The Value of Left Ventricular Mechanical Dyssynchrony and Scar Burden in the Combined Assessment of Factors Associated with Cardiac Resynchronization Therapy Response in Patients with CRT-D
Abstract
1. Introduction
2. Materials and Methods
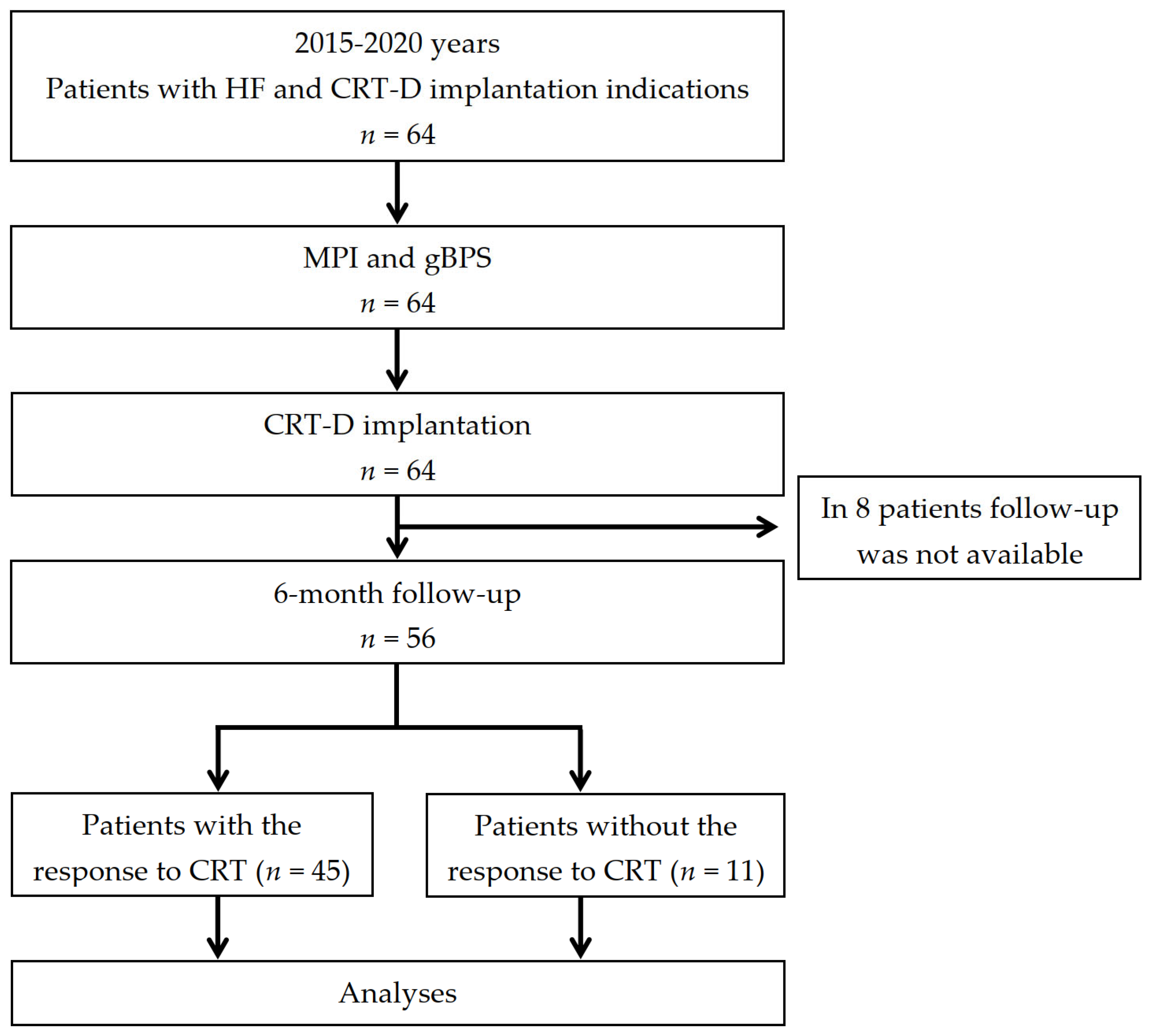
2.1. Patient Population and Study Design
2.2. Consent
2.3. Minute Walk Distance Test
- -
- More than 551 m—the patient has no signs of HF;
- -
- A distance of 426–550 m—I FC of HF;
- -
- A distance of 301–425 m—II FC of HF;
- -
- A distance of 151–300 m—III FC of HF;
- -
- Less than 150 m—IV FC of HF.
