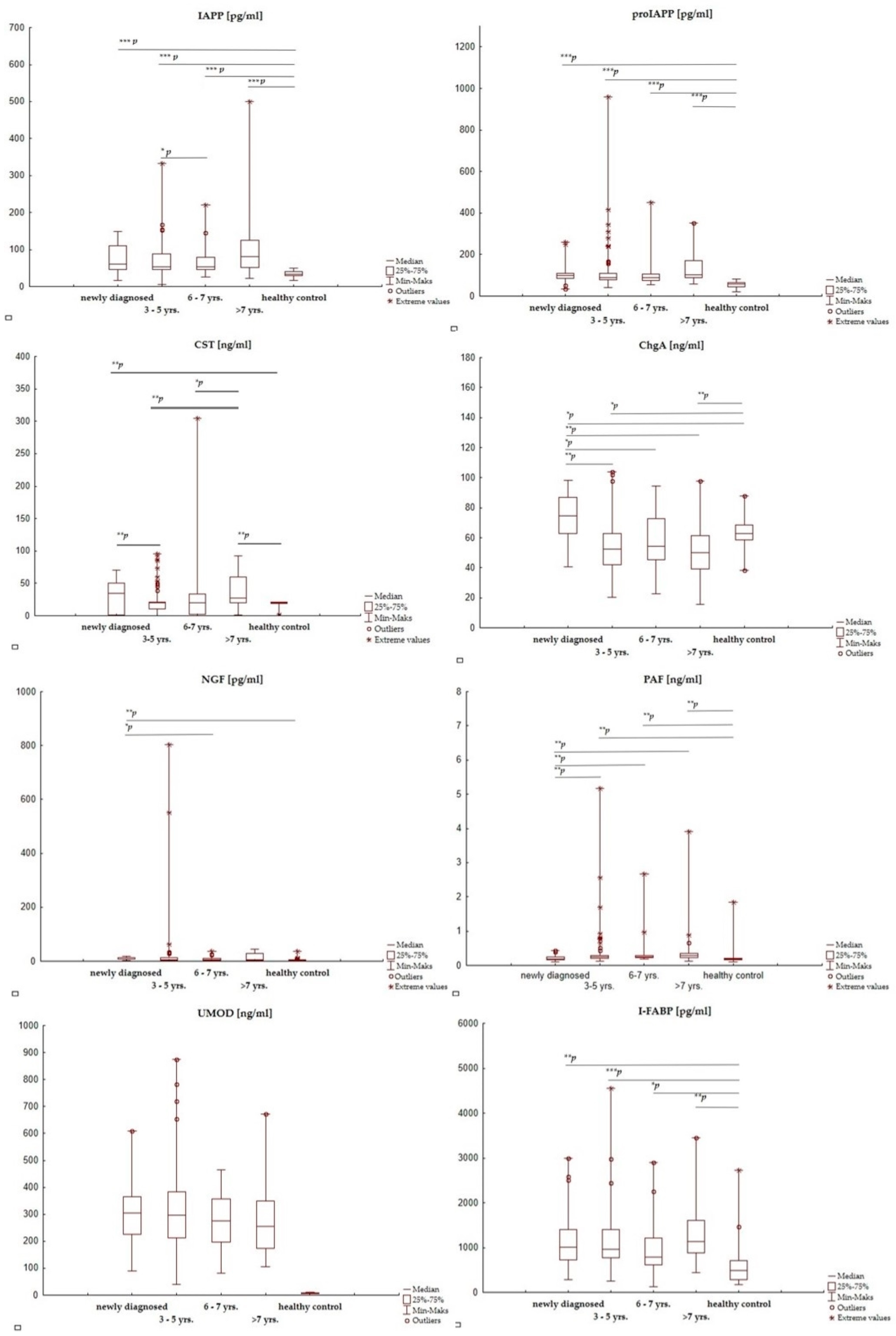
3.3. The Effect of Disease Duration on the Selected Active Substances’ Concentrations in T1D Children
4. Discussion
5. Limitations and Strengths
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Chierico, F.; Rapini, N.; Deodati, A.; Matteoli, M.C.; Cianfarani, S.; Putignani, L. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. Int. J. Mol. Sci. 2022, 23, 14650. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2022; Available online: https://Diabetesatlas.Org/ (accessed on 1 February 2023).
