Oral Cell Lysates Reduce the Inflammatory Response of Activated Macrophages
Abstract
1. Introduction
2. Methods
2.1. Cell Lines
2.2. Cell Lysates
2.3. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
2.4. Immunoassay
2.5. Immunofluorescent Analysis
2.6. Statistical Analysis
3. Results
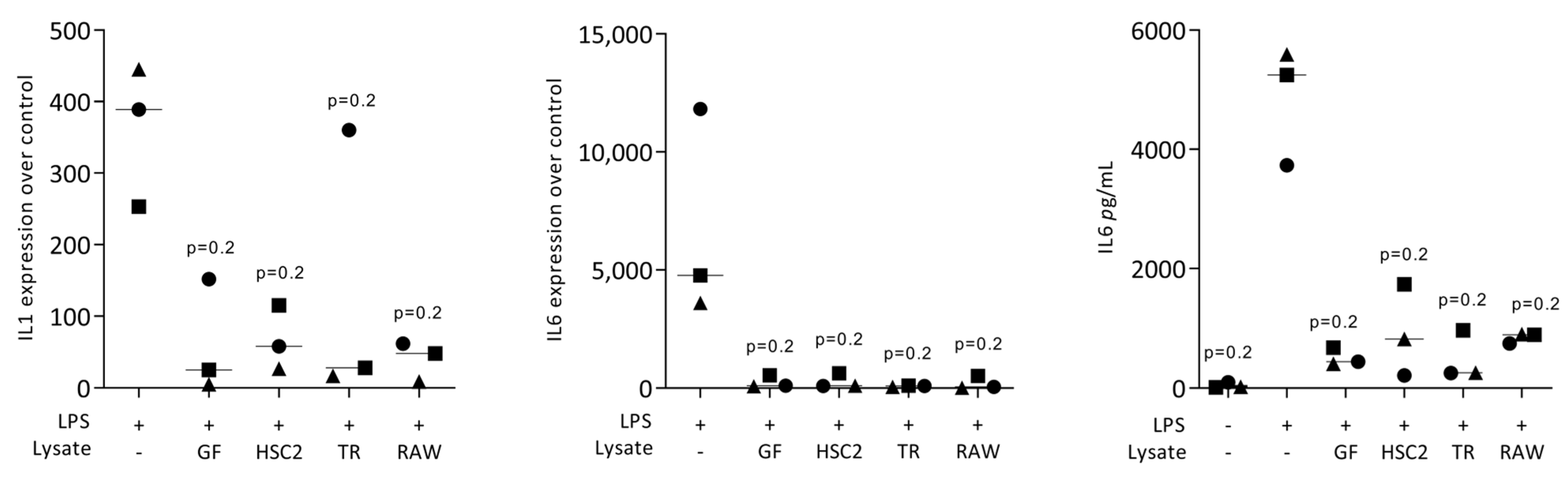
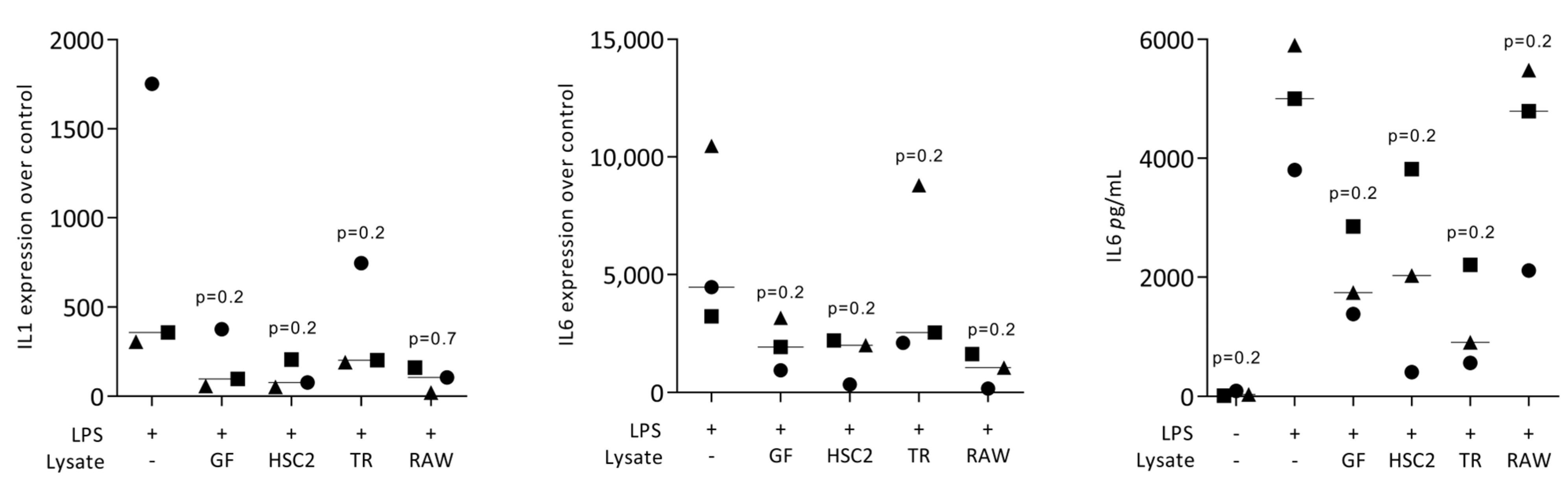
3.1. Necrotic Cell Lysates Reduce LPS-Induced IL1 and IL6 in RAW264.7 Cells
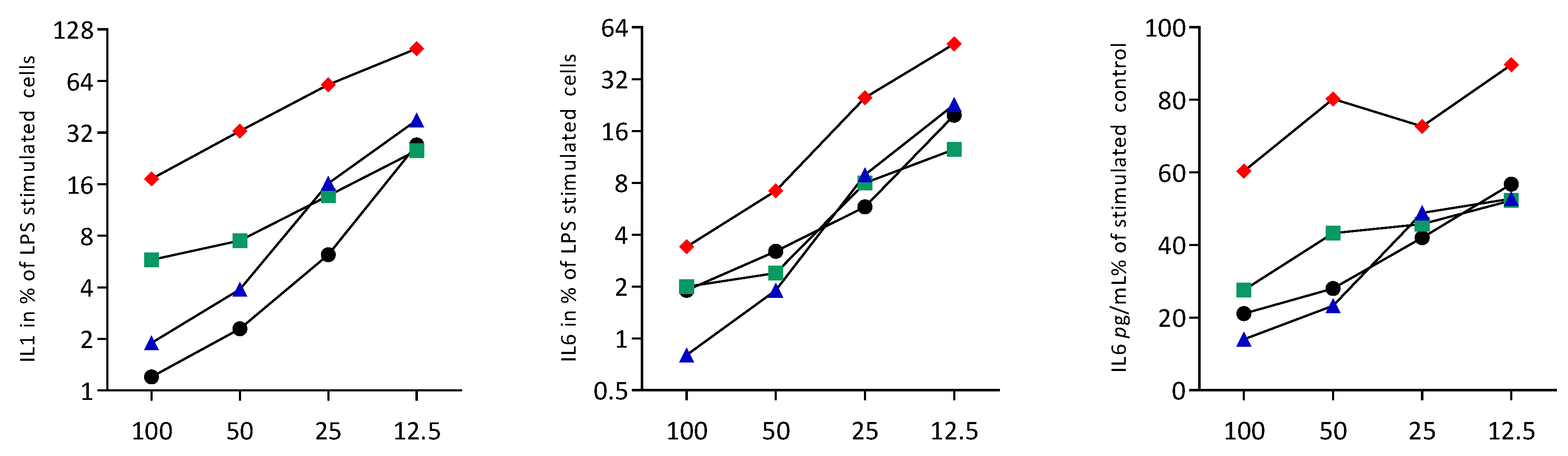
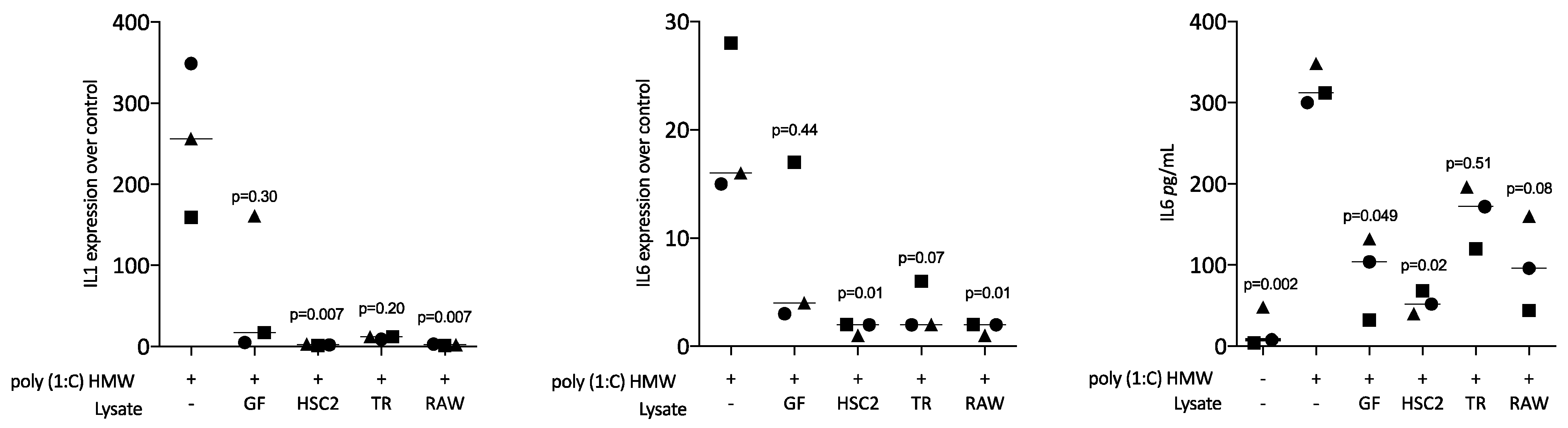
3.2. Sonicated Cell Lysates Reduce TLR3 Agonist-Induced Cytokine Expression in RAW264.7 Cells
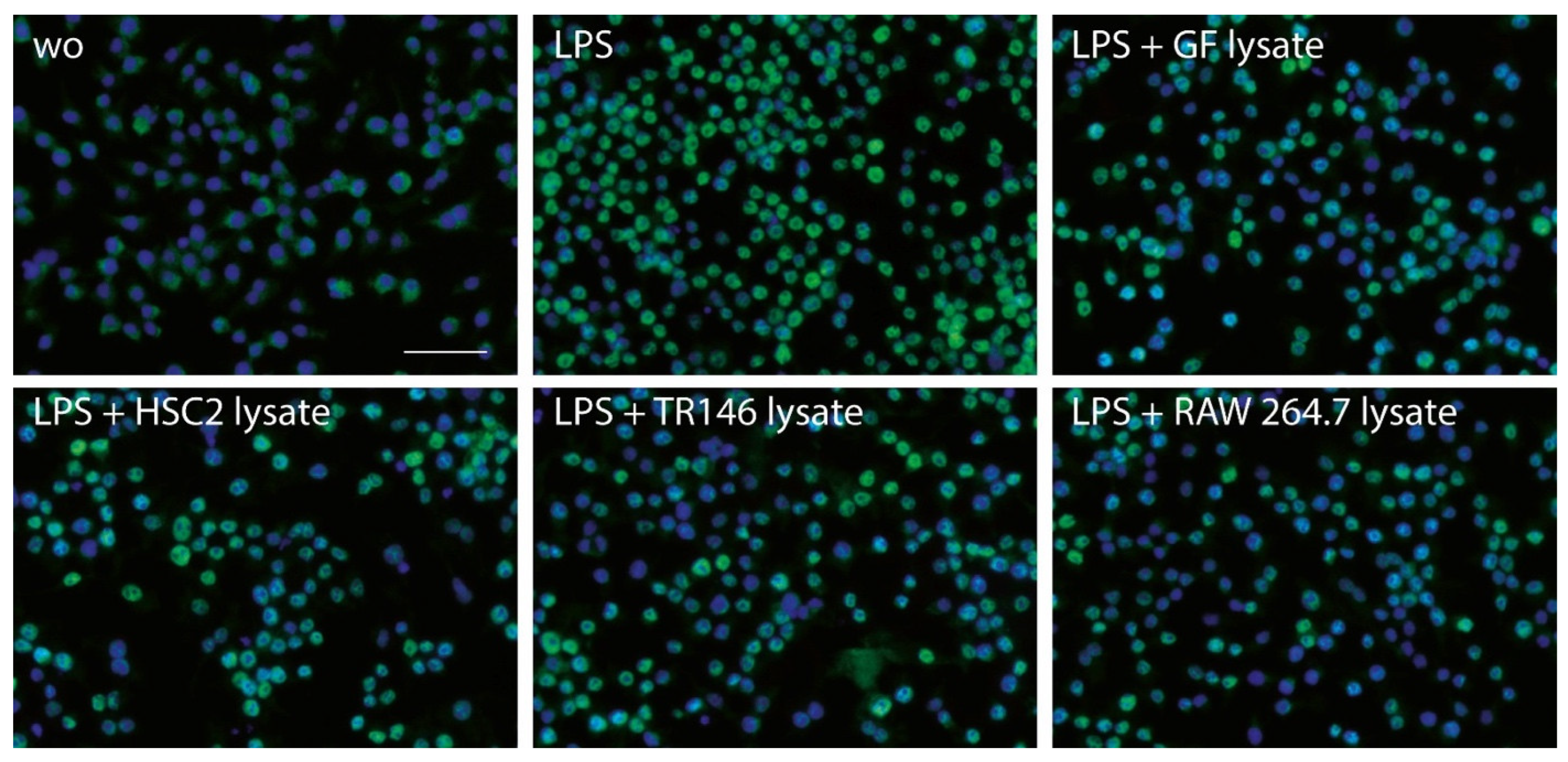
3.3. Necrotic Cell Lysates Reduce LPS-Induced p65 Nuclear Translocation in RAW264.7 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachet, M.; Liang, Y.Y.; Oehler, R. The immune response to secondary necrotic cells. Apoptosis 2017, 22, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, J.; Hoffmann, W.; Gasser, A.; Brunski, J.B.; Helms, J.A. Mechanical and Biological Advantages of a Tri-Oval Implant Design. J. Clin. Med. 2019, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Coyac, B.R.; Arioka, M.; Leahy, B.; Tulu, U.S.; Aghvami, M.; Holst, S.; Hoffmann, W.; Quarry, A.; Bahat, O.; et al. A Novel Osteotomy Preparation Technique to Preserve Implant Site Viability and Enhance Osteogenesis. J. Clin. Med. 2019, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Coyac, B.R.; Sun, Q.; Leahy, B.; Salvi, G.; Yuan, X.; Brunski, J.B.; Helms, J.A. Optimizing autologous bone contribution to implant osseointegration. J. Periodontol. 