Liver X Receptor Agonist Inhibits Oxidized Low-Density Lipoprotein Induced Choroidal Neovascularization via the NF-κB Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. Induction of Choroidal Neovascularization
2.4. Severity Assessment of CNV
2.5. Frozen Section Preparation and Immunofluorescence Staining
2.6. Cytoplasmic and Nuclear Protein Extraction and Western Blotting
2.7. Real-Time Quantification of Polymerase Chain Reactions
2.8. Tube Formation
2.9. Oil Red O Staining
2.10. RNAi
2.11. Protein Array Analysis
2.12. Data Analysis
3. Results
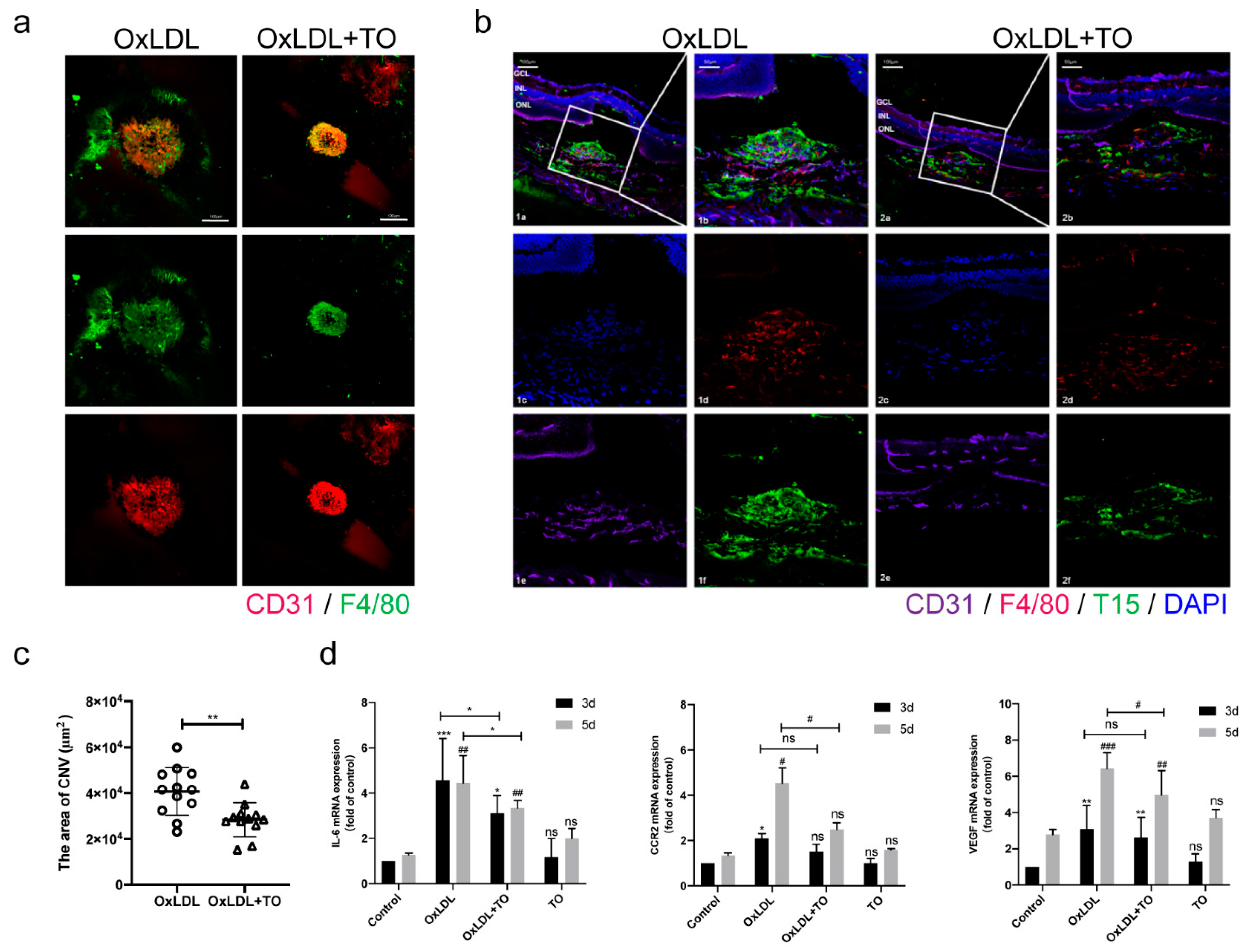
3.1. LXR Agonist Inhibits CNV Formation and Inflammation
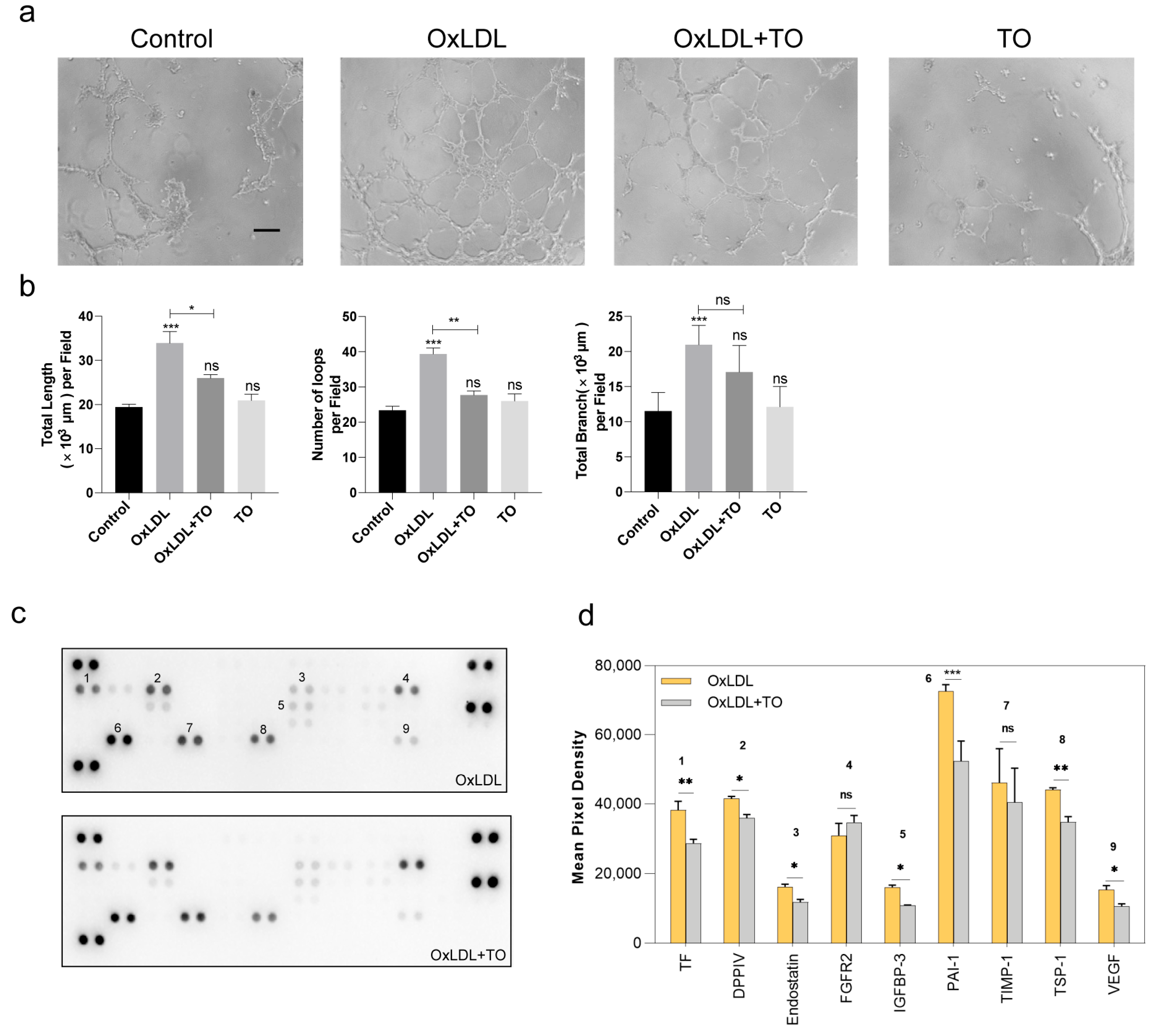
3.2. LXR Agonist Inhibits Angiogenesis in RPE Cells
3.3. LXR Agonist Inhibits Lipid Accumulation via the ABCG1 Pathway and Inflammation in RPE Cells
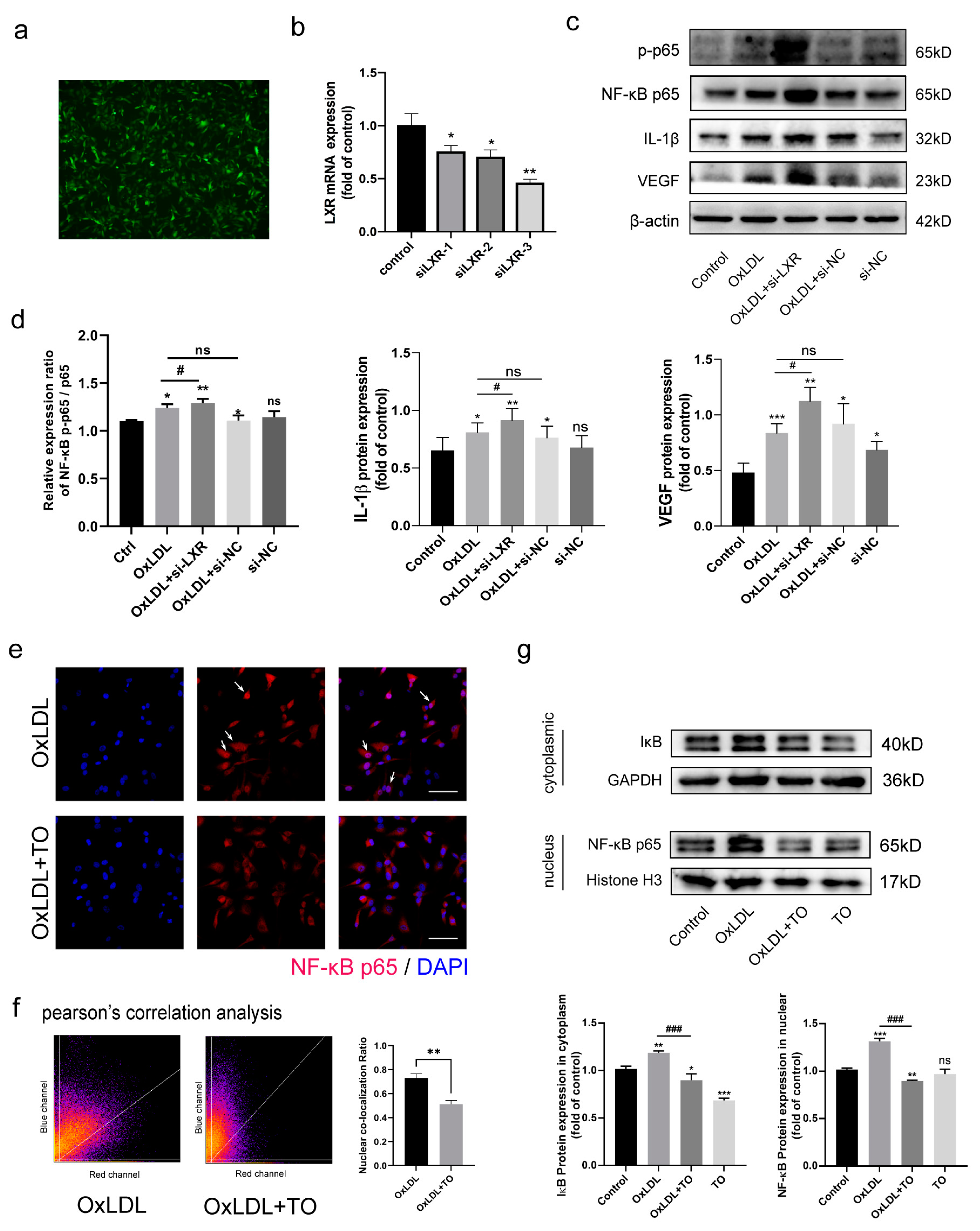
3.4. LXR Agonist Inhibits the OxLDL-Activated NF-κB Pathway
3.5. LXR Knockout Aggravated NF-κB Pathway-Mediated IL-1β and VEGF Expression
3.6. LXR Agonist Inhibits OxLDL-Mediated Activation of NF-κB p65 Nuclear Translocation
3.7. LXR Agonist Reduces the CNV Volume in Vldlr−/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Finnemann, S.C.; Febbraio, M.; Shan, L.; Annangudi, S.P.; Podrez, E.A.; Hoppe, G.; Darrow, R.; Organisciak, D.T.; Salomon, R.G.; et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: A potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J. Biol. Chem. 2006, 281, 4222–4230. [Google Scholar] [CrossRef]
- Gu, X.; Neric, N.J.; Crabb, J.S.; Crabb, J.W.; Bhattacharya, S.K.; Rayborn, M.E.; Hollyfield, J.G.; Bonilha, V.L. Age-related changes in the retinal pigment epithelium (RPE). PLoS ONE 2012, 7, e38673. [Google Scholar] [CrossRef]