- Cusick, M.; Meleth, A.D.; Agrón, E.; Fisher, M.R.; Reed, G.F.; Knatterud, G.L.; Barton, F.B.; Davis, M.D.; Ferris, F.L.; Chew, E.Y.; et al. Associations of Mortality and Diabetes Complications in Patients with Type 1 and Type 2 Diabetes: Early Treatment Diabetic Retinopathy Study Report No. 27. Diabetes Care 2005, 28, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Karges, B.; Durinovic-Belló, I.; Heinze, E.; Debatin, K.-M.; Boehm, B.; Karges, W. Immunological Mechanisms Associated with Long-Term Remission of Human Type 1 Diabetes. Diabetes/Metab. Res. Rev. 2006, 22, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Maharlooei, M.; Madley, R.; Borsotti, C.; Ferreira, L.M.R.; Sharp, R.C.; Brehm, M.A.; Greiner, D.L.; Parent, A.V.; Anderson, M.S.; Sykes, M.; et al. Modeling Human T1D-Associated Autoimmune Processes. Mol. Metab. 2021, 56, 101417. [Google Scholar] [CrossRef] [PubMed]
- Yapanis, M.; James, S.; Craig, M.E.; O’Neal, D.; Ekinci, E.I. Complications of Diabetes and Metrics of Glycemic Management Derived From Continuous Glucose Monitoring. J. Clin. Endocrinol. Metab. 2022, 107, e2221–e2236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Natarajan, R. Epigenetic Modifications in Metabolic Memory: What Are the Memories, and Can We Erase Them? Am. J. Physiol.-Cell Physiol. 2022, 323, C570–C582. [Google Scholar] [CrossRef]
- Piona, C.; Ventrici, C.; Marcovecchio, L.; Chiarelli, F.; Maffeis, C.; Bonfanti, R.; Rabbone, I. Long-Term Complications of Type 1 Diabetes: What Do We Know and What Do We Need to Understand? Minerva Pediatr. 2021, 73, 504–522. [Google Scholar] [CrossRef]
- Yi, L.; Swensen, A.C.; Qian, W.-J. Serum Biomarkers for Diagnosis and Prediction of Type 1 Diabetes. Transl. Res. 2018, 201, 13–25. [Google Scholar] [CrossRef]
- Bonifacio, E. Predicting Type 1 Diabetes Using Biomarkers. Diabetes Care 2015, 38, 989–996. [Google Scholar] [CrossRef]
- Grauslund, J.; Jørgensen, T.M.M.; Nybo, M.; Green, A.; Rasmussen, L.M.; Sjølie, A.K. Risk Factors for Mortality and Ischemic Heart Disease in Patients with Long-Term Type 1 Diabetes. J. Diabetes Its Complicat. 2010, 24, 223–228. [Google Scholar] [CrossRef]
- Cichocka, E.; Gumprecht, J. Is HbA1c the Only Choice? Alternative Biomarkers for Glycaemic Control Assessment. Clin. Diabetol. 2017, 6, 136–141. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark Insights 2016, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.J.; Basu, A. Biomarkers in diabetes: Hemoglobin A1c, vascular and tissue markers. Transl. Res. 2012, 159, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Bronský, J.; Chada, M.; Kotaska, K.; Průsa, R. Amylin--Its physiological role in humans. Cesk. Fysiol. 2002, 51, 176–180. [Google Scholar] [PubMed]
- Otto-Buczkowska, E.; Jarosz-Chobot, P.; Machnica, Ł. The Role of Amylin in Glucose Homeostasis Regulation and Possible Future Usage in Adolescents with Type 1 Diabetes. Diabetol. Dosw. I Klin. 2009, 9, 41–45. [Google Scholar]
