Demodicosis in Different Age Groups and Alternative Treatment Options—A Review
Abstract
1. Introduction
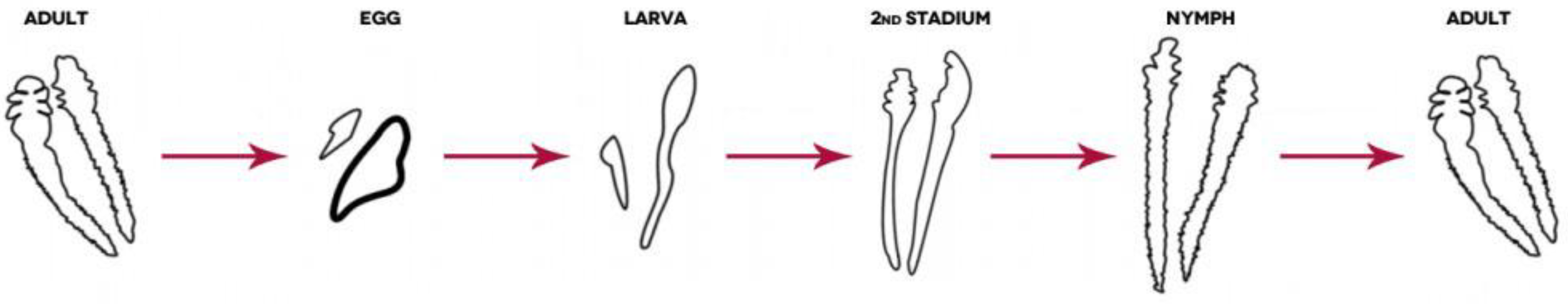
Demodex spp.
2. Literature Search Strategy
3. Demodicosis Etiology and Coinfections
3.1. Immunological Response
3.2. Demodex spp. and Coinfections
4. Incidence of Demodicosis in Different Patient Groups
4.1. Demodicosis in Children and Teenagers
4.2. Demodicosis in Adults and Elderly
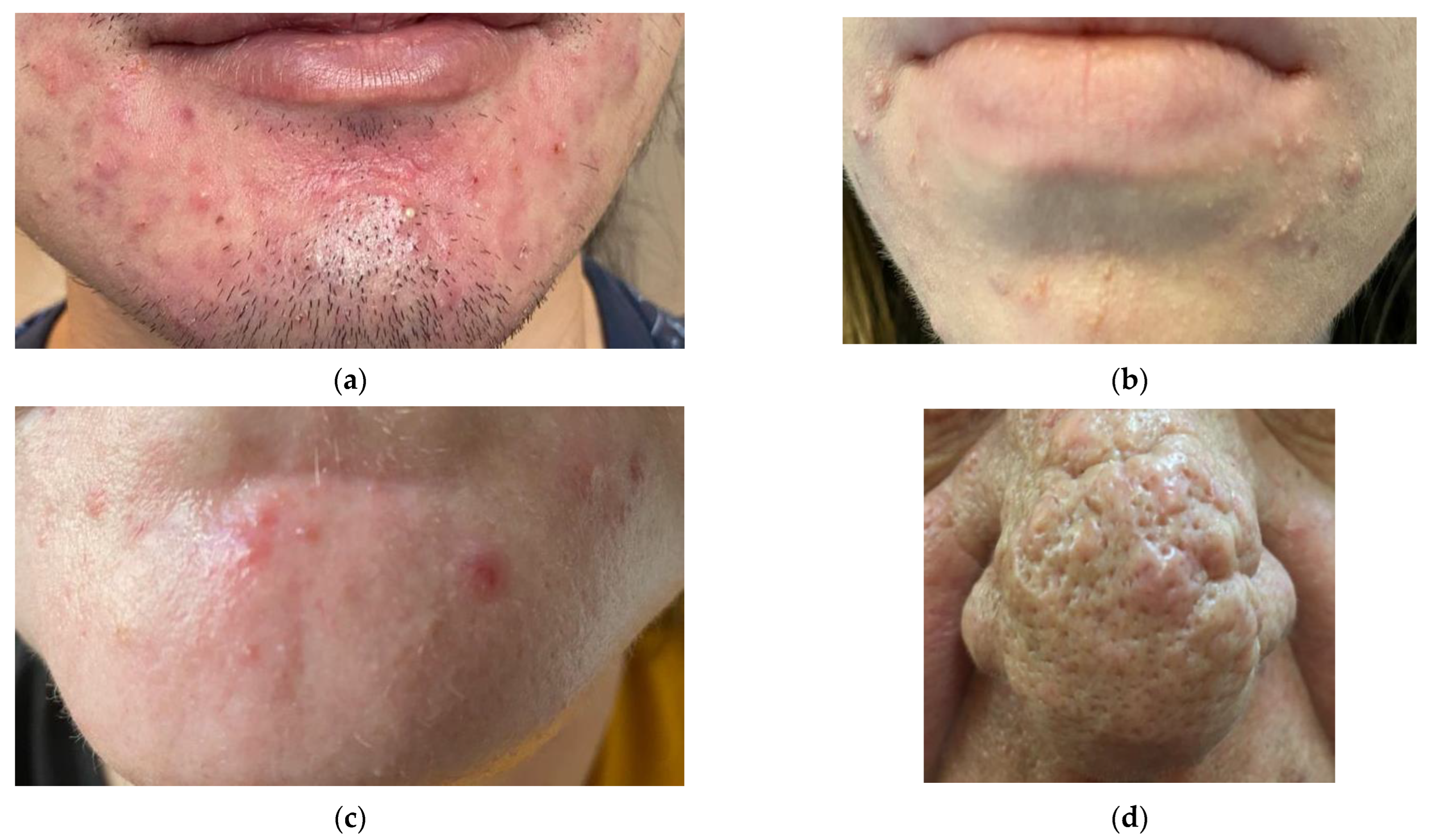
5. Demodicosis—Predisposing Factors and Clinical Presentation
6. Diagnosis
7. Treatment
7.1. Antibiotics
7.2. Essential Oils
| Available Treatment Options in Demodicosis: | |||
|---|---|---|---|
| Antibiotics: | Properties | Treatment | References |
| Ivermectin |
|
| [100,118,119,120] |
| Metronidazole |
|
| [9,107,121,122,123] |
| Alternative treatment: | Properties | Treatment | References |
| Tea tree oil |
|
| [103,105,106] |
| Salvia oil |
|
| [9] |
| Peppermint oil |
|
| [9,105,108] |
| Castor oil |
|
| [106,110] |
| Black seed oil |
|
| [111,112,113] |
| Bergamot oil (BEO) |
|
| [108,116,117] |
8. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Sheha, H.; Tseng, S.C. Pathogenic role of Demodex mites in blepharitis. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 505. [Google Scholar] [CrossRef]
- Thoemmes, M.S.; Fergus, D.J.; Urban, J.; Trautwein, M.; Dunn, R.R. Ubiquity and diversity of human-associated Demodex mites. PLoS ONE 2014, 9, e106265. [Google Scholar] [CrossRef]
- Lacey, N.; Kavanagh, K.; Tseng, S.C. Under the lash: Demodex mites in human diseases. Biochemist 2009, 31, 20–24. [Google Scholar] [CrossRef]
- Forton, F. The pathogenic role of Demodex mites in Rosacea: A potential therapeutic target already in erythematotelangiectatic Rosacea? Dermatol. Ther. 2020, 10, 1229–1253. [Google Scholar] [CrossRef]
- Patrizi, A.; Neri, I.; Chieregato, C.; Misciali, M. Demodicidosis in immunocompetent young children: Report of eight cases. Dermatology 1997, 195, 239–242. [Google Scholar] [CrossRef]
- Kaya, S.; Selimoglu, M.A.; Kaya, O.A.; Ozgen, U. Prevalence of Demodex folliculorum and Demodex brevis in childhood malnutrition and malignancy. Pediatr. Int. 2013, 55, 85–89. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Y.; Wen, K.; Jin, L.; Chen, C.; Chen, Q.; Zeng, Y.; Liang, L. Prevalence of Ocular Demodex Infestation in Children: An Epidemiological Survey in South China. Eye Contact Lens 2021, 47, 60–64. [Google Scholar] [CrossRef]
- Navel, V.; Mulliez, A.; d’Azy, C.B.; Baker, J.S.; Malecaze, J.; Chiambaretta, F.; Dutheil, F. Efficacy of treatments for Demodex blepharitis: A systematic review and meta-analysis. Ocul. Surf. 2019, 17, 655–669. [Google Scholar] [CrossRef]
- Sedzikowska, A.; Oseka, M.; Roman, B.; Jaremko, E. Impact of Salvia and peppermint oil on the in vitro survival of Demodex mites. J. Bacteriol. Parasitol. 2015, 6, 1. [Google Scholar]
- Lacey, N.; Delaney, S.; Kavanagh, K.; Powell, F. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br. J. Dermatol. 2007, 157, 474–481. [Google Scholar] [CrossRef]
- Raszeja-Kotelba, B.; Jenerowicz, D.; Izdebska, J.N.; Bowszyc-Dmochowska, M.; Tomczak, M.; Dembińska, M. Some aspects of the skin infestation by Demodex folliculorum. Wiad. Parazytol. 2004, 50, 41–54. [Google Scholar]
- Cheng, S.; Zhang, M.; Chen, H.; Fan, W.; Huang, Y. The correlation between the microstructure of meibomian glands and ocular Demodex infestation: A retrospective case-control study in a Chinese population. Medicine 2019, 98, e15595. [Google Scholar] [CrossRef]
- Jing, X.; Shuling, G.; Ying, L. Environmental scanning electron microscopy observation of the ultrastructure of Demodex. Microsc. Res. Tech. 2005, 68, 284–289. [Google Scholar] [CrossRef]
- Lacey, N.; Raghallaigh, S.N.; Powell, F.C. Demodex mites-commensals, parasites or mutualistic organisms? Dermatology 2011, 222, 128. [Google Scholar] [CrossRef]
