Association of Serum BAFF Levels with Cardiovascular Events in ST-Segment Elevation Myocardial Infarction
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Study Endpoints and Follow-Up
2.4. BAFF Measurement
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association between Serum BAFF Levels and the Risk of MACEs
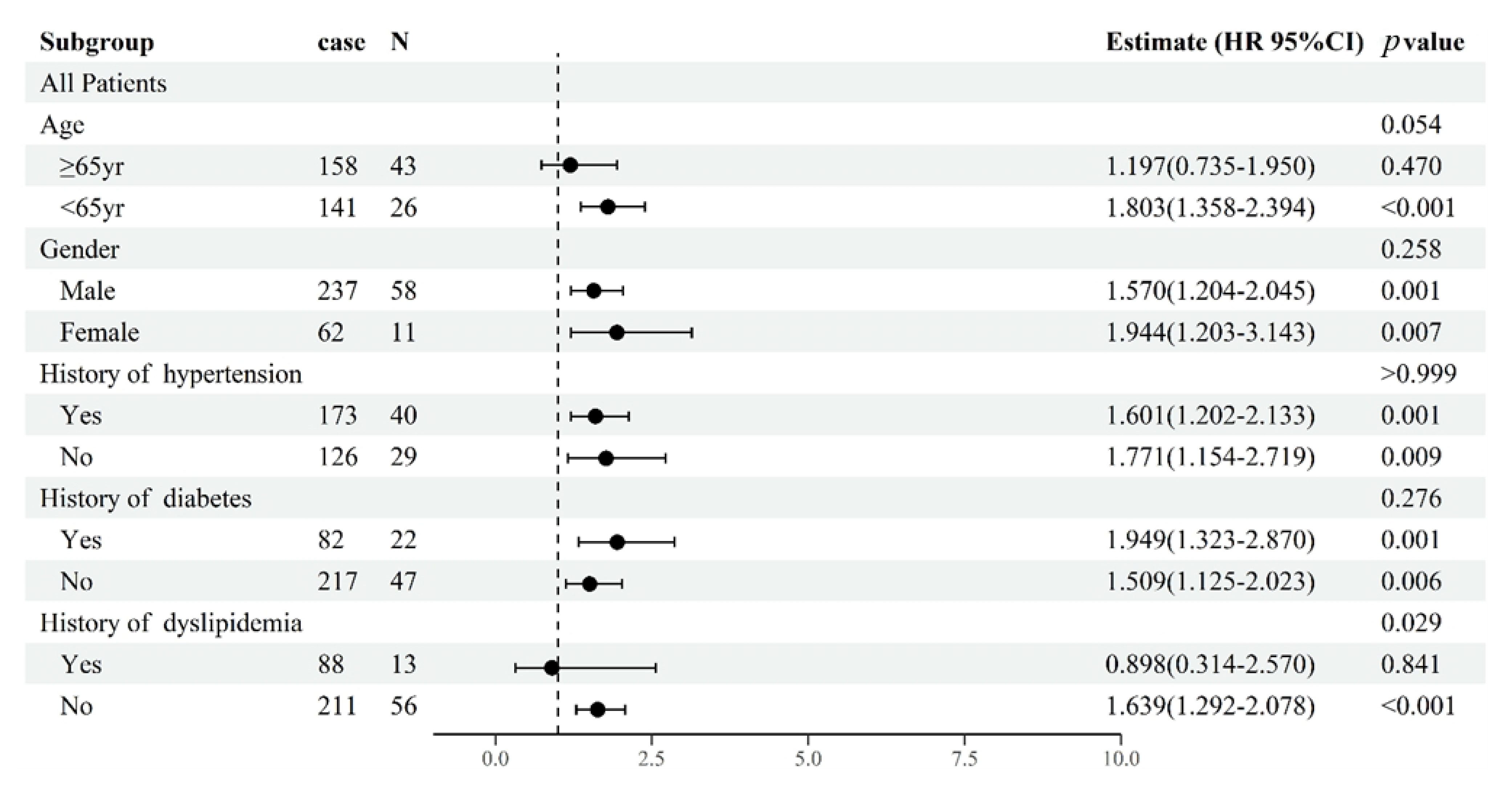
3.3. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Pinto, C.; Camus, S.; Duval, V.; Alayrac, P.; Zlatanova, I.; Loyer, X.; Vilar, J.; Lemitre, M.; Levoye, A.; et al. Splenic Marginal Zone B Lymphocytes Regulate Cardiac Remodeling after Acute Myocardial Infarction in Mice. J. Am. Coll. Cardiol. 2022, 79, 632–647. [Google Scholar] [CrossRef]
- Huan, T.; Zhang, B.; Wang, Z.; Joehanes, R.; Zhu, J.; Johnson, A.D.; Ying, S.; Munson, P.J.; Raghavachari, N.; Wang, R.; et al. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, T.; Toh, B.H.; Bobik, A. Evolving BAFF targeted therapies for preventing acute myocardial infarctions and ischemic strokes. Expert Opin. Ther. Targets 2020, 24, 7–12. [Google Scholar] [CrossRef]
- Theodorou, E.; Nezos, A.; Antypa, E.; Ioakeimidis, D.; Koutsilieris, M.; Tektonidou, M.; Moutsopoulos, H.M.; Mavragani, C.P. B-cell activating factor and related genetic variants in lupus related atherosclerosis. J. Autoimmun. 2018, 92, 87–92. [Google Scholar] [CrossRef]
- Samy, E.; Wax, S.; Huard, B.; Hess, H.; Schneider, P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int. Rev. Immunol. 2017, 36, 3–19. [Google Scholar] [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef] [PubMed]
- Ohata, J.; Zvaifler, N.J.; Nishio, M.; Boyle, D.L.; Kalled, S.L.; Carson, D.A.; Kipps, T.J. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J. Immunol. 2005, 174, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Eilertsen, G.; Van Ghelue, M.; Strand, H.; Nossent, J.C. Increased levels of BAFF in patients with systemic lupus erythematosus are associated with acute-phase reactants, independent of BAFF genetics: A case-control study. Rheumatology 2011, 50, 2197–2205. [Google Scholar] [CrossRef]
- Tay, C.; Liu, Y.H.; Kanellakis, P.; Kallies, A.; Li, Y.; Cao, A.; Hosseini, H.; Tipping, P.; Toh, B.H.; Bobik, A.; et al. Follicular B Cells Promote Atherosclerosis via T Cell-Mediated Differentiation into Plasma Cells and Secreting Pathogenic Immunoglobulin G. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e71–e84. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Katus, H.A.; Lindahl, B.; Morrow, D.A.; Clemmensen, P.M.; et al. Third universal definition of myocardial infarction. Circulation 2012, 126, 2020–2035. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I., III; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.A.; Tcheng, J.E.; Bozkurt, B.; Chaitman, B.R.; Cutlip, D.E.; Farb, A.; Fonarow, G.C.; Jacobs, J.P.; Jaff, M.R.; Lichtman, J.H.; et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation 2015, 132, 302–361. [Google Scholar]
- Pencina, M.J.; D’Agostino, R.B.; D’Agostino, R.B., Sr.; Vasan, R.S., Jr. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172, discussion 207–212. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Tian, L.; Cai, T.; Kohane, I.S.; Wei, L.J. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat. Med. 2013, 32, 2430–2442. [Google Scholar] [CrossRef]
- Groom, J.; Kalled, S.L.; Cutler, A.H.; Olson, C.; Woodcock, S.A.; Schneider, P.; Tschopp, J.; Cachero, T.G.; Batten, M.; Wheway, J.; et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J. Clin. Investig. 2002, 109, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Möckel, T.; Basta, F.; Weinmann-Menke, J.; Schwarting, A. B cell activating factor (BAFF): Structure, functions, autoimmunity and clinical implications in Systemic Lupus Erythematosus (SLE). Autoimmun. Rev. 2021, 20, 102736. [Google Scholar] [CrossRef]
- Li, K.; Yu, W.; Cao, R.; Zhu, Z.; Zhao, G. Microglia-mediated BAFF-BAFFR ligation promotes neuronal survival in brain ischemia injury. Neuroscience 2017, 363, 87–96. [Google Scholar] [CrossRef]
- Wada, K.; Maeda, K.; Tajima, K.; Kato, T.; Kobata, T.; Yamakawa, M. Expression of BAFF-R and TACI in reactive lymphoid tissues and B-cell lymphomas. Histopathology 2009, 54, 221–232. [Google Scholar] [CrossRef]
- Paiva, C.; Rowland, T.A.; Sreekantham, B.; Godbersen, C.; Best, S.R.; Kaur, P.; Loriaux, M.M.; Spurgeon, S.E.F.; Danilova, O.V.; Danilov, A.V. SYK inhibition thwarts the BAFF—B-cell receptor crosstalk and thereby antagonizes Mcl-1 in chronic lymphocytic leukemia. Haematologica 2017, 102, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.W.; Davidson, A. BAFF inhibition in SLE-Is tolerance restored? Immunol. Rev. 2019, 292, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Pattarabanjird, T.; Li, C.; McNamara, C. B Cells in Atherosclerosis: Mechanisms and Potential Clinical Applications. JACC Basic Transl. Sci. 2021, 6, 546–563. [Google Scholar] [CrossRef] [PubMed]