- Courtade, J.A.; Klimek-Abercrombie, A.M.; Chen, Y.-C.; Patel, N.; Lu, P.Y.T.; Speake, C.; Orban, P.C.; Najafian, B.; Meneilly, G.; Greenbaum, C.J.; et al. Measurement of Pro-Islet Amyloid Polypeptide (1-48) in Diabetes and Islet Transplants. J. Clin. Endocrinol. Metab. 2017, 102, 2595–2603. [Google Scholar] [CrossRef]
- Ramzy, A.; Kieffer, T.J. Altered Islet Prohormone Processing: A Cause or Consequence of Diabetes? Physiol. Rev. 2022, 102, 155–208. [Google Scholar] [CrossRef]
- Stadinski, B.D.; Delong, T.; Reisdorph, N.; Reisdorph, R.; Powell, R.L.; Armstrong, M.; Piganelli, J.D.; Barbour, G.; Bradley, B.; Crawford, F.; et al. Chromogranin A Is an Autoantigen in Type 1 Diabetes. Nat. Immunol. 2010, 11, 225–231. [Google Scholar] [CrossRef]
- Baker, R.L.; Bradley, B.; Wiles, T.A.; Lindsay, R.S.; Barbour, G.; Delong, T.; Friedman, R.S.; Haskins, K. NOD Mice Deficient in Chromogranin A Are Protected from Autoimmune Diabetes. J. Immunol. 2016, 196, 39–43. [Google Scholar] [CrossRef]
- Srivastava, N.; Hu, H.; Vomund, A.N.; Peterson, O.J.; Baker, R.L.; Haskins, K.; Teyton, L.; Wan, X.; Unanue, E.R. Chromogranin A Deficiency Confers Protection From Autoimmune Diabetes via Multiple Mechanisms. Diabetes 2021, 70, 2860–2870. [Google Scholar] [CrossRef]
- Gallo, M.P.; Femminò, S.; Antoniotti, S.; Querio, G.; Alloatti, G.; Levi, R. Catestatin Induces Glucose Uptake and GLUT4 Trafficking in Adult Rat Cardiomyocytes. Biomed. Res. Int. 2018, 2018, 2086109. [Google Scholar] [CrossRef] [PubMed]
- Laslop, A.; Doblinger, A.; Weiss, U. Proteolytic Processing of Chromogranins. In Chromogranins: Functional and Clinical Aspects; Helle, K.B., Aunis, D., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2002; pp. 155–166. [Google Scholar] [CrossRef]
- Ochocińska, A.; Wysocka-Mincewicz, M.; Groszek, A.; Rybak, A.; Konopka, E.; Bierła, J.B.; Trojanowska, I.; Szalecki, M.; Cukrowska, B. Could I-FABP Be an Early Marker of Celiac Disease in Children with Type 1 Diabetes? Retrospective Study from the Tertiary Reference Centre. Nutrients 2022, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Chaldakov, G.N.; Aloe, L. Nerve Growth Factor as a Signaling Molecule for Nerve Cells and Also for the Neuroendocrine-Immune Systems. Rev. Neurosci. 2009, 20, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Nathan, N.; Denizot, Y.; Huc, M.C.; Claverie, C.; Laubie, B.; Benveniste, J.; Arnoux, B. Elevated Levels of Paf-Acether in Blood of Patients with Type 1 Diabetes Mellitus. Diabete. Metab. 1992, 18, 59–62. [Google Scholar]
- Spangenberg, P.; Schymik, C.; Hofmann, B.; Ostermann, G.; Rühling, K.; Till, U. Blood Platelet Behaviour in Patients with a Type I Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 1989, 94, 329–337. [Google Scholar] [CrossRef]