2.4. TTE Acquisition and Analysis
2.5. Scintigraphic Data Acquisition
2.6. MPI Acquisition and Analysis
2.7. gBPS Acquisition and Analysis
2.8. CRT-D Implantation and Programming
2.9. Clinical Follow-Up, CRT-D Data Acquisition, and Analysis
2.10. Statistical Analysis
3. Results
3.1. Patients’ Baseline Clinical and Follow-Up Characteristics
3.2. Events According to the CRT-D Interrogation Data
3.3. CRT Response Predictors
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef]
- Sayago-Silva, I.; López, F.J.G.; Segovia-Cubero, J. Epidemiology of Heart Failure in Spain Over the Last 20 Years. Rev. Esp. Cardiol. 2013, 66, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the Impact of Heart Failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Fomin, I.V. Chronic heart failure in Russian Federation: What do we know and what to do. Russ. J. Cardiol. 2016, 8, 7–13. [Google Scholar] [CrossRef]
- Kashani, A.; Barold, S.S. Significance of QRS complex duration in patients with heart failure. J. Am. Coll. Cardiol. 2005, 46, 2183–2192. [Google Scholar] [CrossRef]
- Prinzen, F.W.; Vernooy, K.; Auricchio, A. Cardiac resynchronization therapy: State-of-the-art of current applications, guidelines, ongoing trials, and areas of controversy. Circulation 2013, 128, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Herweg, B.; Welter-Frost, A.; Vijayaraman, P. The evolution of cardiac resynchronization therapy and an introduction to conduction system pacing: A conceptual review. EP Eur. 2020, 23, 496–510. [Google Scholar] [CrossRef]
- Poole, J.E.; Singh, J.P.; Birgersdotter-Green, U. QRS Duration or QRS Morphology: What really matters in cardiac resynchronization therapy? J. Am. Coll. Cardiol. 2016, 67, 1104–1117. [Google Scholar] [CrossRef]
- Linde, C.; Gold, M.R.; Abraham, W.T.; Sutton, M.S.J.; Ghio, S.; Cerkvenik, J.; Daubert, C. Long-term impact of cardiac resynchronization therapy in mild heart failure: 5-year results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Eur. Heart J. 2013, 34, 2592–2599. [Google Scholar] [CrossRef]
- Tang, A.S.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.-A.; Cleland, J.G.F.; Deharo, J.-C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur. Heart J. 2013, 34, 2281–2329. [Google Scholar] [CrossRef] [PubMed]
- Eldadah, Z.A.; Rosen, B.; Hay, I.; Edvardsen, T.; Jayam, V.; Dickfeld, T.; Meininger, G.R.; Judge, D.; Hare, J.; Lima, J.B.; et al. The benefit of upgrading chronically right ventricle–paced heart failure patients to resynchronization therapy demonstrated by strain rate imaging. Heart Rhythm 2006, 3, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zou, T.; Zhong, Y.; Yang, C.; Ren, Y.; Wang, F. Prevention of non-response to cardiac resynchronization therapy: Points to remember. Heart Fail. Rev. 2020, 25, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Orszulak, M.; Filipecki, A.; Wróbel, W.; Berger-Kucza, A.; Orszulak, W.; Urbańczyk-Swić, D.; Kwaśniewski, W.; Płońska-Gościniak, E.; Mizia-Stec, K. Regional Strain Pattern Index—A Novel Technique to Predict CRT Response. Int. J. Environ. Res. Public Health 2021, 18, 926. [Google Scholar] [CrossRef]