2020, 91, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Gil, A.; Hämmerle, C.H.F.; Jung, R.E. Management and prevention of soft tissue complications in implant dentistry. Periodontol. 2000 2022, 88, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Strauss, F.J.; Mancini, L.; Gasser, T.J.W.; Jung, R.E. Minimal invasiveness in soft tissue augmentation at dental implants: A systematic review and meta-analysis of patient-reported outcome measures. Periodontol. 2000, 2022; ahead of print. [Google Scholar] [CrossRef]
- Cobb, C.M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002, 29 (Suppl. 2), 6–16. [Google Scholar] [CrossRef]
- Williams, V.D. Electrosurgery and wound healing: A review of the literature. J. Am. Dent. Assoc. 1984, 108, 220–222. [Google Scholar] [CrossRef]
- Kujan, O.; Azzeghaiby, S.N.; Tarakji, B.; Abuderman, A.; Sakka, S. Cryosurgery of the oral and peri-oral region: A literature review of the mechanism, tissue response, and clinical applications. J. Investig. Clin. Dent. 2013, 4, 71–77. [Google Scholar] [CrossRef]
- Lucas, H.; Bartold, P.; Dharmapatni, A.; Holding, C.; Haynes, D. Inhibition of Apoptosis in Periodontitis. J. Dent. Res. 2010, 89, 29–33. [Google Scholar] [CrossRef]