- Schiel, R.; Block, M.; Stein, G.; Steveling, A.; Lücking, S.; Scherberich, J. Serum Uromodulin in Children and Adolescents with Type 1 Diabetes Mellitus and Controls: Its Potential Role in Kidney Health. Exp. Clin. Endocrinol. Diabetes 2022. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Budzyński, A.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G.; Dziedzic, T.; Franek, E.; et al. 2020 Guidelines on the Management of Diabetic Patients. A Position of Diabetes Poland. Clin. Diabetol. 2020, 9, 1–101. [Google Scholar]
- Mahmud, F.H.; Elbarbary, N.S.; Fröhlich-Reiterer, E.; Holl, R.W.; Kordonouri, O.; Knip, M.; Simmons, K.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Other Complications and Associated Conditions in Children and Adolescents with Type 1 Diabetes. Pediatr. Diabetes 2018, 19 (Suppl. 27), 275–286. [Google Scholar] [CrossRef]
- Bennet, W.M.; Smith, D.M.; Bloom, S.R. Islet Amyloid Polypeptide: Does It Play a Pathophysiological Role in the Development of Diabetes? Diabet. Med. 1994, 11, 825–829. [Google Scholar] [CrossRef]
- Scherbaum, W.A. The Role of Amylin in the Physiology of Glycemic Control. Exp. Clin. Endocrinol. Diabetes 1998, 106, 97–102. [Google Scholar] [CrossRef]
- Denroche, H.C.; Verchere, C.B. IAPP and Type 1 Diabetes: Implications for Immunity, Metabolism and Islet Transplants. J. Mol. Endocrinol. 2018, 60, R57–R75. [Google Scholar] [CrossRef] [PubMed]
- Khemtémourian, L.; Killian, J.A.; Höppener, J.W.M.; Engel, M.F.M. Recent Insights in Islet Amyloid Polypeptide-Induced Membrane Disruption and Its Role in Beta-Cell Death in Type 2 Diabetes Mellitus. Exp. Diabetes Res. 2008, 2008, 421287. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.D.; Butler, P.C.; Westermark, P.; Johnson, K.H. Islet Amyloid Polypeptide: A Review of Its Biology and Potential Roles in the Pathogenesis of Diabetes Mellitus. Vet. Pathol. 1993, 30, 317–332. [Google Scholar] [CrossRef]
- Zraika, S.; Hull, R.L.; Verchere, C.B.; Clark, A.; Potter, K.J.; Fraser, P.E.; Raleigh, D.P.; Kahn, S.E. Toxic Oligomers and Islet Beta Cell Death: Guilty by Association or Convicted by Circumstantial Evidence? Diabetologia 2010, 53, 1046–1056. [Google Scholar] [CrossRef]
- Westermark, P.; Andersson, A.; Westermark, G.T. Islet Amyloid Polypeptide, Islet Amyloid, and Diabetes Mellitus. Physiol. Rev. 2011, 91, 795–826. [Google Scholar] [CrossRef]
- Watanabe, T. The Emerging Roles of Chromogranins and Derived Polypeptides in Atherosclerosis, Diabetes, and Coronary Heart Disease. Int. J. Mol. Sci. 2021, 22, 6118. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.K.; Vu, C.U.; Gentile, S.; Lee, H.; Biswas, N.; Chi, N.-W.; O’Connor, D.T.; Mahata, S.K. Catestatin (Chromogranin A(352-372)) and Novel Effects on Mobilization of Fat from Adipose Tissue through Regulation of Adrenergic and Leptin Signaling. J. Biol. Chem. 2012, 287, 23141–23151. [Google Scholar] [CrossRef] [PubMed]