- Saidoune, F.; Even, G.; Lamri, Y.; Chezel, J.; Gaston, A.T.; Escoubet, B.; Papo, T.; Charles, N.; Nicoletti, A.; Sacre, K. Effects of BAFF Neutralization on Atherosclerosis Associated with Systemic Lupus Erythematosus. Arthritis Rheumatol. 2021, 73, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.; Schneider, P. Function, occurrence and inhibition of different forms of BAFF. Curr. Opin. Immunol. 2021, 71, 75–80. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Herbin, O.; Bouaziz, J.D.; Binder, C.J.; Uyttenhove, C.; Laurans, L.; Taleb, S.; Van Vré, E.; Esposito, B.; Vilar, J.; et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 2010, 207, 1579–1587. [Google Scholar] [CrossRef]
- Kyaw, T.; Tay, C.; Khan, A.; Dumouchel, V.; Cao, A.; To, K.; Kehry, M.; Dunn, R.; Agrotis, A.; Tipping, P.; et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J. Immunol. 2010, 185, 4410–4419. [Google Scholar] [CrossRef]
- Tay, C.; Liu, Y.H.; Hosseini, H.; Kanellakis, P.; Cao, A.; Peter, K.; Tipping, P.; Bobik, A.; Toh, B.H.; Kyaw, T. B-cell-specific depletion of tumour necrosis factor alpha inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation. Cardiovasc. Res. 2016, 111, 385–397. [Google Scholar] [CrossRef]
- Kyaw, T.; Cui, P.; Tay, C.; Kanellakis, P.; Hosseini, H.; Liu, E.; Rolink, A.G.; Tipping, P.; Bobik, A.; Toh, B.H. BAFF receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE(−/−) mice. PLoS ONE 2013, 8, e60430. [Google Scholar] [CrossRef]
- Tsiantoulas, D.; Sage, A.P.; Göderle, L.; Ozsvar-Kozma, M.; Murphy, D.; Porsch, F.; Pasterkamp, G.; Menche, J.; Schneider, P.; Mallat, Z.; et al. B Cell-Activating Factor Neutralization Aggravates Atherosclerosis. Circulation 2018, 138, 2263–2273. [Google Scholar] [CrossRef]
- Srikakulapu, P.; Hu, D.; Yin, C.; Mohanta, S.K.; Bontha, S.V.; Peng, L.; Beer, M.; Weber, C.; McNamara, C.A.; Grassia, G.; et al. Artery Tertiary Lymphoid Organs Control Multilayered Territorialized Atherosclerosis B-Cell Responses in Aged ApoE−/− Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Natorska, J.; Marek, G.; Sadowski, J.; Undas, A. Presence of B cells within aortic valves in patients with aortic stenosis: Relation to severity of the disease. J. Cardiol. 2016, 67, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Bryl-Górecka, P.; James, K.; Torngren, K.; Haraldsson, I.; Gan, L.M.; Svedlund, S.; Olde, B.; Laurell, T.; Omerovic, E.; Erlinge, D. Microvesicles in plasma reflect coronary flow reserve in patients with cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H2147–H2160. [Google Scholar] [CrossRef] [PubMed]
- Kindermann, I.; Kindermann, M.; Kandolf, R.; Klingel, K.; Bültmann, B.; Müller, T.; Lindinger, A.; Böhm, M. Predictors of outcome in patients with suspected myocarditis. Circulation 2008, 118, 639–648. [Google Scholar] [CrossRef]