- Udomwech, L.; Phasuk, N. Multiple Eyelid Signs are Suggestive of Demodex Infestation. Clin. Ophthalmol. 2021, 15, 671. [Google Scholar] [CrossRef]
- Amescua, G.; Akpek, E.K.; Farid, M.; Garcia-Ferrer, F.J.; Lin, A.; Rhee, M.K.; Varu, D.M.; Musch, D.C.; Dunn, S.P.; Mah, F.S. Blepharitis preferred practice pattern®. Ophthalmology 2019, 126, P56–P93. [Google Scholar] [CrossRef]
- Jimenez-Acosta, F.; Planas, L.; Penneys, N. Demodex mites contain immunoreactive lipase. Arch. Dermatol. 1989, 125, 1436–1437. [Google Scholar] [CrossRef]
- Paichitrojjana, A. Demodex: The worst enemies are the ones that used to be friends. Dermatol. Rep. 2022, 14, 9339. [Google Scholar] [CrossRef]
- Aktaş Karabay, E.; Aksu Çerman, A. Demodex folliculorum infestations in common facial dermatoses: Acne vulgaris, rosacea, seborrheic dermatitis. An. Bras. De Dermatol. 2020, 95, 187–193. [Google Scholar] [CrossRef]
- Ricardo-Gonzalez, R.R.; Kotas, M.E.; O’Leary, C.E.; Singh, K.; Damsky, W.; Liao, C.; Arouge, E.; Tenvooren, I.; Marquez, D.M.; Schroeder, A.W. Innate type 2 immunity controls hair follicle commensalism by Demodex mites. Immunity 2022, 55, 1891–1908.e12. [Google Scholar] [CrossRef]
- Rather, P.A.; Hassan, I. Human demodex mite: The versatile mite of dermatological importance. Indian J. Dermatol. 2014, 59, 60. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Aguh, C. Approach to treatment of refractory dissecting cellulitis of the scalp: A systematic review. J. Dermatol. Treat. 2021, 32, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, J.; Chen, C.; Tseng, S.; Liang, L. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea 2017, 36 (Suppl. S1), S9. [Google Scholar] [CrossRef]
- Kim, J.T.; Lee, S.H.; Chun, Y.S.; Kim, J.C. Tear cytokines and chemokines in patients with Demodex blepharitis. Cytokine 2011, 53, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-B.; Ding, Y.-H.; He, W. The association between demodex infestation and ocular surface manifestations in meibomian gland dysfunction. Int. J. Ophthalmol. 2018, 11, 589. [Google Scholar] [PubMed]
- Kot, K.; Czepita, M.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Czepita, D. Blepharitis and Demodex spp. infection. Ophthalmol. J. 2017, 2, 22–27. [Google Scholar] [CrossRef]
- Cheng, A.M.; Hwang, J.; Dermer, H.; Galor, A. Prevalence of ocular demodicosis in an older population and its association with symptoms and signs of dry eye. Cornea 2021, 40, 995–1001. [Google Scholar] [CrossRef]
- Yamasaki, K.; Gallo, R.L. Rosacea as a disease of cathelicidins and skin innate immunity. In Journal of Investigative Dermatology Symposium Proceedings; Elsevier: Amsterdam, The Netherlands, 2011; pp. 12–15. [Google Scholar]
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea: Part I. Introduction, categorization, histology, pathogenesis, and risk factors. J. Am. Acad. Dermatol. 2015, 72, 749–758. [Google Scholar] [CrossRef]
- Tsutsumi, Y. Deposition of IgD, alpha-1-antitrypsin and alpha-1-antichymotrypsin on Demodex folliculorum and D. brevis infesting the pilosebaceous unit. Pathol. Int. 2004, 54, 32–34. [Google Scholar] [CrossRef]
- Lacey, N.; Russell-Hallinan, A.; Zouboulis, C.; Powell, F. Demodex mites modulate sebocyte immune reaction: Possible role in the pathogenesis of rosacea. Br. J. Dermatol. 2018, 179, 420–430. [Google Scholar] [CrossRef]
- Wolf, R.; Ophir, J.; Avigad, J.; Lengy, J.; Krakowski, A. The hair follicle mites (Demodex spp.). Could they be vectors of pathogenic microorganisms? Acta Derm. Venereol. 1988, 68, 535–537. [Google Scholar]
- Liang, L.; Safran, S.; Gao, Y.; Sheha, H.; Raju, V.; Tseng, S.C. Ocular demodicosis as a potential cause of pediatric blepharoconjunctivitis. Cornea 2010, 29, 1386–1391. [Google Scholar] [CrossRef]