- Herold, Z.; Herold, M.; Nagy, P.; Patocs, A.; Doleschall, M.; Somogyi, A. Serum Chromogranin A Level Continuously Rises with the Progression of Type 1 Diabetes, and Indicates the Presence of Both Enterochromaffin-like Cell Hyperplasia and Autoimmune Gastritis. J. Diabetes Investig. 2020, 11, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Herold, Z.; Doleschall, M.; Kovesdi, A.; Patocs, A.; Somogyi, A. Chromogranin A and Its Role in the Pathogenesis of Diabetes Mellitus. Endokrynol. Pol. 2018, 69, 598–610. [Google Scholar] [CrossRef]
- Herold, Z.; Nagy, P.; Patócs, A.; Somogyi, A. The role of chromogranin-A and its derived peptide, WE-14 in the development of type 1 diabetes mellitus. Orv. Hetil. 2015, 156, 163–170. [Google Scholar] [CrossRef]
- Pittenger, G.; Vinik, A. Nerve Growth Factor and Diabetic Neuropathy. Exp. Diabesity Res. 2003, 4, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Faradji, V.; Sotelo, J. Low Serum Levels of Nerve Growth Factor in Diabetic Neuropathy. Acta Neurol. Scand. 1990, 81, 402–406. [Google Scholar] [CrossRef]
- Schmidt, R.E. The Role of Nerve Growth Factor in the Pathogenesis and Therapy of Diabetic Neuropathy. Diabet. Med. 1993, 10 (Suppl. 2), 10S–13S. [Google Scholar] [CrossRef] [PubMed]
- Cavallo-Perin, P.; Lupia, E.; Gruden, G.; Olivetti, C.; De Martino, A.; Cassader, M.; Furlani, D.; Servillo, L.; Quagliuolo, L.; Iorio, E.; et al. Increased Blood Levels of Platelet-activating Factor in Insulin-dependent Diabetic Patients with Microalbuminuria. Nephrol. Dial. Transplant. 2000, 15, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, B.; Hüseyinov, A.; Darcan, Ş. The Role of Platelet-Activating Factor in Pathogenesis of Type 1 Diabetes. Diabetes Care 2005, 28, 980. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Singh, S.K.; Snell-Bergeon, J.K.; Lovshin, J.A.; Lytvyn, Y.; Lovblom, L.E.; Rewers, M.J.; Boulet, G.; Lai, V.; Tse, J.; et al. The Relationships between Markers of Tubular Injury and Intrarenal Hemodynamic Function in Adults with and without Type 1 Diabetes: Results from the Canadian Study of Longevity in Type 1 Diabetes. Obes. Metab. 2019, 21, 575–583. [Google Scholar] [CrossRef]
- Bjornstad, P.; Wiromrat, P.; Johnson, R.J.; Sippl, R.; Cherney, D.Z.I.; Wong, R.; Rewers, M.J.; Snell-Bergeon, J.K. Serum Uromodulin Predicts Less Coronary Artery Calcification and Diabetic Kidney Disease Over 12 Years in Adults With Type 1 Diabetes: The CACTI Study. Diabetes Care 2019, 42, 297–302. [Google Scholar] [CrossRef]
- Paulsson, J.F.; Ludvigsson, J.; Carlsson, A.; Casas, R.; Forsander, G.; Ivarsson, S.A.; Kockum, I.; Lernmark, Å.; Marcus, C.; Lindblad, B.; et al. High Plasma Levels of Islet Amyloid Polypeptide in Young with New-Onset of Type 1 Diabetes Mellitus. PLoS ONE 2014, 9, e93053. [Google Scholar] [CrossRef]
