High-Flow Nasal Cannula Oxygen Therapy versus Non-Invasive Ventilation in AIDS Patients with Acute Respiratory Failure: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
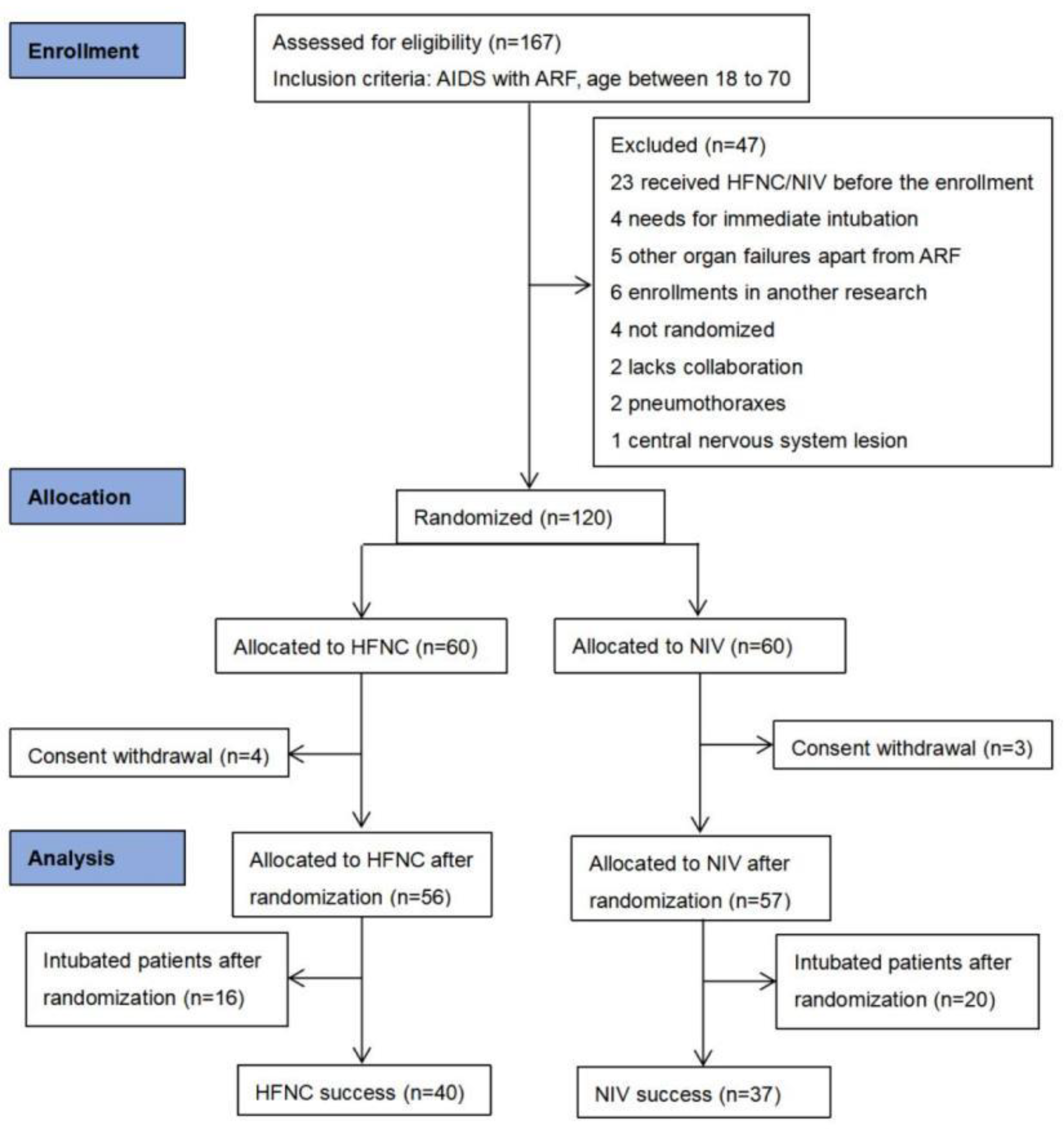
2.2. Screening of Patients
2.3. Study Treatments
2.4. Data Collection
2.5. Outcomes
2.6. Sample Size, Randomization, and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatments
3.3. Primary Outcome
3.4. Secondary Outcomes
VAS Scores, Vital Signs, and Blood Gas Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.S.; Tubiana, R.; Katlama, C.; Calvez, V.; Ait Mohand, H.; Autran, B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet 1998, 351, 1682–1686. [Google Scholar] [CrossRef]
- Palella, F.J., Jr.; Delaney, K.M.; Moorman, A.C.; Loveless, M.O.; Fuhrer, J.; Satten, G.A.; Aschman, D.J.; Holmberg, S.D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 1998, 338, 853–860. [Google Scholar] [CrossRef]
- Collett, L.W.; Simpson, T.; Camporota, L.; Meadows, C.I.; Ioannou, N.; Glover, G.; Kulasegaram, R.; Barrett, N.A. The use of extracorporeal membrane oxygenation in HIV-positive patients with severe respiratory failure: A retrospective observational case series. Int. J. STD AIDS 2019, 30, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Orsini, J.; Ahmad, N.; Butala, A.; Flores, R.; Tran, T.; Llosa, A.; Fishkin, E. Etiology and Outcome of Patients with HIV Infection and Respiratory Failure Admitted to the Intensive Care Unit. Interdiscip. Perspect. Infect. Dis. 2013, 2013, 732421. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Coquet, I.; Legriel, S.; Pavie, J.; Darmon, M.; Mayaux, J.; Molina, J.M.; Schlemmer, B.; Azoulay, E. Etiologies and outcome of acute respiratory failure in HIV-infected patients. Intensive Care Med. 2009, 35, 1678–1686. [Google Scholar] [CrossRef]
- Chiang, H.H.; Hung, C.C.; Lee, C.M.; Chen, H.Y.; Chen, M.Y.; Sheng, W.H.; Hsieh, S.M.; Sun, H.Y.; Ho, C.C.; Yu, C.J. Admissions to intensive care unit of HIV-infected patients in the era of highly active antiretroviral therapy: Etiology and prognostic factors. Crit. Care 2011, 15, R202. [Google Scholar] [CrossRef] [PubMed]
- Akgün, K.M.; Pisani, M.; Crothers, K. The changing epidemiology of HIV-infected patients in the intensive care unit. J. Intensive Care Med. 2011, 26, 151–164. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Luo, K.; He, J.; Ma, Y.; Li, Z.; Zhao, N.; Xu, Q.; Li, Y.; Yu, X. Noninvasive versus invasive mechanical ventilation for immunocompromised patients with acute respiratory failure: A systematic review and meta-analysis. BMC Pulm. Med. 2016, 16, 129. [Google Scholar] [CrossRef]