- Rabensteiner, D.F.; Aminfar, H.; Boldin, I.; Nitsche-Resch, M.; Berisha, B.; Schwantzer, G.; Horwath-Winter, J. Demodex mite infestation and its associations with tear film and ocular surface parameters in patients with ocular discomfort. Am. J. Ophthalmol. 2019, 204, 7–12. [Google Scholar] [CrossRef]
- Zhu, M.; Cheng, C.; Yi, H.; Lin, L.; Wu, K. Quantitative analysis of the bacteria in blepharitis with Demodex infestation. Front. Microbiol. 2018, 9, 1719. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, D.H.; Jung, J.Y.; Kim, J.C.; Jeon, C.O. Comparative ocular microbial communities in humans with and without blepharitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5585–5593. [Google Scholar] [CrossRef]
- Vanam, H.P.; Mohanram, K.; Poojari, S.S.; Anuradha, P.; Kandi, V. First report of concomitant tinea faciei and pityriasis folliculorum: A dermatomicrobiological rarity. Cureus 2018, 10, e3017. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Xiong, K.; Chen, S.; Li, Z.; Zhang, Z.; Xia, Z.; Yi, G.; Fu, M. Demodex infection changes ocular surface microbial communities, in which meibomian gland dysfunction may play a role. Ophthalmol. Ther. 2021, 10, 601–617. [Google Scholar] [CrossRef]
- Oakley, C.; Nicholls, S.; Tan, A.; Vote, B. Demodex species in human ocular disease: New clinicopathological aspects. Int. Ophthalmol. 2017, 37, 303–312. [Google Scholar]
- Hung, K.-H.; Lan, Y.-H.; Lin, J.-Y.; Kang, E.Y.-C.; Tan, H.-Y.; Chen, H.-C.; Hsiao, C.-H.; Yeh, L.-K. Potential Role and Significance of Ocular Demodicosis in Patients with Concomitant Refractory Herpetic Keratitis. Clin. Ophthalmol. 2020, 14, 4469. [Google Scholar] [CrossRef]
- Kim, H.S. Microbiota in rosacea. Am. J. Clin. Dermatol. 2020, 21, 25–35. [Google Scholar] [CrossRef]
- O’reilly, N.; Bergin, D.; Reeves, E.; McElvaney, N.; Kavanagh, K. Demodex-associated bacterial proteins induce neutrophil activation. Br. J. Dermatol. 2012, 166, 753–760. [Google Scholar] [CrossRef]
- McMahon, F.; Banville, N.; Bergin, D.A.; Smedman, C.; Paulie, S.; Reeves, E.; Kavanagh, K. Activation of neutrophils via IP3 pathway following exposure to demodex-associated bacterial proteins. Inflammation 2016, 39, 425–433. [Google Scholar] [CrossRef]
- Whitfeld, M.; Gunasingam, N.; Leow, L.J.; Shirato, K.; Preda, V. Staphylococcus epidermidis: A possible role in the pustules of rosacea. J. Am. Acad. Dermatol. 2011, 64, 49–52. [Google Scholar] [CrossRef]
- Dahl, M.V.; Ross, A.J.; Schlievert, P.M. Temperature regulates bacterial protein production: Possible role in rosacea. J. Am. Acad. Dermatol. 2004, 50, 266–272. [Google Scholar] [CrossRef]
- Holmes, A.D. Potential role of microorganisms in the pathogenesis of rosacea. J. Am. Acad. Dermatol. 2013, 69, 1025–1032. [Google Scholar] [CrossRef]
- Yan, Y.; Yao, Q.; Lu, Y.; Shao, C.; Sun, H.; Li, Y.; Fu, Y. Association between Demodex infestation and ocular surface microbiota in patients with Demodex blepharitis. Front. Med. 2020, 7, 592759. [Google Scholar] [CrossRef]
- Czepita, D.; Kuźna-Grygiel, W.; Kosik-Bogacka, D. Badania nad występowaniem oraz rolą Demodex folliculorum i Demodex brevis w patogenezie przewlekłego zapalenia brzegów powiek. Klin. Ocz. 2005, 107, 80–82. [Google Scholar]
- Karincaoglu, Y.; Esrefoglu Seyhan, M.; Bayram, N.; Aycan, O.; Taskapan, H. Incidence of Demodex folliculorum in patients with end stage chronic renal failure. Ren. Fail. 2005, 27, 495–499. [Google Scholar] [CrossRef]
- Morrás, P.G.; Santos, S.P.; Imedio, I.L.; Echeverría, M.L.; Hermosa, J.M.H. Rosacea-like demodicidosis in an immunocompromised child. Pediatr. Dermatol. 2003, 20, 28–30. [Google Scholar] [CrossRef]
- Ashack, R.; Frost, M.; Norins, A. Papular pruritic eruption of Demodex folliculitis in patients with acquired immunodeficiency syndrome. J. Am. Acad. Dermatol. 1989, 21, 306–307. [Google Scholar] [CrossRef]