- Gottlieb, P.A.; Delong, T.; Baker, R.L.; Fitzgerald-Miller, L.; Wagner, R.; Cook, G.; Rewers, M.R.; Michels, A.; Haskins, K. Chromogranin A Is a T Cell Antigen in Human Type 1 Diabetes. J. Autoimmun. 2014, 50, 38–41. [Google Scholar] [CrossRef]
- Xu, G.; Grimes, T.D.; Grayson, T.B.; Chen, J.; Thielen, L.A.; Tse, H.M.; Li, P.; Kanke, M.; Lin, T.-T.; Schepmoes, A.A.; et al. Exploratory Study Reveals Far Reaching Systemic and Cellular Effects of Verapamil Treatment in Subjects with Type 1 Diabetes. Nat. Commun. 2022, 13, 1159. [Google Scholar] [CrossRef]
- Delong, T.; Baker, R.L.; He, J.; Haskins, K. Novel Autoantigens for Diabetogenic CD4 T Cells in Autoimmune Diabetes. Immunol. Res. 2013, 55, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Wang, Y.; Crawford, F.; White, J.; Marrack, P.; Dai, S.; Kappler, J.W. N-Terminal Additions to the WE14 Peptide of Chromogranin A Create Strong Autoantigen Agonists in Type 1 Diabetes. Proc. Natl. Acad. Sci. USA 2015, 112, 13318. [Google Scholar] [CrossRef] [PubMed]
- Herold, Z.; Doleschall, M.; Somogyi, A. Role and Function of Granin Proteins in Diabetes Mellitus. World J. Diabetes 2021, 12, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Muntjewerff, E.M.; Dunkel, G.; Nicolasen, M.J.T.; Mahata, S.K.; van den Bogaart, G. Catestatin as a Target for Treatment of Inflammatory Diseases. Front. Immunol. 2018, 9, 2199. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Mahata, S.; Bandyopadhyay, G.K.; Zhou, Z.; Wollam, J.; Vu, J.; Mayoral, R.; Chi, N.-W.; Webster, N.J.G.; Corti, A.; et al. Catestatin Inhibits Obesity-Induced Macrophage Infiltration and Inflammation in the Liver and Suppresses Hepatic Glucose Production, Leading to Improved Insulin Sensitivity. Diabetes 2018, 67, 841–848. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.T.; Kailasam, M.T.; Kennedy, B.P.; Ziegler, M.G.; Yanaihara, N.; Parmer, R.J. Early Decline in the Catecholamine Release-Inhibitory Peptide Catestatin in Humans at Genetic Risk of Hypertension. J. Hypertens. 2002, 20, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.T.; Zhu, G.; Rao, F.; Taupenot, L.; Fung, M.M.; Das, M.; Mahata, S.K.; Mahata, M.; Wang, L.; Zhang, K.; et al. Heritability and Genome-Wide Linkage in US and Australian Twins Identify Novel Genomic Regions Controlling Chromogranin A. Circulation 2008, 118, 247–257. [Google Scholar] [CrossRef]
- Zivkovic, P.M.; Matetic, A.; Tadin Hadjina, I.; Rusic, D.; Vilovic, M.; Supe-Domic, D.; Borovac, J.A.; Mudnic, I.; Tonkic, A.; Bozic, J. Serum Catestatin Levels and Arterial Stiffness Parameters Are Increased in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2020, 9, 628. [Google Scholar] [CrossRef]
- Aloe, L.; Bracci-Laudiero, L.; Alleva, E.; Lambiase, A.; Micera, A.; Tirassa, P. Emotional Stress Induced by Parachute Jumping Enhances Blood Nerve Growth Factor Levels and the Distribution of Nerve Growth Factor Receptors in Lymphocytes. Proc. Natl. Acad. Sci. USA 1994, 91, 10440–10444. [Google Scholar] [CrossRef]