- Fulati, Z.; Liu, Y.; Sun, N.; Kang, Y.; Su, Y.; Chen, H.; Shu, X. Speckle tracking echocardiography analyses of myocardial contraction efficiency predict response for cardiac resynchronization therapy. Cardiovasc. Ultrasound 2018, 16, 30. [Google Scholar] [CrossRef]
- Van Everdingen, W.M.; Zweerink, A.; Nijveldt, R.; Salden, O.A.E.; Meine, M.; Maass, A.H.; Vernooy, K.; De Lange, F.J.; Van Rossum, A.C.; Croisille, P.; et al. Comparison of strain imaging techniques in CRT candidates: CMR tagging, CMR feature tracking and speckle tracking echocardiography. Int. J. Cardiovasc. Imaging 2017, 34, 443–456. [Google Scholar] [CrossRef]
- Zweerink, A.; van Everdingen, W.; Nijveldt, R.; Salden, O.A.; Meine, M.; Maass, A.H.; Vernooy, K.; De Lange, F.J.; Vos, M.A.; Croisille, P.; et al. Strain imaging to predict response to cardiac resynchronization therapy: A systematic comparison of strain parameters using multiple imaging techniques. ESC Heart Fail. 2018, 5, 1130–1140. [Google Scholar] [CrossRef]
- Tada, T.; Osuda, K.; Nakata, T.; Muranaka, I.; Himeno, M.; Muratsubaki, S.; Murase, H.; Sato, K.; Hirose, M.; Fukuma, T. A novel approach to the selection of an appropriate pacing position for optimal cardiac resynchronization therapy using CT coronary venography and myocardial perfusion imaging: FIVE STaR method (fusion image using CT coronary venography and perfusion SPECT applied for cardiac resynchronization therapy). J. Nucl. Cardiol. 2019, 28, 1438–1445. [Google Scholar] [CrossRef]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.-P.; Nihoyannopoulos, P.; Merlino, J.; Abraham, W.T.; Ghio, S.; Leclercq, C.; Bax, J.J.; et al. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation 2008, 117, 2608–2616. [Google Scholar] [CrossRef]
- Pang, B.J.; Joshi, S.B.; Lui, E.H.; Tacey, M.; Ling, L.-H.; Alison, J.; Seneviratne, S.K.; Cameron, J.; Mond, H.G. Validation of conventional fluoroscopic and ECG criteria for right ventricular pacemaker lead position using cardiac computed tomography. Pacing Clin. Electrophysiol. 2013, 37, 495–504. [Google Scholar] [CrossRef]