- Damian, D.; Rogers, M. Demodex infestation in a child with leukaemia: Treatment with ivermectin and permethrin. Int. J. Dermatol. 2003, 42, 724–726. [Google Scholar] [CrossRef]
- Guerrero-González, G.A.; Herz-Ruelas, M.E.; Gómez-Flores, M.; Ocampo-Candiani, J. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep. Dermatol. Med. 2014, 2014, 458046. [Google Scholar] [CrossRef]
- Huang, J.; Guo, M.-X.; Xiang, D.-M.; Yan, L.-F.; Yu, Y.; Han, L.; Wang, J.-X.; Lu, X.-H. The association of demodex infestation with pediatric chalazia. BMC Ophthalmol. 2022, 22, 124. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, L.; Shen, L.; Yang, C. High Load of Demodex in Young Children With Chalazia. J. Pediatr. Ophthalmol. Strabismus 2022, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Liao, Y.; Liang, L. Age differences in ocular demodicosis: Demodex profiles and clinical manifestations. Ann. Transl. Med. 2021, 9, 791. [Google Scholar] [CrossRef]
- Elston, D.M. Demodex mites: Facts and controversies. Clin. Dermatol. 2010, 28, 502–504. [Google Scholar] [CrossRef]
- Özdemir, M.H.; Aksoy, U.; Sönmez, E.; Aksu, Ç.; Yorulmaz, C.; Hilal, A. Prevalence of Demodex in health personnel working in the autopsy room. Am. J. Forensic Med. Pathol. 2005, 26, 18–23. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Bartosik, K.; Przydatek-Tyrajska, R.; Dybicz, M. Shared makeup cosmetics as a route of Demodex folliculorum infections. Acta Parasitol. 2021, 66, 631–637. [Google Scholar] [CrossRef]
- Vargas-Arzola, J.; Segura-Salvador, A.; Torres-Aguilar, H.; Urbina-Mata, M.; Aguilar-Ruiz, S.; Díaz-Chiguer, D.L.; Márquez-Navarro, A.; Morales-Reyes, L.; Alvarado-Vásquez, N.; Nogueda-Torres, B. Prevalence and risk factors to Demodex folliculorum infection in eyelash follicles from a university population of Mexico. Acta Microbiol. Et Immunol. Hung. 2020, 67, 156–160. [Google Scholar] [CrossRef]
- Biernat, M.M.; Rusiecka-Ziółkowska, J.; Piątkowska, E.; Helemejko, I.; Biernat, P.; Gościniak, G. Occurrence of Demodex species in patients with blepharitis and in healthy individuals: A 10-year observational study. Jpn. J. Ophthalmol. 2018, 62, 628–633. [Google Scholar] [CrossRef]
- Horváth, A.; Neubrandt, D.; Ghidán, Á.; Nagy, K. Risk factors and prevalence of Demodex mites in young adults. Acta Microbiol. Et Immunol. Hung. 2011, 58, 145–155. [Google Scholar] [CrossRef]
- Zhao, Y.E.; Guo, N.; Wu, L.P. The effect of temperature on the viability of Demodex folliculorum and Demodex brevis. Parasitol. Res. 2009, 105, 1623–1628. [Google Scholar] [CrossRef]
- Erbagci, Z.; Erbagci, I.; Erkiliç, S. High incidence of demodicidosis in eyelid basal cell carcinomas. Int. J. Dermatol. 2003, 42, 567–571. [Google Scholar] [CrossRef]
- Sun, J.; Gui, X.; He, J.; Liu, H.; Yu, H.; Xia, C.; Xu, Y. The relationship between infestation of Demodex folliculorum and epidermal neoplasm on face. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi Chin. J. Parasitol. Parasit. Dis. 2005, 23, 428–431. [Google Scholar]
- Kubanov, A.; Gallyamova, Y.; Kravchenko, A. Clinical picture, diagnosis and treatment of rosacea, complicated by Demodex mites. Dermatol. Rep. 2019, 11, 7675. [Google Scholar] [CrossRef]
- Akilov, O.; Mumcuoglu, K. Association between human demodicosis and HLA class I. Clin. Exp. Dermatol. 2003, 28, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Araviiskaia, E.; Berardesca, E.; Gontijo, G.; Sanchez Viera, M.; Xiang, L.; Martin, R.; Bieber, T. Microbiome in healthy skin, update for dermatologists. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2038–2047. [Google Scholar] [CrossRef]
- Kupfer, T.R.; Fessler, D.M. Ectoparasite defence in humans: Relationships to pathogen avoidance and clinical implications. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170207. [Google Scholar] [CrossRef]