- Azar, S.T.; Major, S.C.; Safieh-Garabedian, B. Altered Plasma Levels of Nerve Growth Factor and Transforming Growth Factor-Β2 in Type-1 Diabetes Mellitus. Brain Behav. Immun. 1999, 13, 361–366. [Google Scholar] [CrossRef]
- Kim, H.C.; Cho, Y.J.; Ahn, C.W.; Park, K.S.; Kim, J.C.; Nam, J.S.; Im, Y.S.; Lee, J.E.; Lee, S.C.; Lee, H.K. Nerve Growth Factor and Expression of Its Receptors in Patients with Diabetic Neuropathy. Diabet. Med. 2009, 26, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.; Lambiase, A.; Bonini, S.; Angelucci, F.; Magrini, L.; Manni, L.; Aloe, L. Circulating Nerve Growth Factor Levels Are Increased in Humans with Allergic Diseases and Asthma. Proc. Natl. Acad. Sci. USA 1996, 93, 10955–10960. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-T.; Kuo, H.-C. Increased Urine and Serum Nerve Growth Factor Levels in Interstitial Cystitis Suggest Chronic Inflammation Is Involved in the Pathogenesis of Disease. PLoS ONE 2012, 7, e44687. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Pace, A.; Bove, L.; Cognetti, F.; Properzi, F.; Fiore, M.; Triaca, V.; Savarese, A.; Simone, M.D.; Jandolo, B.; et al. Patients Treated with Antitumor Drugs Displaying Neurological Deficits Are Characterized by a Low Circulating Level of Nerve Growth Factor. Clin. Cancer Res. 2000, 6, 90–95. [Google Scholar] [PubMed]
- Lang, U.E.; Gallinat, J.; Kuhn, S.; Jockers-Scherübl, M.C.; Hellweg, R. Nerve Growth Factor and Smoking Cessation. AJP 2002, 159, 674-a. [Google Scholar] [CrossRef] [PubMed]
| Parameters | T1D (n = 156) a | Newly Diagnosed (n = 30) b | Disease Duration (in Years) | ||
|---|---|---|---|---|---|
| 3–5 (n = 77) c | 6–7 (n = 25) d | >7 (n = 24) e | |||
| Blood glucose (mg/dL) | 266 (75.6–792) | 337 (153–792) | 243 (119–640) | 221 (144–569) | 311 (75.6–512) |
| Glycated hemoglobin (%) | 7.80 (5.90–18.8) | 12.8 (6.60–18.8) | 7.40 (6.10–14.3) | 7.10 (5.90–10.5) | 8.00 (5.9–11.5) |
| Serum peptide-C (ng/mL) | 0.50 (0.06–3.78) | 0.51 (0.30–3.22) | 0.54 (0.20–3.78) | 0.49 (0.19–2.15) | 0.38 (0.06–0.88) |
| Serum insulin (mIU/L) | 4.14 (2.00–21.9) | 4.90 (3.39–21.9) | 3.87 (2.00–11.9) | 4.34 (3.00–7.34) | 3.56 (2.00–6.89) |
| Serum total cholesterol (mg/dL) | 158 (85.0–323) | 156 (110–210) | 159 (85.0–257) | 168 (117–260) | 159 (105–323) |
| Serum triglycerides (mg/dL) | 70.0 (33.0–244) | 86.0 (40.0–150) | 61.0 (33–244) | 77.0 (40–214) | 73.0 (49.0–194) |
| Serum HDL cholesterol (mg/dL) | 61.6 (23.5–115) | 48.0 (23.5–99.5) | 64.5 (26.2–98.5) | 62.8 (40.4–90.2) | 60.7 (47.7–115) |
| Serum LDL cholesterol (mg/dL) | 82.5 (32.5–474) | 88.5 (45.9–152) | 82.2 (32.5–145) | 90.0 (46.1–163) | 76.3 (40.7–474) |
| Serum non-HDL cholesterol (mg/dL) | 98.5 (41.9–267) | 103 (57.9–168) | 94.6 (41.9–186) | 105 (55.1–194) | 90.7 (50.5–267) |
| Serum CRP (mg/dL) | 0.04 (0.03–0.53) | 0.08 (0.03–0.48) | 0.04 (0.03–0.53) | 0.05 (0.03–0.15) | 0.04 (0.03–0.11) |