- Seddon, J.M.; Reynolds, R.; Yu, Y.; Daly, M.J.; Rosner, B. Risk models for progression to advanced age-related macular degeneration using demographic, environmental, genetic, and ocular factors. Ophthalmology 2011, 118, 2203–2211. [Google Scholar] [CrossRef]
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2017, 1862, 430–440. [Google Scholar] [CrossRef]
- AnandBabu, K.; Bharathidevi, S.R.; Sripriya, S.; Sen, P.; Prakash, V.J.; Bindu, A.; Viswanathan, N.; Angayarkanni, N. Serum Paraoxonase activity in relation to lipid profile in Age-related Macular Degeneration patients. Exp. Eye Res. 2016, 152, 100–112. [Google Scholar] [CrossRef]
- Nowak, M.; Swietochowska, E.; Marek, B.; Szapska, B.; Wielkoszynski, T.; Kos-Kudla, B.; Karpe, J.; Kajdaniuk, D.; Sieminska, L.; Glogowska-Szelag, J.; et al. Changes in lipid metabolism in women with age-related macular degeneration. Clin. Exp. Med. 2005, 4, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Que, X.; Hung, M.Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Määttä, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Wu, T.; Dang, K.R.; Wang, Y.F.; Lyu, B.Z.; Xu, W.Q.; Dou, G.R.; Zhou, J.; Hui, Y.N.; Du, H.J. A modified laser-induced choroidal neovascularization animal model with intravitreal oxidized low-density lipoprotein. Int. J. Ophthalmol. 2020, 13, 1187–1194. [Google Scholar] [CrossRef]
- Zelcer, N.; Hong, C.; Boyadjian, R.; Tontonoz, P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 2009, 325, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Breevoort, S.R.; Angdisen, J.; Fu, M.; Schmidt, D.R.; Holmstrom, S.R.; Kliewer, S.A.; Mangelsdorf, D.J.; Schulman, I.G. Liver LXRα expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J. Clin. Investig. 