- Putnam, C.M. Diagnosis and management of blepharitis: An optometrist’s perspective. Clin. Optom. 2016, 8, 71. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Huang, Y.-C. Role of Demodex mite infestation in rosacea: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 441–447.e6. [Google Scholar] [CrossRef]
- Enginyurt, O.; Karaman, U.; Cetin, F.; Ozer, A. The prevalence of Demodex species and its relationship with the metabolic syndrome in women of Malatya province, Turkey. Jundishapur J. Microbiol. 2015, 8, e24322. [Google Scholar] [CrossRef]
- Zhong, J.; Tan, Y.; Li, S.; Peng, L.; Wang, B.; Deng, Y.; Yuan, J. The prevalence of Demodex folliculorum and Demodex brevis in cylindrical dandruff patients. J. Ophthalmol. 2019, 2019, 8949683. [Google Scholar] [CrossRef]
- AYRES, S. Demodectic Eruptions (Demodicidosis) in the human: 30 years’ experience with 2 commonly unrecognized entities: Pityriasis Folliculorum (Demodex) and Acne Rosacea (Demodex type). Arch. Dermatol. 1961, 83, 816–827. [Google Scholar] [CrossRef]
- Forton, F.; Germaux, M.-A.; Brasseur, T.; De Liever, A.; Laporte, M.; Mathys, C.; Sass, U.; Stene, J.-J.; Thibaut, S.; Tytgat, M. Demodicosis and rosacea: Epidemiology and significance in daily dermatologic practice. J. Am. Acad. Dermatol. 2005, 52, 74–87. [Google Scholar] [CrossRef]
- Forton, F. Papulopustular rosacea, skin immunity and Demodex: Pityriasis folliculorum as a missing link. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 19–28. [Google Scholar] [CrossRef]
- Hsu, C.-K.; Hsu, M.M.-L.; Lee, J.Y.-Y. Demodicosis: A clinicopathological study. J. Am. Acad. Dermatol. 2009, 60, 453–462. [Google Scholar] [CrossRef]
- Chen, W.; Plewig, G. Human demodicosis: Revisit and a proposed classification. Br. J. Dermatol. 2014, 170, 1219–1225. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Casas, V.; Li, W.; Raju, V.K.; Tseng, S.C. Corneal manifestations of ocular demodex infestation. Am. J. Ophthalmol. 2007, 143, 743–749.e1. [Google Scholar] [CrossRef]
- English, F. Demodex folliculorum and oedema of the eyelash. Br. J. Ophthalmol. 1971, 55, 742. [Google Scholar] [CrossRef]
- Kosik-Bogacka, D.I.; Łanocha, N.; Łanocha, A.; Czepita, D.; Grobelny, A.; Zdziarska, B.; Kalisińska, E. Demodex folliculorum and Demodex brevis in healthy and immunocompromised patients. Ophthalmic Epidemiol. 2013, 20, 159–163. [Google Scholar] [CrossRef]
- Cheng, A.M.; Sheha, H.; Tseng, S.C. Recent advances on ocular Demodex infestation. Curr. Opin. Ophthalmol. 2015, 26, 295–300. [Google Scholar] [CrossRef]
- Fromstein, S.R.; Harthan, J.S.; Patel, J.; Opitz, D.L. Demodex blepharitis: Clinical perspectives. Clin. Optom. 2018, 10, 57. [Google Scholar] [CrossRef]
- Inceboz, T.; Yaman, A.; Over, L.; Ozturk, A.T.; Akisu, C. Diagnosis and treatment of demodectic blepharitis. Turk. Parazitol. Derg. 2009, 33, 32–36. [Google Scholar]
- Karincaoglu, Y.; Bayram, N.; Aycan, O.; Esrefoglu, M. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J. Dermatol. 2004, 31, 618–626. [Google Scholar] [CrossRef]
- Gerber, P.A.; Kukova, G.; Buhren, B.A.; Homey, B. Density of Demodex folliculorum in patients receiving epidermal growth factor receptor inhibitors. Dermatology 2011, 222, 144–147. [Google Scholar] [CrossRef]
- Segal, R.; Mimouni, D.; Feuerman, H.; Pagovitz, O.; David, M. Report: Dermoscopy as a Diagnostic Tool in Demodicidosis; Wiley Online Library: Hoboken, NJ, USA, 2010. [Google Scholar]
- Nazzaro, G.; Farnetani, F.; Moltrasio, C.; Passoni, E.; Pellacani, G.; Berti, E. Image gallery: Demodex folliculorum longitudinal appearance with reflectance confocal microscopy. Br. J. Dermatol. 2018, 179, e230. [Google Scholar] [CrossRef]