- Schönhofer, B.; Kuhlen, R.; Neumann, P.; Westhoff, M.; Berndt, C.; Sitter, H. Clinical practice guideline: Non-invasive mechanical ventilation as treatment of acute respiratory failure. Dtsch. Ärzteblatt Int. 2008, 105, 424–433. [Google Scholar]
- Keenan, S.P.; Sinuff, T.; Burns, K.E.; Muscedere, J.; Kutsogiannis, J.; Mehta, S.; Cook, D.J.; Ayas, N.; Adhikari, N.K.; Hand, L. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ 2011, 183, E195–E214. [Google Scholar] [CrossRef]
- Anjos, C.F.; Schettino, G.P.; Park, M.; Souza, V.S.; Scalabrini Neto, A. A randomized trial of noninvasive positive end expiratory pressure in patients with acquired immune deficiency syndrome and hypoxemic respiratory failure. Respir. Care 2012, 57, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, Y.; Xiao, G.; Ouyang, H.; Zhang, C. Analysis of the clinical effect of noninvasive mechanical ventilation in AIDS patients complicated with pneumonia. Am. J. Transl. Res. 2021, 13, 3794–3799. [Google Scholar] [CrossRef] [PubMed]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Longhini, F.; Madotto, F.; Groff, P.; Scala, R.; Crimi, C.; Carlucci, A.; Bruni, A.; Garofalo, E.; Raineri, S.M.; et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: A multicenter non-inferiority randomized trial. Crit. Care 2020, 24, 692. [Google Scholar] [CrossRef]
- Tan, D.; Walline, J.H.; Ling, B.; Xu, Y.; Sun, J.; Wang, B.; Shan, X.; Wang, Y.; Cao, P.; Zhu, Q.; et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: A multicenter, randomized controlled trial. Crit. Care 2020, 24, 489. [Google Scholar] [CrossRef]
- Bräunlich, J.; Köhler, M.; Wirtz, H. Nasal highflow improves ventilation in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1077–1085. [Google Scholar] [CrossRef]
- Longhini, F.; Liu, L.; Pan, C.; Xie, J.; Cammarota, G.; Bruni, A.; Garofalo, E.; Yang, Y.; Navalesi, P.; Qiu, H. Neurally-Adjusted Ventilatory Assist for Noninvasive Ventilation via a Helmet in Subjects with COPD Exacerbation: A Physiologic Study. Respir. Care 2019, 64, 582–589. [Google Scholar] [CrossRef]
- Coudroy, R.; Jamet, A.; Petua, P.; Robert, R.; Frat, J.P.; Thille, A.W. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: An observational cohort study. Ann. Intensive Care 2016, 6, 45. [Google Scholar] [CrossRef]