- Yan, X.; Anzai, A.; Katsumata, Y.; Matsuhashi, T.; Ito, K.; Endo, J.; Yamamoto, T.; Takeshima, A.; Shinmura, K.; Shen, W.; et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell Cardiol. 2013, 62, 24–35. [Google Scholar] [CrossRef]
- Adamo, L.; Staloch, L.J.; Rocha-Resende, C.; Matkovich, S.J.; Jiang, W.; Bajpai, G.; Weinheimer, C.J.; Kovacs, A.; Schilling, J.D.; Barger, P.M.; et al. Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight 2018, 3, e120137. [Google Scholar] [CrossRef]
- Mo, F.; Luo, Y.; Yan, Y.; Li, J.; Lai, S.; Wu, W. Are activated B cells involved in the process of myocardial fibrosis after acute myocardial infarction? An in vivo experiment. BMC Cardiovasc. Disord. 2021, 21, 5. [Google Scholar] [CrossRef]
- Fox, K.A.; Carruthers, K.F.; Dunbar, D.R.; Graham, C.; Manning, J.R.; De Raedt, H.; Buysschaert, I.; Lambrechts, D.; Van de Werf, F. Underestimated and under-recognized: The late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur. Heart J. 2010, 31, 2755–2764. [Google Scholar] [CrossRef]
- Kaziród-Wolski, K.; Sielski, J.; Gąsior, M.; Bujak, K.; Hawranek, M.; Pyka, Ł.; Gierlotka, M.; Pawłowski, T.; Siudak, Z. Factors affecting short- and long-term survival of patients with acute coronary syndrome treated invasively using intravascular ultrasound and fractional flow reserve: Analysis of data from the Polish Registry of Acute Coronary Syndromes 2017–2020. Kardiol. Pol. 2022. [Google Scholar] [CrossRef]
- Köktürk, U.; Püşüroğlu, H.; Somuncu, M.U.; Akgül, Ö.; Uygur, B.; Özyılmaz, S.; Işıksaçan, N.; Sürgit, Ö.; Yıldırım, A. Short and Long-Term Prognostic Significance of Galectin-3 in Patients with ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Angiology 2023. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]




| Total (n = 299) | BAFF ≤ 0.79 ng/mL (n = 100) | 0.79 < BAFF ≤ 1.38 ng/mL (n = 99) | BAFF > 1.38 ng/mL (n = 100) | p-Value | |
|---|---|---|---|---|---|
| Demographic and risk factors | |||||
| Age (years) | 65.43 ± 12.57 | 59.46 ± 12.02 | 65.39 ± 12.35 | 71.44 ± 10.38 | <0.001 |
| Male, sex (n, %) | 237 (79.3) | 83 (83.0) | 75 (75.8) | 79 (79.0) | 0.451 |
| BMI (kg/m2) | 24.08 ± 2.98 | 24.87 ± 2.60 | 24.19 ± 2.92 | 23.18 ± 3.18 | <0.001 |
| Smoking (n, %) | 135 (45.2) | 52 (52.0) | 47 (47.5) | 36 (36.0) | 0.064 |
| Alcohol (n, %) | 75 (25.1) | 21 (21.0) | 31 (31.3) | 23 (23.0) | 0.206 |
| Heart rate (beats/minute) | 83.21 ± 16.18 | 80.64 ± 13.35 | 82.31 ± 15.64 | 86.48 ± 18.71 | 0.033 |