- Aşkın, Ü.; Seçkin, D. Comparison of the two techniques for measurement of the density of Demodex folliculorum: Standardized skin surface biopsy and direct microscopic examination. Br. J. Dermatol. 2010, 162, 1124–1126. [Google Scholar] [CrossRef]
- NORN, M.S. Incidence of Demodex folliculorum on skin of lids and nose. Acta Ophthalmol. 1982, 60, 575–583. [Google Scholar] [CrossRef]
- Yun, C.H.; Yun, J.H.; Baek, J.O.; Roh, J.Y.; Lee, J.R. Demodex mite density determinations by standardized skin surface biopsy and direct microscopic examination and their relations with clinical types and distribution patterns. Ann. Dermatol. 2017, 29, 137–142. [Google Scholar] [CrossRef]
- Rufli, T.; Mumcuoglu, Y. The hair follicle mites Demodex folliculorum and Demodex brevis: Biology and medical importance. Dermatologica 1981, 162, 1–11. [Google Scholar] [CrossRef]
- Forton, F.; Seys, B. Density of Demodex folliculorum in rosacea: A case-control study using standardized skin-surface biopsy. Br. J. Dermatol. 1993, 128, 650–659. [Google Scholar] [CrossRef]
- Nashat, M.A.; Ricart Arbona, R.J.; Riedel, E.R.; Francino, O.; Ferrer, L.; Luchins, K.R.; Lipman, N.S. Comparison of diagnostic methods and sampling sites for the detection of Demodex musculi. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 173–185. [Google Scholar] [PubMed]
- Rivero, A.L.; Whitfeld, M. An update on the treatment of rosacea. Aust. Prescr. 2018, 41, 20. [Google Scholar] [CrossRef]
- Jacob, S.; VanDaele, M.A.; Brown, J.N. Treatment of Demodex-associated inflammatory skin conditions: A systematic review. Dermatol. Ther. 2019, 32, e13103. [Google Scholar] [CrossRef]
- Savla, K.; Le, J.T.; Pucker, A.D. Tea tree oil for Demodex blepharitis. Cochrane Database Syst. Rev. 2020, 2020, CD013333. [Google Scholar] [CrossRef]
- Carly, A.; Elston, B.; Dirk, M.; Elston, M. Demodex mites. Clin. Dermatol. 2014, 32, 739–743. [Google Scholar]
- Gao, Y.; Di Pascuale, M.; Li, W.; Baradaran-Rafii, A.; Elizondo, A.; Kuo, C.; Raju, V.; Tseng, S. In Vitro and In Vivo killing of ocular Demodex by tea tree oil. Br. J. Ophthalmol. 2005, 89, 1468–1473. [Google Scholar] [CrossRef]
- Salem, D.A.-B.; El-Shazly, A.; Nabih, N.; El-Bayoumy, Y.; Saleh, S. Evaluation of the efficacy of oral ivermectin in comparison with ivermectin–metronidazole combined therapy in the treatment of ocular and skin lesions of Demodex folliculorum. Int. J. Infect. Dis. 2013, 17, e343–e347. [Google Scholar] [CrossRef]
- Samuelson, J. Why metronidazole is active against both bacteria and parasites. Antimicrob. Agents Chemother. 1999, 43, 1533–1541. [Google Scholar] [CrossRef]
- Borchman, D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul. Surf. 2019, 17, 360–364. [Google Scholar] [CrossRef]
- Marchese, E.; D’onofrio, N.; Balestrieri, M.L.; Castaldo, D.; Ferrari, G.; Donsì, F. Bergamot essential oil nanoemulsions: Antimicrobial and cytotoxic activity. Z. Für Nat. C 2020, 75, 279–290. [Google Scholar] [CrossRef]
- Liu, J.X.; Sun, Y.H.; Li, C.P. Volatile oils of Chinese crude medicines exhibit antiparasitic activity against human Demodex with no adverse effects In Vivo. Exp. Ther. Med. 2015, 9, 1304–1308. [Google Scholar] [CrossRef]
- Liang, R.; Xu, S.; Shoemaker, C.F.; Li, Y.; Zhong, F.; Huang, Q. Physical and antimicrobial properties of peppermint oil nanoemulsions. J. Agric. Food Chem. 2012, 60, 7548–7555. [Google Scholar] [CrossRef]
- Sandford, E.C.; Muntz, A.; Craig, J.P. Therapeutic potential of castor oil in managing blepharitis, meibomian gland dysfunction and dry eye. Clin. Exp. Optom. 2021, 104, 315–322. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Graziano, A.C.; Formisano, C.; Rigano, D.; Canzoneri, M.; Bruno, M.; Senatore, F. Comparison of essential oil components and In Vitro anticancer activity in wild and cultivated Salvia verbenaca. Nat. Prod. Res. 2015, 29, 1630–1640. [Google Scholar] [CrossRef]