2012, 122, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Rader, D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005, 96, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Cedó, L.; Metso, J.; Santos, D.; García-León, A.; Plana, N.; Sabate-Soler, S.; Rotllan, N.; Rivas-Urbina, A.; Méndez-Lara, K.A.; Tondo, M.; et al. LDL Receptor Regulates the Reverse Transport of Macrophage-Derived Unesterified Cholesterol via Concerted Action of the HDL-LDL Axis: Insight From Mouse Models. Circ. Res. 2020, 127, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; McKilligin, E.; Pei, L.; Watson, M.A.; Collins, A.R.; Laffitte, B.A.; Chen, M.; Noh, G.; Goodman, J.; Hagger, G.N.; et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 7604–7609. [Google Scholar] [CrossRef]
- Tangirala, R.K.; Bischoff, E.D.; Joseph, S.B.; Wagner, B.L.; Walczak, R.; Laffitte, B.A.; Daige, C.L.; Thomas, D.; Heyman, R.A.; Mangelsdorf, D.J.; et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. USA 2002, 99, 11896–11901. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.M.; Long, P.; Chen, M.Z.; Wei, D.Y.; Wang, J.C.; Zhang, Z.M.; Zhang, L.; Chen, T. Retinal neovascularization induced by mutant Vldlr gene inhibited in an inherited retinitis pigmentosa mouse model: An in-vivo study. Int. J. Ophthalmol. 2021, 14, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Poor, S.H.; Qiu, Y.; Fassbender, E.S.; Shen, S.; Woolfenden, A.; Delpero, A.; Kim, Y.; Buchanan, N.; Gebuhr, T.C.; Hanks, S.M.; et al. Reliability of the mouse model of choroidal neovascularization induced by laser photocoagulation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6525–6534. [Google Scholar] [CrossRef]