- Li, J.; Ke, X.; Yan, F.; Lei, L.; Li, H. Necroptosis in the periodontal homeostasis: Signals emanating from dying cells. Oral Dis. 2018, 24, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Sordi, M.B.; Magini, R.D.S.; Panahipour, L.; Gruber, R. Pyroptosis-Mediated Periodontal Disease. Int. J. Mol. Sci. 2021, 23, 372. [Google Scholar] [CrossRef] [PubMed]
- Bantel, H.; Beikler, T.; Flemmig, T.F.; Schulze-Osthoff, K. Caspase activation is involved in chronic periodontitis. FEBS Lett. 2005, 579, 5559–5564. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, J.; Su, W.; Zhao, S.; Li, H.; Lei, L. Loss of periodontal ligament fibroblasts by RIPK3-MLKL-mediated necroptosis in the progress of chronic periodontitis. Sci. Rep. 2019, 9, 2902. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Shu, R.; Xie, Y. The expression of NLRP3, NLRP1 and AIM2 in the gingival tissue of periodontitis patients: RT-PCR study and immunohistochemistry. Arch. Oral Biol. 2015, 60, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, B.; Liu, Y.; Wang, H.; He, M.; Liu, Y.; Sun, Y.; Meng, W. Porphyromonas gingivalis lipopolysaccharide affects oral epithelial connections via pyroptosis. J. Dent. Sci. 2021, 16, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Rider, P.; Carmi, Y.; Braiman, A.; Dotan, S.; White, M.R.; Voronov, E.; Martin, M.U.; Dinarello, C.A.; Apte, R.N. Differential release of chromatin-bound IL-1α discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2574–2579. [Google Scholar] [CrossRef]