- da Silva Ramos, R.; Rodrigues, A.B.L.; Farias, A.L.F.; Simões, R.C.; Pinheiro, M.T.; Ferreira, R.M.d.A.; Costa Barbosa, L.M.; Picanço Souto, R.N.; Fernandes, J.B.; Santos, L.d.S. Chemical composition and In Vitro antioxidant, cytotoxic, antimicrobial, and larvicidal activities of the essential oil of Mentha piperita L. (Lamiaceae). Sci. World J. 2017, 2017, 4927214. [Google Scholar] [CrossRef]
- Thosar, N.; Basak, S.; Bahadure, R.N.; Rajurkar, M. Antimicrobial efficacy of five essential oils against oral pathogens: An In Vitro study. Eur. J. Dent. 2013, 7, S071–S077. [Google Scholar] [CrossRef]
- Muntz, A.; Sandford, E.; Claassen, M.; Curd, L.; Jackson, A.K.; Watters, G.; Wang, M.T.; Craig, J.P. Randomized trial of topical periocular castor oil treatment for blepharitis. Ocul. Surf. 2021, 19, 145–150. [Google Scholar] [CrossRef]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (black cumin): A promising natural remedy for wide range of illnesses. Evid.—Based Complement. Altern. Med. 2019, 2019, 1528635. [Google Scholar]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P. Black cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Eid, A.M.; Elmarzugi, N.A.; Abu Ayyash, L.M.; Sawafta, M.N.; Daana, H.I. A Review on the Cosmeceutical and External Applications of Nigella sativa. J. Trop. Med. 2017, 2017, 7092514. [Google Scholar] [CrossRef]
- Nawarathne, N.W.; Wijesekera, K.; Wijayaratne, W.M.D.G.B.; Napagoda, M. Development of novel topical cosmeceutical formulations from Nigella sativa L. with antimicrobial activity against acne-causing microorganisms. Sci. World J. 2019, 2019. [Google Scholar]
- Kausar, H.; Mujeeb, M.; Ahad, A.; Moolakkadath, T.; Aqil, M.; Ahmad, A.; Akhter, M.H. Optimization of ethosomes for topical thymoquinone delivery for the treatment of skin acne. J. Drug Deliv. Sci. Technol. 2019, 49, 177–187. [Google Scholar] [CrossRef]
- Aquilina, C.; Viraben, R.; Sire, S. Ivermectin-responsive Demodex infestation during human immunodeficiency virus infection. Dermatology 2002, 205, 394–397. [Google Scholar] [CrossRef]
- van Atteveld, J.E.; de Graaf, M.; van Grotel, M.; van den Heuvel-Eibrink, M.M. Demodicosis in pediatric cancer. J. Pediatr. Hematol. Oncol. 2017, 39, 402–406. [Google Scholar] [CrossRef]
- Forstinger, C.; Kittler, H.; Binder, M. Treatment of rosacea-like demodicidosis with oral ivermectin and topical permethrin cream. J. Am. Acad. Dermatol. 1999, 41, 775–777. [Google Scholar] [CrossRef]
- Brown, M.; Hernández-Martín, A.; Clement, A.; Colmenero, I.; Torrelo, A. Severe Demodexfolliculorum–Associated Oculocutaneous Rosacea in a Girl Successfully Treated With Ivermectin. JAMA Dermatol. 2014, 150, 61–63. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Topical ivermectin: Data supporting dual modes of action in rosacea. J. Clin. Aesthetic Dermatol. 2017, 10, 39. [Google Scholar]
- Gazi, U.; Gureser, A.S.; Oztekin, A.; Karasartova, D.; Kosar-Acar, N.; Derici, M.K.; Artuz, F.; Mumcuoglu, K.Y.; Taylan-Ozkan, A. Skin-homing T-cell responses associated with Demodex infestation and rosacea. Parasite Immunol. 2019, 41, e12658. [Google Scholar] [CrossRef]
- Gazi, U.; Taylan-Ozkan, A.; Mumcuoglu, K.Y. Immune mechanisms in human and canine demodicosis: A review. Parasite Immunol. 2019, 41, e12673. [Google Scholar] [CrossRef]
- Shamriz, O.; Lev, A.; Simon, A.J.; Barel, O.; Javasky, E.; Matza-Porges, S.; Shaulov, A.; Davidovics, Z.; Toker, O.; Somech, R. Chronic demodicosis in patients with immune dysregulation: An unexpected infectious manifestation of Signal transducer and activator of transcription (STAT) 1 gain-of-function. Clin. Exp. Immunol. 2021, 206, 56–67. [Google Scholar] [CrossRef]
- Douglas, A.; Zaenglein, A.L. A case series of demodicosis in children. Pediatr. Dermatol. 2019, 36, 651–654. [Google Scholar] [CrossRef]