- Castro, K.G.; Ward, J.W.; Slutsker, L.; Buehler, J.W.; Jaffe, H.W.; Berkelman, R.L.; Curran, J.W. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm. Rep. 1992, 41, 1–19. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Schoenfeld, D.A.; Bernard, G.R. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit. Care Med. 2002, 30, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Piaggio, G.; Elbourne, D.R.; Pocock, S.J.; Evans, S.J. Reporting of noninferiority and equivalence randomized trials: Extension of the CONSORT 2010 statement. JAMA 2012, 308, 2594–2604. [Google Scholar] [CrossRef] [PubMed]
- Sklar, M.C.; Mohammed, A.; Orchanian-Cheff, A.; Del Sorbo, L.; Mehta, S.; Munshi, L. The Impact of High-Flow Nasal Oxygen in the Immunocompromised Critically Ill: A Systematic Review and Meta-Analysis. Respir. Care 2018, 63, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Crimi, C.; Sanfilippo, F.; Noto, A.; Di Falco, D.; Grasselli, G.; Gregoretti, C.; Giarratano, A. High flow nasal therapy in immunocompromised patients with acute respiratory failure: A systematic review and meta-analysis. J. Crit. Care 2019, 50, 250–256. [Google Scholar] [CrossRef]
- Frat, J.P.; Brugiere, B.; Ragot, S.; Chatellier, D.; Veinstein, A.; Goudet, V.; Coudroy, R.; Petitpas, F.; Robert, R.; Hille, A.W.; et al. Sequential application of oxygen therapy via high-flow nasal cannula and noninvasive ventilation in acute respiratory failure: An observational pilot study. Respir. Care 2015, 60, 170–178. [Google Scholar] [CrossRef]
- Coudroy, R.; Frat, J.P.; Ehrmann, S.; Pène, F.; Decavèle, M.; Terzi, N.; Prat, G.; Garret, C.; Contou, D.; Gacouin, A.; et al. High-flow nasal oxygen alone or alternating with non-invasive ventilation in critically ill immunocompromised patients with acute respiratory failure: A randomised controlled trial. Lancet Respir. Med. 2022, 10, 641–649. [Google Scholar] [CrossRef]
- Azoulay, E.; Lemiale, V.; Mokart, D.; Nseir, S.; Argaud, L.; Pène, F.; Kontar, L.; Bruneel, F.; Klouche, K.; Barbier, F.; et al. Effect of High-Flow Nasal Oxygen vs. Standard Oxygen on 28-Day Mortality in Immunocompromised Patients With Acute Respiratory Failure: The HIGH Randomized Clinical Trial. JAMA 2018, 320, 2099–2107. [Google Scholar] [CrossRef]
- Lemiale, V.; Mokart, D.; Mayaux, J.; Lambert, J.; Rabbat, A.; Demoule, A.; Azoulay, E. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: A multicenter randomized trial. Crit. Care 2015, 19, 380. [Google Scholar] [CrossRef]
- Confalonieri, M.; Calderini, E.; Terraciano, S.; Chidini, G.; Celeste, E.; Puccio, G.; Gregoretti, C.; Meduri, G.U. Noninvasive ventilation for treating acute respiratory failure in AIDS patients with Pneumocystis carinii pneumonia. Intensive Care Med. 2002, 28, 1233–1238. [Google Scholar] [CrossRef]