| Systolic pressure (mmHg) | 123.91 ± 21.46 | 126.54 ± 20.16 | 123.65 ± 21.30 | 121.19 ± 22.89 | 0.212 |
| Diastolic pressure (mmHg) | 74.92 ± 13.60 | 75.92 ± 14.86 | 75.22 ± 11.00 | 73.63 ± 14.60 | 0.476 |
| Medical history | |||||
| Hypertension (n, %) | 173 (57.9) | 47 (47.0) | 60 (60.6) | 66 (66.0) | 0.020 |
| Diabetes (n, %) | 82 (27.4) | 26 (26.0) | 28 (28.3) | 28 (28.0) | 0.925 |
| Dyslipidemia (n, %) | 88 (29.4) | 33 (33.0) | 30 (30.0) | 25 (25.0) | 0.450 |
| Clinical presentation | |||||
| WBC (×109/L) | 9.20 (7.10–11.20) | 9.50 (7.33–11.90) | 9.10 (6.86–10.79) | 8.90 (6.98–11.21) | 0.454 |
| Hemoglobin (g/L) | 137.00 (122.00–147.00) | 140.08 (132.00–149.00) | 135.00 (122.00–147.00) | 130.00 (112.75–142.25) | <0.001 |
| Platelet (×109/L) | 190.00 (157.50–221.00) | 192.50 (150.00–224.00) | 196.00 (170.00–232.00) | 180.50 (152.75–210.00) | 0.090 |
| HbA1c (%) | 6.00 (5.65–6.80) | 5.90 (5.50–6.90) | 6.20 (5.70–6.78) | 6.05 (5.70–6.80) | 0.386 |
| Fasting glucose (mmol/L) | 6.09 (5.24–7.33) | 5.64 (4.98–7.28) | 6.09 (5.25–7.50) | 6.45 (5.58–7.50) | 0.037 |
| hs-CRP (mg/L) | 3.00 (1.07–7.96) | 2.50 (1.11–5.08) | 2.80 (0.72–6.00) | 6.16 (1.30–19.21) | <0.001 |
| Triglyceride (mmol/L) | 1.29 (0.97–1.82) | 1.42 (1.083–2.09) | 1.33 (1.02–1.74) | 1.22 (0.87–1.60) | 0.100 |
| Total cholesterol (mmol/L) | 4.58 (3.72–5.36) | 4.49 (3.86–5.39) | 4.73 (3.73–5.59) | 4.50 (3.36–5.27) | 0.107 |
| HDL-C (mmol/L) | 1.05 (0.92–1.25) | 1.08 (0.90–1.21) | 1.03 (0.94–1.30) | 1.04 (0.92–1.25) | 0.962 |
| LDL-C (mmol/L) | 2.87 (2.22–3.52) | 3.03 (2.47–3.46) | 3.01 (2.32–3.70) | 2.78 (2.05–3.26) | 0.091 |
| Lp(a) (mmol/L) | 0.14 (0.07–0.29) | 0.11 (0.07–0.27) | 0.15 (0.08–0.25) | 0.14 (0.06–0.38) | 0.440 |
| Creatine (μmol/L) | 81.00 (70.00–100.00) | 77.00 (68.25–88.00) | 76.00 (67.00–76.00) | 97.50 (77.00–136.00) | <0.001 |
| Cystatin C (mg/L) | 1.04 (0.91–1.29) | 0.97 (0.88–1.11) | 1.01 (0.91–1.24) | 1.21 (0.97–1.57) | <0.001 |
| eGFR (mL/minute/1.73 m2) | 82.75 (62.43–94.80) | 91.05 (79.68–98.93) | 87.85 (72.85–95.95) | 61.95 (43.38–81.88) | <0.001 |
| NT-proBNP (pg/mL) | 614.95 (152.85–2026.00) | 406.00 (130.00–1118.00) | 292.10 (107.80–1282.00) | 1551.50 (392.30–4637.75) | <0.001 |
| CK-MB (mg/L) | 107.60 (26.30–275.00) | 103.65 (15.18–211.35) | 66.50 (22.70–266.10) | 194.70 (60.23–318.40) | 0.001 |
| cTnI (ng/L) | 20.27 (6.06–61.22) | 17.14 (3.59–49.71) | 15.80 (2.83–55.93) | 36.76 (11.71–82.00) | <0.001 |
| Syntax score | 19.00 (12.00–27.00) | 17.00 (8.00–26.13) | 19.00 (13.00–26.50) | 21.00 (15.50–29.00) | 0.003 |