- Verberne, H.J.; Acampa, W.; Anagnostopoulos, C.; Ballinger, J.; Bengel, F.; De Bondt, P.; Buechel, R.R.; Cuocolo, A.; van Eck-Smit, B.L.F.; Flotats, A.; et al. EANM procedural guidelines for radionuclide myocardial perfusion imaging with SPECT and SPECT/CT: 2015 revision. Eur. J. Nucl. Med. 2015, 42, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Boogers, M.M.; Van Kriekinge, S.D.; Henneman, M.M.; Ypenburg, C.; Van Bommel, R.J.; Boersma, E.; Dibbets-Schneider, P.; Stokkel, M.P.; Schalij, M.J.; Berman, D.S.; et al. Quantitative gated SPECT-derived phase analysis on gated myocardial perfusion SPECT detects left ventricular dyssynchrony and predicts response to cardiac resynchronization therapy. J. Nucl. Med. 2009, 50, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, J.; Ge, J.; Tang, H.; He, Z.; Liu, Y.; Zhao, Z.; Li, C.; Gu, K.; Hou, X.; et al. Left ventricular systolic and diastolic dyssynchrony to improve cardiac resynchronization therapy response in heart failure patients with dilated cardiomyopathy. J. Nucl. Cardiol. 2020, 28, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, D.; Cui, C.; Qin, H.-Y.; Zhao, Z.; Hou, X.; Zou, J.; Chen, M.-L.; Wang, C.; Zhou, W. Predictive values of left ventricular mechanical dyssynchrony for CRT response in heart failure patients with different pathophysiology. J. Nucl. Cardiol. 2021, 29, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Peix, A.; Karthikeyan, G.; Massardo, T.; Kalaivani, M.; Patel, C.; Pabon, L.M.; Jiménez-Heffernan, A.; Alexanderson, E.; Butt, S.; Kumar, A.; et al. Value of intraventricular dyssynchrony assessment by gated-SPECT myocardial perfusion imaging in the management of heart failure patients undergoing cardiac resynchronization therapy (VISION-CRT). J. Nucl. Cardiol. 2019, 28, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, E.C.; Tanaka, H.; Soman, P.; Miske, G.; Haberman, S.C.; Saba, S.F.; Gorcsan, J. 3rd. Impact of scar burden by single-photon emission computed tomography myocardial perfusion imaging on patient outcomes following cardiac resynchronization therapy. Eur. Heart J. 2010, 32, 93–103. [Google Scholar] [CrossRef]
- Goldenberg, I.; Moss, A.J.; Hall, W.J.; Foster, E.; Goldberger, J.J.; Santucci, P.; Shinn, T.; Solomon, S.; Steinberg, J.S.; Wilber, D.; et al. Predictors of Response to Cardiac Resynchronization Therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011, 124, 1527–1536. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Henzlova, M.J.; Duvall, W.L.; Einstein, A.J.; Travin, M.I.; Verberne, H.J. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J. Nucl. Cardiol. 2016, 23, 606–639. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Corbett, J.R.; Akinboboye, O.O.; Bacharach, S.L.; Borer, J.S.; Botvinick, E.H.; DePuey, E.G.; Ficaro, E.P.; Hansen, C.L.; Henzlova, M.J.; Van Kriekinge, S. Equilibrium radionuclide angiocardiography. J. Nucl. Cardiol. 2006, 13, e56–e79. [Google Scholar] [CrossRef] [PubMed]