- Fields, J.K.; Günther, S.; Sundberg, E.J. Structural Basis of IL-1 Family Cytokine Signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Biswas, L.; Zeng, Z.; Graham, A.; Shu, X. Gypenosides mediate cholesterol efflux and suppress oxidized LDL induced inflammation in retinal pigment epithelium cells. Exp. Eye Res. 2020, 191, 107931. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qiu, R.; Wang, W.; Liu, J.; Jin, X.; Li, Y.; Li, L.; Lei, B. P2X7 Receptor Antagonist Attenuates Retinal Inflammation and Neovascularization Induced by Oxidized Low-Density Lipoprotein. Oxidative Med. Cell. Longev. 2021, 2021, 5520644. [Google Scholar] [CrossRef] [PubMed]
- van Lookeren Campagne, M.; LeCouter, J.; Yaspan, B.L.; Ye, W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J. Pathol. 2014, 232, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Hogg, R.E.; Woodside, J.V.; Gilchrist, S.E.; Graydon, R.; Fletcher, A.E.; Chan, W.; Knox, A.; Cartmill, B.; Chakravarthy, U. Cardiovascular disease and hypertension are strong risk factors for choroidal neovascularization. Ophthalmology 2008, 115, 1046–1052.e2. [Google Scholar] [CrossRef]
- Shaw, P.X.; Zhang, L.; Zhang, M.; Du, H.; Zhao, L.; Lee, C.; Grob, S.; Lim, S.L.; Hughes, G.; Lee, J.; et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2012, 109, 13757–13762. [Google Scholar] [CrossRef]
- Calkin, A.C.; Tontonoz, P. Liver x receptor signaling pathways and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1513–1518. [Google Scholar] [CrossRef]
- Choudhary, M.; Ismail, E.N.; Yao, P.L.; Tayyari, F.; Radu, R.A.; Nusinowitz, S.; Boulton, M.E.; Apte, R.S.; Ruberti, J.W.; Handa, J.T.; et al. LXRs regulate features of age-related macular degeneration and may be a potential therapeutic target. JCI Insight 2020, 5, e131928. [Google Scholar] [CrossRef] [PubMed]