| Echocardiography | |||||
| LVEF (%) | 56.47 ± 8.20 | 58.08 ± 6.82 | 58.09 ± 7.09 | 53.11 ± 9.54 | <0.001 |
| LAD (mm) | 38.08 ± 3.91 | 37.44 ± 3.20 | 37.80 ± 3.66 | 39.03 ± 4.64 | 0.012 |
| LVEDD (mm) | 49.31 ± 4.16 | 49.06 ± 3.57 | 48.94 ± 3.94 | 49.96 ± 4.86 | 0.182 |
| LVESD (mm) | 34.13 ± 4.69 | 33.23 ± 3.39 | 33.53 ± 4.56 | 35.70 ± 5.56 | <0.001 |
| LVEDV (mL) | 116.80 ± 23.58 | 115.05 ± 19.00 | 114.99 ± 22.59 | 120.50 ± 28.26 | 0.180 |
| LVESV (mL) | 50.17 ± 19.45 | 46.63 ± 11.33 | 47.81 ± 14.84 | 56.31 ± 22.92 | <0.001 |
| Ischemia time | 0.170 | ||||
| 0 h ≤ chest pain to balloon ≤ 6 h | 100 (33.4) | 41 (41.0) | 32 (32.3) | 27 (27.0) | |
| 6 h < chest pain to balloon ≤ 12 h | 99 (33.1) | 34 (34.0) | 31 (31.3) | 34 (34.0) | |
| 12 h < chest pain to balloon ≤ 24 h | 100 (33.4) | 36 (36.0) | 30 (30.3) | 34 (34.0) | |
| Culprit vessel | 0.013 | ||||
| LAD (n, %) | 163 (54.5) | 60 (60.6) | 55 (55.0) | 48 (48.0) | |
| LCX (n, %) | 32 (10.7) | 8 (8.1) | 15 (15.0) | 9 (9.0) | |
| RCA (n, %) | 92 (30.7) | 25 (25.3) | 30 (30.0) | 37 (37.0) | |
| Number of diseased vessels | 0.391 | ||||
| Single vessel | 63 (21.4) | 23 (23.0) | 32 (32.0) | 44 (44.0) | |
| Double vessel | 81 (27.6) | 24 (24.0) | 25 (25.3) | 50 (50.5) | |
| Triple vessel | 149 (50.7) | 16 (16.0) | 24 (25.3) | 55 (57.9) | |
| Killip classification | <0.001 | ||||
| I (n, %) | 205 (68.6) | 89 (89.0) | 81 (81.8) | 35 (35.0) | |
| II (n, %) | 57 (19.1) | 8 (8.0) | 13 (13.1) | 36 (36.0) | |
| III/IV (n, %) | 37 (12.4) | 3 (3.0) | 5 (5.1) | 29 (29.0) | |
| Medication | |||||
| Aspirin (n, %) | 289 (96.7) | 99 (99.0) | 96 (97.0) | 94 (94.0) | >0.999 |
| Clopidogrel (n, %) | 251 (86.9) | 79 (79.0) | 91 (91.9) | 81 (81.0) | 0.008 |
| Ticagrelor (n, %) | 39 (13.6) | 20 (20.0) | 5 (5.1) | 14 (14.0) | 0.010 |
| Statins (n, %) | 283 (97.9) | 97 (97.0) | 96 (97.0) | 90 (90.0) | 0.120 |
| Anticoagulants (n, %) | 109 (37.0) | 25 (25.0) | 41 (41.4) | 41 (41.0) | 0.011 |
| ACEI/ARB (n, %) | 244 (84.4) | 81 (81.0) | 90 (90.9) | 73 (73.0) | 0.006 |
| Beta blockers (n, %) | 268 (92.7) | 93 (93.0) | 91 (91.9) | 84 (84.0) | 0.301 |
| Nitrates (n, %) | 106 (36.6) | 33 (33.0) | 34 (34.3) | 39 (39.0) | 0.515 |
| Unadjusted HR (95%CI) | p-Value | Adjusted for Model 1 HR (95%CI) | p-Value | Adjusted for Model 2 HR (95%CI) | p-Value | |
|---|---|---|---|---|---|---|
| MACEs | ||||||
| Log2 BAFF | 1.828 (1.383–2.415) | <0.001 | 1.628 (1.215–2.183) | 0.001 | 1.525 (1.085–2.145) | 0.015 |
| BAFF cut-off | 3.439 (2.143–5.521) | <0.001 | 2.864 (1.747–4.694) | <0.001 | 2.705 (1.518–4.822) | 0.001 |