- Stiles, M.K.; Fauchier, L.; Morillo, C.A.; Wilkoff, B.L. 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm 2019, 17, e220–e228. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, K.A.; Gold, M.R.; Meyer, T.E.; Lozano, I.F.; Mittal, S.; Waggoner, A.D.; Lemke, B.; Singh, J.P.; Spinale, F.G.; Van Eyk, J.E.; et al. Primary results from the SmartDelay determined AV optimization: A comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: A randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation 2010, 122, 2660–2668. [Google Scholar] [CrossRef]
- Ypenburg, C.; van Bommel, R.J.; Borleffs, C.J.W.; Bleeker, G.B.; Boersma, E.; Schalij, M.J.; Bax, J.J. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J. Am. Coll. Cardiol. 2009, 53, 483–490. [Google Scholar] [CrossRef]
- Verhaert, D.; Grimm, R.A.; Puntawangkoon, C.; Wolski, K.; De, S.; Wilkoff, B.L.; Starling, R.C.; Tang, W.W.; Thomas, J.D.; Popović, Z.B. Long-term reverse remodeling with cardiac resynchronization therapy: Results of extended echocardiographic follow-up. J. Am. Coll. Cardiol. 2010, 55, 1788–1795. [Google Scholar] [CrossRef]
- Said, F.; ter Maaten, J.M.; Martens, P.; Vernooy, K.; Meine, M.; Allaart, C.P.; Geelhoed, B.; Vos, M.A.; Cramer, M.J.; van Gelder, I.C.; et al. Aetiology of heart failure, rather than sex, determines reverse LV remodeling response to CRT. J. Clin. Med. 2021, 10, 5513. [Google Scholar] [CrossRef]
- Wikstrom, G.; Blomström-Lundqvist, C.; Andren, B.; Lönnerholm, S.; Blomström, P.; Freemantle, N.; Remp, T.; Cleland, J.G.; on behalf of the CARE-HF study investigators. The effects of aetiology on outcome in patients treated with cardiac resynchronization therapy in the CARE-HF trial. Eur. Heart J. 2008, 30, 782–788. [Google Scholar] [CrossRef]
- Beltrami, C.A.; Finato, N.; Rocco, M.; Feruglio, G.A.; Puricelli, C.; Cigola, E.; Sonnenblick, E.H.; Olivetti, G.; Anversa, P. The cellular basis of dilated cardiomyopathy in humans. J. Mol. Cell. Cardiol. 1995, 27, 291–305. [Google Scholar] [CrossRef]
- Leyva, F.; Taylor, R.; Foley, P.; Umar, F.; Mulligan, L.J.; Patel, K.; Stegemann, B.; Haddad, T.; Smith, R.E.; Prasad, S.K. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 1659–1667. [Google Scholar] [CrossRef]
- Henneman, M.M.; Chen, J.; Dibbets-Schneider, P.; Stokkel, M.P.; Bleeker, G.B.; Ypenburg, C.; Van Der Wall, E.E.; Schalij, M.J.; Garcia, E.V.; Bax, J.J. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT. J. Nucl. Med. 2007, 48, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.S.; Alraies, M.C.; Cerqueira, M.D.; Jaber, W.A.; AlJaroudi, W.A. Prognostic value of left ventricular mechanical dyssynchrony by phase analysis in patients with non-ischemic cardiomyopathy with ejection fraction 25–50% and QRS < 150 ms. J. Nucl. Cardiol. 2013, 21, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Forleo, G.B.; Schiavone, M.; Della Rocca, D.; Solimene, F.; Schillaci, V.; Covino, G.; Sassara, M.; Savarese, G.; Donzelli, S.; Badolati, S.; et al. Effective nonapical left ventricular pacing with quadripolar leads for cardiac resynchronization therapy. Kardiol. Pol. 2021, 79, 442–448. [Google Scholar] [CrossRef] [PubMed]