- Rohne, P.; Wolf, S.; Dörr, C.; Ringen, J.; Holtz, A.; Gollan, R.; Renner, B.; Prochnow, H.; Baiersdörfer, M.; Koch-Brandt, C. Exposure of vital cells to necrotic cell lysates induce the IRE1α branch of the unfolded protein response and cell proliferation. Cell Stress Chaperon. 2018, 23, 77–88. [Google Scholar] [CrossRef]
- Shim, Y.-J.; Tae, Y.-K.; Kang, B.-H.; Park, J.-S.; Jeon, S.-Y.; Min, B.-H. Toll-like receptor 4 signaling is required for clusterin-induced tumor necrosis factor-α secretion in macrophage. Biochem. Biophys. Res. Commun. 2017, 482, 1407–1412. [Google Scholar] [CrossRef]
- Shim, Y.-J.; Kang, B.-H.; Jeon, H.-S.; Park, I.-S.; Lee, K.-U.; Lee, I.-K.; Park, G.-H.; Lee, K.-M.; Schedin, P.; Min, B.-H. Clusterin induces matrix metalloproteinase-9 expression via ERK1/2 and PI3K/Akt/NF-κB pathways in monocytes/macrophages. J. Leukoc. Biol. 2011, 90, 761–769. [Google Scholar] [CrossRef]
- Škoberne, M.; Beignon, A.-S.; Bhardwaj, N. Danger signals: A time and space continuum. Trends Mol. Med. 2004, 10, 251–257. [Google Scholar] [CrossRef]
- Dhanasekar, C.; Kalaiselvan, S.; Rasool, M. Morin, a Bioflavonoid Suppresses Monosodium Urate Crystal-Induced Inflammatory Immune Response in RAW 264.7 Macrophages through the Inhibition of Inflammatory Mediators, Intracellular ROS Levels and NF-κB Activation. PLoS ONE 2015, 10, e0145093. [Google Scholar] [CrossRef] [PubMed]