- Malek, G.; Lad, E.M. Emerging roles for nuclear receptors in the pathogenesis of age-related macular degeneration. Cell. Mol. Life Sci. CMLS 2014, 71, 4617–4636. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef]
- Do, J.Y.; Kim, J.; Kim, M.J.; Lee, J.Y.; Park, S.Y.; Yanai, R.; Lee, I.K.; Park, S.; Park, D.H. Fursultiamine Alleviates Choroidal Neovascularization by Suppressing Inflammation and Metabolic Reprogramming. Investig. Ophthalmol. Vis. Sci. 2020, 61, 24. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Wei, X.; Dang, K.; Tao, M.; Lv, B.; Chen, T.; Zhang, Z.; Zhou, J.; Du, H. Liver X Receptor Agonist Inhibits Oxidized Low-Density Lipoprotein Induced Choroidal Neovascularization via the NF-κB Signaling Pathway. J. Clin. Med. 2023, 12, 1674. https://doi.org/10.3390/jcm12041674
Wu T, Wei X, Dang K, Tao M, Lv B, Chen T, Zhang Z, Zhou J, Du H. Liver X Receptor Agonist Inhibits Oxidized Low-Density Lipoprotein Induced Choroidal Neovascularization via the NF-κB Signaling Pathway. Journal of Clinical Medicine. 2023; 12(4):1674. https://doi.org/10.3390/jcm12041674
Chicago/Turabian StyleWu, Tong, Xinli Wei, Kuanrong Dang, Mengzhang Tao, Baozhen Lv, Tao Chen, Zuoming Zhang, Jian Zhou, and Hongjun Du. 2023. "Liver X Receptor Agonist Inhibits Oxidized Low-Density Lipoprotein Induced Choroidal Neovascularization via the NF-κB Signaling Pathway" Journal of Clinical Medicine 12, no. 4: 1674. https://doi.org/10.3390/jcm12041674
APA StyleWu, T., Wei, X., Dang, K., Tao, M., Lv, B., Chen, T., Zhang, Z., Zhou, J., & Du, H. (2023). Liver X Receptor Agonist Inhibits Oxidized Low-Density Lipoprotein Induced Choroidal Neovascularization via the NF-κB Signaling Pathway. Journal of Clinical Medicine, 12(4), 1674. https://doi.org/10.3390/jcm12041674