| Demographic and Clinical Characteristics | Total (n = 56) | RESP (n = 45) | Non-RESP (n = 11) | P2–3 |
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Age, year, mean ± SD | 57.0 ± 11.5 | 56.7 ± 11.8 | 58.4 ± 10.8 | 0.680 |
| Male gender, n (%) | 35 (62.5) | 28 (62.2) | 7 (63.6) | 0.950 |
| Ischemic heart failure, n (%) | 22 (39.3) | 15 (33.4) | 7 (63.6) | 0.124 |
| Nonischemic heart failure, n (%) | 34 (60.7) | 30 (66.6) | 4 (36.4) | 0.124 |
| 6 min walk distance test, m, mean ± SD | 284.5 ± 66.3 | 288.3 ± 64.8 | 269.0 ± 73.1 | 0.312 |
| Heart failure: | ||||
| II functional class NYHA, n (%) | 23 (41.1) | 20 (44.4) | 3 (27.3) | 0.386 |
| III functional class NYHA, n (%) | 33 (58.9) | 25 (55.6) | 8 (72.7) | 0.386 |
| QRS duration, ms, Me [Q1; Q3] | 165.0 [160.0; 180.0] | 165.0 [160.0; 180.0] | 165.0 [155.0; 185.0] | 0.657 |
| LVEF, % [Q1; Q3] | 28.0 [22.0; 31.0] | 28.0 [21.0; 31.0] | 28.0 [25.0; 32.0] | 0.502 |
| LVESV, ml [Q1; Q3] | 171.0 [131.5; 217.5] | 169.0 [133.0; 210.0] | 208.0 [121.0; 232.0] | 0.598 |
| History of sustained VT, n (%) | 5 (8.9) | 3 (6.7) | 2 (18.2) | 0.563 |
| History of ventricular fibrillation, n (%) | 1 (1.8) | 1 (2.2) | 0 (0.0) | 0.917 |
| Comorbidities: | ||||
| Hypertension, n (%) | 22 (39.3) | 15 (33.4) | 7 (63.6) | 0.124 |
| Left ventricular hypertrophy, n (%) | 45 (80.3) | 35 (77.8) | 10 (90.9) | 0.509 |
| Diabetes mellitus, n (%) | 8 (14.3) | 6 (13.3) | 2 (18.2) | 0.812 |
| Body mass index, kg/m2, mean ± SD | 28.9 ± 5.0 | 28.7 ± 5.3 | 29.7 ± 3.8 | 0.509 |
| Dyslipidemia, n (%) | 23 (41.0) | 18 (40.0) | 5 (45.4) | 0.788 |
| GFR, ml/min, mean ± SD | 72.2 ± 21.2 | 72.3 ± 22.7 | 71.8 ± 13.7 | 0.804 |
| Therapy: | ||||
| Beta-blockers, n (%) | 53 (94.6) | 43 (95.5) | 10 (90.9) | 0.820 |
| Loop diuretics, n (%) | 44 (78.6) | 33 (73.3) | 11 (100.0) | 0.176 |
| Potassium-sparing diuretics, n (%) | 43 (76.8) | 36 (80.0) | 7 (63.6) | 0.409 |
| ACEI, n (%) | 33 (58.9) | 26 (57.8) | 7 (63.6) | 0.772 |
| Antiplatelet agents, n (%) | 33 (58.9) | 24 (53.3) | 9 (81.8) | 0.148 |
| Statins, n (%) | 31 (55.3) | 25 (55.6) | 6 (54.5) | 0.967 |
| Amiodarone, n (%) | 20 (35.7) | 16 (35.5) | 4 (36.4) | 0.975 |
| Angiotensin II receptor blocker, n (%) | 19 (33.9) | 15 (33.4) | 4 (36.4) | 0.914 |
| LV lead position: | ||||
| Lateral vein, n (%) | 18 (32.1) | 16 (35.5) | 2 (18.2) | 0.380 |
| Posterolateral vein, n (%) | 20 (35.7) | 16 (35.5) | 4 (36.4) | 0.975 |
| Anterolateral vein, n (%) | 10 (17.8) | 8 (17.7) | 2 (18.2) | 0.991 |
| Posterior vein, n (%) | 8 (14.2) | 5 (11.1) | 3 (27.3) | 0.415 |