- Panahipour, L.; Abbasabadi, A.O.; Kaiser, V.; Sordi, M.B.; Kargarpour, Z.; Gruber, R. Damaged Mesenchymal Cells Dampen the Inflammatory Response of Macrophages and the Formation of Osteoclasts. J. Clin. Med. 2022, 11, 4061. [Google Scholar] [CrossRef] [PubMed]
- Sordi, M.B.; Panahipour, L.; Kargarpour, Z.; Gruber, R. Platelet-Rich Fibrin Reduces IL-1β Release from Macrophages Undergoing Pyroptosis. Int. J. Mol. Sci. 2022, 23, 8306. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.A.; Phan, T.K.; Hanssen, E.; Liem, M.; Hulett, M.D.; Mathivanan, S.; Poon, I.K.H. Analysis of extracellular vesicles generated from monocytes under conditions of lytic cell death. Sci. Rep. 2019, 9, 7538. [Google Scholar] [CrossRef] [PubMed]
- Malvicini, R.; Santa-Cruz, D.; De Lazzari, G.; Tolomeo, A.M.; Sanmartin, C.; Muraca, M.; Yannarelli, G.; Pacienza, N. Macrophage bioassay standardization to assess the anti-inflammatory activity of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy 2022, 24, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wang, C.; Zhang, J.; Zhang, J.; Gu, Y.; Guo, X.; Zuo, X.; Pan, H.; Hsu, A.C.-Y.; Wang, G.; et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res. Ther. 2021, 12, 204. [Google Scholar] [CrossRef]