- Lemiale, V.; Mokart, D.; Resche-Rigon, M.; Pène, F.; Mayaux, J.; Faucher, E.; Nyunga, M.; Girault, C.; Perez, P.; Guitton, C.; et al. Effect of Noninvasive Ventilation vs. Oxygen Therapy on Mortality Among Immunocompromised Patients With Acute Respiratory Failure: A Randomized Clinical Trial. JAMA 2015, 314, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.R.; Kacmarek, R.M.; Hess, D.R. Factors affecting oxygen delivery with bi-level positive airway pressure. Respir. Care 2004, 49, 270–275. [Google Scholar] [PubMed]
- Kang, B.J.; Koh, Y.; Lim, C.-M.; Huh, J.W.; Baek, S.; Han, M.; Seo, H.-S.; Suh, H.J.; Seo, G.J.; Kim, E.Y.; et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015, 41, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Synn, A.; Huh, J.W.; Hong, S.B.; Koh, Y.; Lim, C.M. Clinical efficacy of high-flow nasal cannula compared to noninvasive ventilation in patients with post-extubation respiratory failure. Korean J. Intern. Med. 2016, 31, 82–88. [Google Scholar] [CrossRef]
- Nishimura, M. High-flow nasal cannula oxygen therapy in adults. J. Intensive Care 2015, 3, 15. [Google Scholar] [CrossRef]
- Lee, C.C.; Mankodi, D.; Shaharyar, S.; Ravindranathan, S.; Danckers, M.; Herscovici, P.; Moor, M.; Ferrer, G. High flow nasal cannula versus conventional oxygen therapy and non-invasive ventilation in adults with acute hypoxemic respiratory failure: A systematic review. Respir. Med. 2016, 121, 100–108. [Google Scholar] [CrossRef]
- Cuquemelle, E.; Pham, T.; Papon, J.F.; Louis, B.; Danin, P.E.; Brochard, L. Heated and humidified high-flow oxygen therapy reduces discomfort during hypoxemic respiratory failure. Respir. Care 2012, 57, 1571–1577. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Ling, B.; Zhu, Q.; Hu, Y.; Tan, D.; Geng, P.; Xu, J. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: An observational cohort study. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1229–1237. [Google Scholar] [CrossRef]

| HFNC Group (n = 56) | NIV Group (n = 57) | p Value | |
|---|---|---|---|
| Age (years) | 41 ± 12 | 39 ± 12 | 0.478 |
| Weight (kg) | 62 ± 10 | 60 ± 12 | 0.185 |
| Male | 54 (96.4%) | 52 (91.2%) | 0.438 |
| Smoking history | 12 (21.4%) | 13 (22.8%) | 0.860 |
| Comorbidities | 0.810 | ||
| Diabetes mellitus | 4 (7.1%) | 3 (5.3%) | |
| Hypertension | 3 (5.4%) | 3 (5.3%) | |
| Coronary artery disease | 0 (0) | 1 (1.8%) | |
| Chronic kidney disease | 0 (0) | 1 (1.8%) | |
| HIV-related data | |||
| Newly diagnosed HIV infection | 41 (73.2%) | 41 (71.9%) | 0.878 |