| Pacing QRS duration, ms, Me [Q1; Q3] | 140.0 [130.0; 140.0] | 140.0 [130.0; 140.0] | 140.0 [130.0; 140.0] | 0.243 |
| Quadripolar LV lead, n (%) | 28 (50.0) | 24 (53.3) | 4 (36.3) | 0.392 |
| Bipolar LV lead, n (%) | 28 (50.0) | 21 (46.7) | 7 (63.7) | 0.392 |
| Paced AV delay, ms, Me [Q1; Q3] | 150.0 [140.0; 150.0] | 150.0 [140.0; 150.0] | 150.0 [150.0; 150.0] | 0.464 |
| Sensed AV delay, ms, Me [Q1; Q3] | 120.0 [100.0; 120.0] | 120.0 [100.0; 120.0] | 120.0 [100.0; 125.0] | 0.215 |
| Interventricular delay, ms, Me [Q1; Q3] | 22.5 [12.5; 40.0] | 25.0 [15.0; 40.0] | 20.0 [10.0; 40.0] | 0.885 |
| Scintigraphic Parameters | Total (n = 56) | RESP (n = 45) | Non-RESP (n = 11) | P2–3 |
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Gated Blood-Pool SPECT: | ||||
| IVD, ms | 67.1 [38.1; 102.4] | 71.8 [42.0; 112.7] | 39.2 [9.0; 76.7] | 0.022 |
| LV HBW, ° | 203.0 [192.0; 222.0] | 203.0 [186.0; 222.0] | 216.0 [192.0; 234.0] | 0.190 |
| LV PE, % | 72.0 [62.0; 73.0] | 72.0 [62.0; 73.0] | 64.0 [59.0; 66.0] | 0.033 |
| RV HBW, ° | 120.0 [99.0; 198.0] | 120.0 [96.0; 186.0] | 198.0 [198.0; 204.0] | 0.053 |
| RV PE, % | 62.0 [59.5; 67.0] | 62.0 [60.0; 67.0] | 63.0 [59.0; 67.0] | 0.375 |
| RV FW_SD, ° | 28.0 [16.0; 36.5] | 28.0 [20.0; 44.0] | 16.0 [13.0; 26.0] | 0.011 |
| LV S_SD, ° | 35.0 [23.0; 40.5] | 35.0 [25.0; 42.0] | 23.0 [19.0; 32.0] | 0.105 |
| LV S_E, % | 65.0 [56.0; 72.5] | 65.0 [59.0; 73.0] | 56.0 [50.0; 58.0] | 0.061 |
| LV AW_SD, ° | 25.0 [11.5; 28.0] | 25.0 [17.0; 29.0] | 10.0 [10.0; 12.0] | <0.001 |
| LV AW_E, % | 50.0 [36.0; 57.5] | 50.0 [45.0; 61.0] | 36.0 [33.0; 37.0] | 0.001 |
| LV LW_SD, ° | 12.0 [9.0; 16.0] | 12.0 [9.0; 15.0] | 16.0 [9.0; 20.0] | 0.109 |
| LV LW_E, % | 36.0 [32.0; 47.0] | 36.0 [31.0; 47.0] | 37.0 [33.0; 52.0] | 0.327 |
| LV IW_SD, ° | 28.0 [26.0; 37.0] | 28.0 [26.0; 37.0] | 27.0 [25.0; 37.0] | 0.312 |
| LV IW_E, % | 59.0 [55.5; 61.50] | 59.0 [56.0; 61.0] | 60.0 [54.0; 62.0] | 0.470 |
| LV PER | −0.81 [−1.18; −0.53] | −0.78 [−1.13; −0.44] | −1.01 [−1.38; −0.57] | 0.364 |
| LV PFR | 0.745 [0.53; 1.045] | 0.7 [0.53; 1.00] | 0.94 [0.6; 1.27] | 0.154 |
| LV PFR2 | 0.63 [0.535; 0.64] | 0.63 [0.48; 0.63] | 0.64 [0.58; 0.66] | 0.092 |
| RV PER | −1.66 [−2.39; −0.81] | −1.55 [−2.41; −0.6] | −1.72 [−2.17; −1.22] | 0.672 |
| RV PFR | 1.38 [1.01; 1.71] | 1.51 [1.08; 1.77] | 1.01 [0.92; 1.08] | 0.005 |
| RV PFR2 | 1.31 [1.23; 1.63] | 1.31 [1.22; 1.57] | 1.63 [1.39; 1.63] | 0.028 |
| Myocardial perfusion imaging: | ||||
| SRS, % | 7.5 [4.0; 14.0] | 6.0 [3.0; 12.0] | 13.0 [9.0; 16.0] | 0.018 |
| Parameters | 95% CI | Cut-off | AUC | Sensitivity | Specificity | p |
|---|---|---|---|---|---|---|
| SRS | 0.596–0.841 | ≤7.0% | 0.731 | 60.00 | 90.91 | 0.001 |
| RV PFR | 0.639–0.872 | ˃1.15 | 0.771 | 66.67 | 90.91 | ˂0.001 |