- Gao, J.; Dong, X.; Wang, Z. Generation, purification and engineering of extracellular vesicles and their biomedical applications. Methods 2020, 177, 114–125. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Ayad, N.B.; Hyzy, S.L.; Boyan, B.D.; Olivares-Navarrete, R. Dental implant surface chemistry and energy alter macrophage activation in vitro. Clin. Oral Implant. Res. 2016, 28, 414–423. [Google Scholar] [CrossRef]
- Williams, D.W.; Greenwell-Wild, T.; Brenchley, L.; Dutzan, N.; Overmiller, A.; Sawaya, A.P.; Webb, S.; Martin, D.; Hajishengallis, G.; Divaris, K.; et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell 2021, 184, 4090–4104.e15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panahipour, L.; Oladzad Abbasabadi, A.; Gruber, R. Oral Cell Lysates Reduce the Inflammatory Response of Activated Macrophages. J. Clin. Med. 2023, 12, 1701. https://doi.org/10.3390/jcm12041701
Panahipour L, Oladzad Abbasabadi A, Gruber R. Oral Cell Lysates Reduce the Inflammatory Response of Activated Macrophages. Journal of Clinical Medicine. 2023; 12(4):1701. https://doi.org/10.3390/jcm12041701
Chicago/Turabian StylePanahipour, Layla, Azarakhsh Oladzad Abbasabadi, and Reinhard Gruber. 2023. "Oral Cell Lysates Reduce the Inflammatory Response of Activated Macrophages" Journal of Clinical Medicine 12, no. 4: 1701. https://doi.org/10.3390/jcm12041701
APA StylePanahipour, L., Oladzad Abbasabadi, A., & Gruber, R. (2023). Oral Cell Lysates Reduce the Inflammatory Response of Activated Macrophages. Journal of Clinical Medicine, 12(4), 1701. https://doi.org/10.3390/jcm12041701