| On HAART before admission | 13 (23.2%) | 13 (22.8%) | 0.959 |
| CD4 (cells/μL) | 16 (6–32) | 14 (8–26) | 0.947 |
| HIV viral load (copies/mL) † | 168,760 (72,472–394,631) | 135,992 (70,661–304,846) | 0.651 |
| Clinical and laboratory data | |||
| Cough | 42 (75.0%) | 43 (75.4%) | 0.957 |
| Abnormal breath sound | 26 (46.4%) | 30 (52.6%) | 0.510 |
| APACHE II score | 14 (11–18) | 14 (10–17) | 0.429 |
| SOFA score | 3 (2–3) | 3 (2–4) | 0.108 |
| Respiratory rate (per min) | 31 ± 7 | 31 ± 10 | 0.939 |
| SpO2 (%) ‡ | 87 (83–90) | 88 (85–90) | 0.863 |
| PaO2 (mmHg) ‡ | 56 (48–60) | 57 (51–62) | 0.266 |
| Lactate dehydrogenase (U/L) | 453 (354–572) | 446 (301–67) | 0.503 |
| Albumin (g/dL) | 29.5 (26.8–32.5) | 30.9 (27.1–34.0) | 0.291 |
| Serum 1,3-d-glucan (pg/mL) | 63 (21–172) | 80 (20–231) | 0.641 |
| Cause of ARF | 0.513 | ||
| PCP | 53 (94.6%) | 54 (94.7%) | |
| Pulmonary tuberculosis | 2 (3.6%) | 2 (3.5%) | |
| Bacterial pneumonia | 1 (1.8%) | 1 (1.8%) | |
| Time before ICU (days) | 0 (0–2) | 0 (0–2) | 1.000 |
| HFNC Group (n = 56) | NIV Group (n = 57) | |||||
|---|---|---|---|---|---|---|
| Baseline | At 2 h | At 24 h † | Baseline | At 2 h | At 24 h † | |
| VAS score for dyspnea ‡ | 6 (5–7) | 6 (5–7) * | 5 (2–6) *,** | 6 (5–7) | 5 (4–7) * | 5 (3–8) *,** |
| VAS score for device discomfort ‡ | 5 (5–7) | 4 (4–5) *,*** | 4 (3–4) *,**,*** | 5 (5–7) | 5 (4–7) * | 4 (3–6) *,** |
| SBP (mmHg) | 116 ± 13 | 114 ± 14 | 109 ± 9 | 117 ± 16 | 113 ± 13 | 111 ± 13 |
| DBP (mmHg) | 71 ± 10 | 69 ± 9 | 70 ± 9 | 73 ± 12 | 70 ± 9 | 71 ± 8 |
| Heart rate (per min) | 100 ± 16 | 98 ± 19 | 85 ± 16 * | 106 ± 23 | 98 ± 19 | 88 ± 15 * |
| Respiratory rate (per min) | 31 ± 7 | 28 ± 5 * | 25 ± 4 *,**,*** | 32 ± 9 | 30 ± 6 * | 27 ± 5 *,** |
| Arterial pH | 7.45 (7.42–7.48) | 7.45 (7.42–7.48) | 7.43 (7.42–7.48) | 7.45 (7.42–7.46) | 7.44 (7.42–7.46) | 7.44 (7.42–7.47) |
| PaCO2 (mmHg) | 31.4 ± 5.5 | 32.5 ± 7.3 | 31.3 ± 5.0 | 32.8 ± 6.3 | 33.6 ± 6.5 | 31.2 ± 2.7 |
| PaO2/FiO2 | 186 ± 56 | 202 ± 66 * | 211 ± 66 * | 177 ± 66 | 198 ± 71 * | 203 ± 42 * |
| HACOR score | 2 (0.25–5) | 2 (0–4.25) | 2 (0–5) | 4 (0–6) | 2 (0, 5) * | 2 (0–5) * |
| ROX index | 5.11 (4.22–6.32) | 7.27 (4.55–12.50) * | 7.08 (4.53–12.73) * | 5.64 (4.13–8.55) | 7.58 (5.07–13.42) * | 7.58 (5.13–14.63) * |
| HFNC Group (n = 56) | NIV Group (n = 57) | Odds Ratio (95% CI) | p Value | |
|---|---|---|---|---|
| Intubation on day 28 | 16 (28.6%) | 20 (35.1%) | 0.740 (0.334 to 1.639) | 0.457 |
| Interval between enrollment and intubation (days) † | 2.9 ± 2.5 | 3.1 ± 2.5 | 0.894 | |