- Wu, M.; Wang, X.; Han, J.; Shao, T.; Wang, Y. Evaluation of the ocular surface characteristics and Demodex infestation in paediatric and adult blepharokeratoconjunctivitis. BMC Ophthalmol. 2019, 19, 67. [Google Scholar] [CrossRef]
- Forton, F.M.; De Maertelaer, V. Rosacea and demodicosis: Little-known diagnostic signs and symptoms. Acta Derm. Venereol. 2019, 99, 47–52. [Google Scholar]
- Jorge, M.F.S.; Miguel, L.M.Z.; Braghiroli, C.S.; Schmitt, J.V. Demodicosis as treatment complication of amicrobial pustulosis of the folds. An. Bras. De Dermatol. 2018, 93, 566–569. [Google Scholar] [CrossRef]
- Ergun, S.B.; Saribas, G.S.; Yarayici, S.; Elmazoglu, Z.; Cardak, A.; Ozogul, C.; Ilhan, M.N.; Karasu, C.; Evren Kemer, O. Comparison of efficacy and safety of two tea tree oil-based formulations in patients with chronic blepharitis: A double-blinded randomized clinical trial. Ocul. Immunol. Inflamm. 2020, 28, 888–897. [Google Scholar] [CrossRef]
- Wróblewska, J.; Nuszkiewicz, J.; Wróblewski, M.; Woźniak, A. Activity of Plant Essential Oils Against Demodex folliculorum and Demodex brevis. Pielęgniarstwo Neurol. I Neurochir. 2020, 9, 160–165. [Google Scholar] [CrossRef]
- Ostlere, L.; Ashrafzadeh, P.; Harris, D.; Rustin, M.H. Response of uremic follicular hyperkeratosis to peritoneal dialysis. J. Am. Acad. Dermatol. 1992, 26, 782–783. [Google Scholar] [CrossRef] [PubMed]


| Microorganism | Symptoms | References |
|---|---|---|
| Staphylococcus spp. (S. aureus, S. epidermidis) | blepharitis, conjunctivitis, cutaneous diseases | [24,32,33,34] |
| Streptococcus spp. | blepharitis | [34] |
| Bacillus oleronius | blepharitis, rosacea | [8,33,34] |
| Propionibacterium acnes (Cutibacterium acnes) | blepharitis, acne | [35] |
| Corynebacterium spp. | blepharitis | [36] |
Funghi:
| cutaneous diseases, dermatophytosis, dermatological disorders, pityriasis folliculorum | [21,32,37] |
| Symptoms of Demodicosis—Summary: | |
|---|---|
| Skin demodicosis: | |
| rash, acne, rosacea, unilateral rosacea (unilateral Demodex sp. folliculitis), pustules, purulent eruptions, erythema, boils, crusts | [35,83,84] |
| swelling around skin lesions, burning and itching of the skin, a tickling feeling | [85] |
| calluses, hyperplasia, peeling of the epidermis (hyperkeratosis), dry skin | [3] |
| inflammation of follicles | [85,86] |
| hair loss | [21,83] |
| blockage of the sebaceous ducts, the formation of blackheads | [83,87,88] |
| widening of blood vessels leading to skin hyperemia | [21,84] |
| skin infections caused by bacteria and fungi | [24,34] |
| Eye demodicosis: | |
| loss of eyelashes and eyebrows | [21,83] |
| posterior and/or anterior blepharitis | [24,32,33,34,83] |
| eye infections caused by bacteria and fungi | [24,34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudzicka-Strugała, I.; Gołębiewska, I.; Brudecki, G.; Elamin, W.; Zwoździak, B. Demodicosis in Different Age Groups and Alternative Treatment Options—A Review. J. Clin. Med. 2023, 12, 1649. https://doi.org/10.3390/jcm12041649
Chudzicka-Strugała I, Gołębiewska I, Brudecki G, Elamin W, Zwoździak B. Demodicosis in Different Age Groups and Alternative Treatment Options—A Review. Journal of Clinical Medicine. 2023; 12(4):1649. https://doi.org/10.3390/jcm12041649
Chicago/Turabian StyleChudzicka-Strugała, Izabela, Iwona Gołębiewska, Grzegorz Brudecki, Wael Elamin, and Barbara Zwoździak. 2023. "Demodicosis in Different Age Groups and Alternative Treatment Options—A Review" Journal of Clinical Medicine 12, no. 4: 1649. https://doi.org/10.3390/jcm12041649
APA StyleChudzicka-Strugała, I., Gołębiewska, I., Brudecki, G., Elamin, W., & Zwoździak, B. (2023). Demodicosis in Different Age Groups and Alternative Treatment Options—A Review. Journal of Clinical Medicine, 12(4), 1649. https://doi.org/10.3390/jcm12041649