| Serum witamin D (ng/mL) | 27.9 (10.1–66.1) | 29.5 (11.5–42.3) | 29.5 (15.5–66.1) | 25.3 (10.1–39.7) | 26.2 (13.0–41.9) |
| Serum creatinine (mg/dL) | 0.57 (0.23–1.05) | 0.47 (0.23–0.85) | 0.56 (0.33–1.01) | 0.66 (0.38–1.05) | 0.72 (0.47–1.05) |
| T1D (n = 156) | T1D—Newly Diagnosed (n = 30) | T1D > 3 Years from Diagnosis (n = 126) | HC (n = 30) | |
|---|---|---|---|---|
| IAPP [pg/mL] | 75.0 (6.16–499) p < 0.000001 | 60.1 (17.3–148) p < 0.0000001 | 65.0 (6.16–499) p = 0.000005 | 33.5 (16.9–49.1) |
| * p = 0.697 | ||||
| proIAPP [pg/mL] | 155 (34.8–958) p < 0.000001 | 99.6 (34.8–258) p < 0.0000001 | 92.6 (42.10–958) p = 0.000021 | 57.6 (19.5–82.3) |
| * p = 0.513 | ||||
| CST [ng/mL] | 20.3 (0.001–305) p < 0.000001 | 35.2 (0.001–70.1) p = 0.003 | 20.1 (0.001–305) p = 0.617 | 20.2 (0.003–21.5) |
| * p = 0.516 | ||||
| ChgA [ng/mL] | 55.7 (15.5–104) p < 0.000001 | 74.5 (40.5–98.5) p = 0.005 | 74.5 (40.5–98.5) p = 0.123 | 34.5 (11.5–88.0) |
| * p < 0.000001 | ||||
| NGF [pg/mL] | 15.2 (0.52–804) p = 0.056 | 12.7 (3.45–17.9) p = 0.000004 | 4.69 (0.52–804) p = 0.036 | 4.30 (3.03–37.9) |
| * p = 0.003 | ||||
| PAF [ng/mL] | 0.37 (0.11–5.18) p < 0.000001 | 0.20 (0.11–0.43) p = 0.588 | 0.25 (0.12–5.18) p = 0.194 | 0.19 (0.11–1.83) |
| * p = 0.00001 | ||||
| UMOD [ng/mL] | 287 (39.0–875) p = 0.847 | 305 (90.5–610) p = 0.796 | 297 (39.0–875) p = 0.440 | 278 (51.0–555) |
| * p = 0.601 | ||||
| I-FABP [pg/mL] | 970 (130–4560) p < 0.000001 | 1015 (280–2990) p = 0.000095 | 955 (130–4560) p = 0.000001 | 485 (170–2730) |
| * p = 0.857 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochocińska, A.; Wysocka-Mincewicz, M.; Świderska, J.; Cukrowska, B. Selected Serum Markers Associated with Pathogenesis and Clinical Course of Type 1 Diabetes in Pediatric Patients—The Effect of Disease Duration. J. Clin. Med. 2023, 12, 2151. https://doi.org/10.3390/jcm12062151
Ochocińska A, Wysocka-Mincewicz M, Świderska J, Cukrowska B. Selected Serum Markers Associated with Pathogenesis and Clinical Course of Type 1 Diabetes in Pediatric Patients—The Effect of Disease Duration. Journal of Clinical Medicine. 2023; 12(6):2151. https://doi.org/10.3390/jcm12062151
Chicago/Turabian StyleOchocińska, Agnieszka, Marta Wysocka-Mincewicz, Jolanta Świderska, and Bożena Cukrowska. 2023. "Selected Serum Markers Associated with Pathogenesis and Clinical Course of Type 1 Diabetes in Pediatric Patients—The Effect of Disease Duration" Journal of Clinical Medicine 12, no. 6: 2151. https://doi.org/10.3390/jcm12062151
APA StyleOchocińska, A., Wysocka-Mincewicz, M., Świderska, J., & Cukrowska, B. (2023). Selected Serum Markers Associated with Pathogenesis and Clinical Course of Type 1 Diabetes in Pediatric Patients—The Effect of Disease Duration. Journal of Clinical Medicine, 12(6), 2151. https://doi.org/10.3390/jcm12062151