| Length of IMV (hours) † | 300 ± 199 | 228 ± 193 | 0.278 | |
| Reason for intubation † | - | 0.513 | ||
| Respiratory failure | 54 (96.4%) | 55 (96.5%) | 0.982 (0.133 to 7.223) | 1.000 |
| Circulatory failure | 2 (3.6%) | 1 (1.8%) | 2.074 (0.183 to 23.545) | 0.618 |
| Neurologic failure | 0 (0) | 1 (1.8%) | - | 1.000 |
| Ventilator-free days on day 28 | 15.9 ± 8.7 | 15.9 ± 10.2 | 0.999 | |
| Ventilator-free days on day 90 | 58.4 ± 37.4 | 58.6 ± 38.7 | 0.969 | |
| Mortality on day 28 | 11 (19.6%) | 15 (26.3%) | 0.684 (0.283 to 1.657) | 0.399 |
| Mortality on day 90 | 16 (28.6%) | 17 (29.8%) | 0.941 (0.418 to 2.118) | 0.884 |
| Airway care interventions | 6 (5–7) | 8 (6–9) | <0.001 | |
| Adverse events | 10 (17.9%) | 17 (29.8%) | 0.512 (0.210 to 1.244) | 0.186 |
| Nasofacial skin breakdown | 3 (5.4%) | 5 (8.8%) | 0.589 (0.134 to 2.590) | 0.716 |
| Intolerance ‡ | 1 (1.8%) | 8 (14.0%) | 0.111 (0.013 to 0.922) | 0.032 |
| Nasal prongs broken | 1 (1.8%) | 0 (0) | - | 0.496 |
| Air leaks | 5 (8.9%) | 4 (7.0%) | 1.299 (0.330 to 5.111) | 0.742 |
| ICU length of stay (days) | 11 (8–15) | 7 (6–13) | 0.005 | |
| Hospital length of stay (days) | 21 (16–33) | 25 (13–34) | 0.669 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Liu, J.; Pu, L.; Li, C.; Zhang, M.; Tan, J.; Wang, H.; Yin, N.; Sun, Y.; Liu, Y.; et al. High-Flow Nasal Cannula Oxygen Therapy versus Non-Invasive Ventilation in AIDS Patients with Acute Respiratory Failure: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 1679. https://doi.org/10.3390/jcm12041679
Hao J, Liu J, Pu L, Li C, Zhang M, Tan J, Wang H, Yin N, Sun Y, Liu Y, et al. High-Flow Nasal Cannula Oxygen Therapy versus Non-Invasive Ventilation in AIDS Patients with Acute Respiratory Failure: A Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(4):1679. https://doi.org/10.3390/jcm12041679
Chicago/Turabian StyleHao, Jingjing, Jingyuan Liu, Lin Pu, Chuansheng Li, Ming Zhang, Jianbo Tan, Hongyu Wang, Ningning Yin, Yao Sun, Yufeng Liu, and et al. 2023. "High-Flow Nasal Cannula Oxygen Therapy versus Non-Invasive Ventilation in AIDS Patients with Acute Respiratory Failure: A Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 4: 1679. https://doi.org/10.3390/jcm12041679
APA StyleHao, J., Liu, J., Pu, L., Li, C., Zhang, M., Tan, J., Wang, H., Yin, N., Sun, Y., Liu, Y., Guo, H., & Li, A. (2023). High-Flow Nasal Cannula Oxygen Therapy versus Non-Invasive Ventilation in AIDS Patients with Acute Respiratory Failure: A Randomized Controlled Trial. Journal of Clinical Medicine, 12(4), 1679. https://doi.org/10.3390/jcm12041679