| LV AW_E | 0.689–0.906 | ˃39.0% | 0.815 | 80.00 | 90.91 | ˂0.001 |
| LV AW_SD | 0.757–0.947 | ˃13.0° | 0.873 | 84.44 | 90.91 | ˂0.001 |
| RV FW_SD | 0.616–0.856 | ˃27.0° | 0.749 | 64.44 | 90.91 | 0.004 |
| LV_E | 0.572–0.823 | ˃68.0% | 0.709 | 68.89 | 90.91 | 0.006 |
| IVD | 0.590–0.836 | ˃91.8 | 0.725 | 40.00 | 100.00 | 0.005 |
| RV PFR 2 | 0.580–0.829 | ≤1.38 | 0.716 | 73.33 | 81.82 | 0.013 |
| AD | MSE | 95% CI | p | |
|---|---|---|---|---|
| CRT RPI~NIHF | 0.298 | 0.0811 | 0.139–0.457 | <0.001 |
| CRT RPI~SRS | 0.218 | 0.0670 | 0.0869–0.349 | 0.001 |
| CRT RPI~LV AW_SD | 0.0768 | 0.0453 | −0.0119–0.165 | 0.089 |
| CRT RPI~IVD | 0.224 | 0.0881 | 0.0516–0.397 | 0.010 |
| CRT RPI~LVE | 0.240 | 0.0623 | 0.118–0.362 | <0.001 |
| CRT RPI~RV PFR 2 | 0.233 | 0.0804 | 0.0757–0.391 | 0.003 |
| CRT RPI~RV PFR | 0.179 | 0.0638 | 0.0538–0.304 | 0.005 |
| CRT RPI~LV AW_E | 0.134 | 0.0646 | 0.00765–0.261 | 0.037 |
| CRT RPI~RV FW_SD | 0.200 | 0.0817 | 0.0398–0.360 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atabekov, T.A.; Khlynin, M.S.; Mishkina, A.I.; Batalov, R.E.; Sazonova, S.I.; Krivolapov, S.N.; Saushkin, V.V.; Varlamova, Y.V.; Zavadovsky, K.V.; Popov, S.V. The Value of Left Ventricular Mechanical Dyssynchrony and Scar Burden in the Combined Assessment of Factors Associated with Cardiac Resynchronization Therapy Response in Patients with CRT-D. J. Clin. Med. 2023, 12, 2120. https://doi.org/10.3390/jcm12062120
Atabekov TA, Khlynin MS, Mishkina AI, Batalov RE, Sazonova SI, Krivolapov SN, Saushkin VV, Varlamova YV, Zavadovsky KV, Popov SV. The Value of Left Ventricular Mechanical Dyssynchrony and Scar Burden in the Combined Assessment of Factors Associated with Cardiac Resynchronization Therapy Response in Patients with CRT-D. Journal of Clinical Medicine. 2023; 12(6):2120. https://doi.org/10.3390/jcm12062120
Chicago/Turabian StyleAtabekov, Tariel A., Mikhail S. Khlynin, Anna I. Mishkina, Roman E. Batalov, Svetlana I. Sazonova, Sergey N. Krivolapov, Victor V. Saushkin, Yuliya V. Varlamova, Konstantin V. Zavadovsky, and Sergey V. Popov. 2023. "The Value of Left Ventricular Mechanical Dyssynchrony and Scar Burden in the Combined Assessment of Factors Associated with Cardiac Resynchronization Therapy Response in Patients with CRT-D" Journal of Clinical Medicine 12, no. 6: 2120. https://doi.org/10.3390/jcm12062120
APA StyleAtabekov, T. A., Khlynin, M. S., Mishkina, A. I., Batalov, R. E., Sazonova, S. I., Krivolapov, S. N., Saushkin, V. V., Varlamova, Y. V., Zavadovsky, K. V., & Popov, S. V. (2023). The Value of Left Ventricular Mechanical Dyssynchrony and Scar Burden in the Combined Assessment of Factors Associated with Cardiac Resynchronization Therapy Response in Patients with CRT-D. Journal of Clinical Medicine, 12(6), 2120. https://doi.org/10.3390/jcm12062120