| Tertiles of BAFF | ||||||
| T1 | 1 (Ref) | 1 (Ref) | 1 (Ref) | |||
| T2 | 3.380 (1.525–7.495) | 0.003 | 3.061 (1.370–6.839) | 0.006 | 3.341 (1.412–7.903) | 0.006 |
| T3 | 5.602 (2.603–12.058) | <0.001 | 4.373 (1.973–9.691) | <0.001 | 4.116 (1.690–10.022) | 0.002 |
| Cardiovascular death | ||||||
| Log2 BAFF | 2.366 (1.482–3.779) | <0.001 | 1.957 (1.181–3.242) | 0.009 | 2.083 (1.014–4.279) | 0.046 |
| BAFF cut-off | 4.594 (2.059–10.245) | <0.001 | 3.102 (1.351–7.120) | 0.008 | 3.632 (1.132–11.650) | 0.030 |
| Tertiles of BAFF | ||||||
| T1 | 1 (Ref) | 1 (Ref) | 1 (Ref) | |||
| T2 | 4.336 (0.920–20.425) | 0.064 | 3.329 (0.701–15.802) | 0.130 | 4.330 (0.491–38.208) | 0.187 |
| T3 | 9.112 (2.082–39.886) | 0.003 | 5.107 (0.944–8.767) | 0.063 | 6.607 (0.755–57.830) | 0.088 |
| C-Statistic | p-Value | IDI (95%CI) | p-Value | |
|---|---|---|---|---|
| Log2 cTnI | 0.623 | NA | Ref | NA |
| Log2 BAFF | 0.680 | 0.263 | 0.030 (0.001, 0.058) | 0.044 |
| Log2 NT-proBNP | 0.612 | 0.807 | 0.007 (−0.015, 0.029) | 0.536 |
| Log2 CK-MB | 0.588 | 0.114 | −0.013 (−0.023, −0.004) | 0.008 |
| Log2 cTnI+ Log2 BAFF | 0.675 | 0.126 | 0.042 (0.020, 0.064) | <0.001 |
| Log2 cTnI+ Log2 NTproBNP+ Log2 CK-MB+ Log2 BAFF | 0.675 | 0.158 | 0.059 (0.031, 0.086) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, Y.; Cui, Y.; Chen, Z.; Yi, L.; Zhu, Z.; Ni, J.; Du, R.; Wang, X.; Zhu, J.; et al. Association of Serum BAFF Levels with Cardiovascular Events in ST-Segment Elevation Myocardial Infarction. J. Clin. Med. 2023, 12, 1692. https://doi.org/10.3390/jcm12041692
Wang Z, Wang Y, Cui Y, Chen Z, Yi L, Zhu Z, Ni J, Du R, Wang X, Zhu J, et al. Association of Serum BAFF Levels with Cardiovascular Events in ST-Segment Elevation Myocardial Infarction. Journal of Clinical Medicine. 2023; 12(4):1692. https://doi.org/10.3390/jcm12041692
Chicago/Turabian StyleWang, Ziyang, Yueying Wang, Yuke Cui, Zhiyong Chen, Lei Yi, Zhengbin Zhu, Jingwei Ni, Run Du, Xiaoqun Wang, Jinzhou Zhu, and et al. 2023. "Association of Serum BAFF Levels with Cardiovascular Events in ST-Segment Elevation Myocardial Infarction" Journal of Clinical Medicine 12, no. 4: 1692. https://doi.org/10.3390/jcm12041692
APA StyleWang, Z., Wang, Y., Cui, Y., Chen, Z., Yi, L., Zhu, Z., Ni, J., Du, R., Wang, X., Zhu, J., Ding, F., Quan, W., Zhang, R., Hu, J., & Yan, X. (2023). Association of Serum BAFF Levels with Cardiovascular Events in ST-Segment Elevation Myocardial Infarction. Journal of Clinical Medicine, 12(4), 1692. https://doi.org/10.3390/jcm12041692





