A Systematic Pan-Cancer Analysis of MEIS1 in Human Tumors as Prognostic Biomarker and Immunotherapy Target
Abstract
1. Introduction
2. Materials and Methods
2.1. The Analysis of MEIS1 Expression in Different Cancers
2.2. Association of MEIS1 with Survival in Different Tumors
2.3. Gene Alteration and Immune Infiltration Analysis of MEIS1
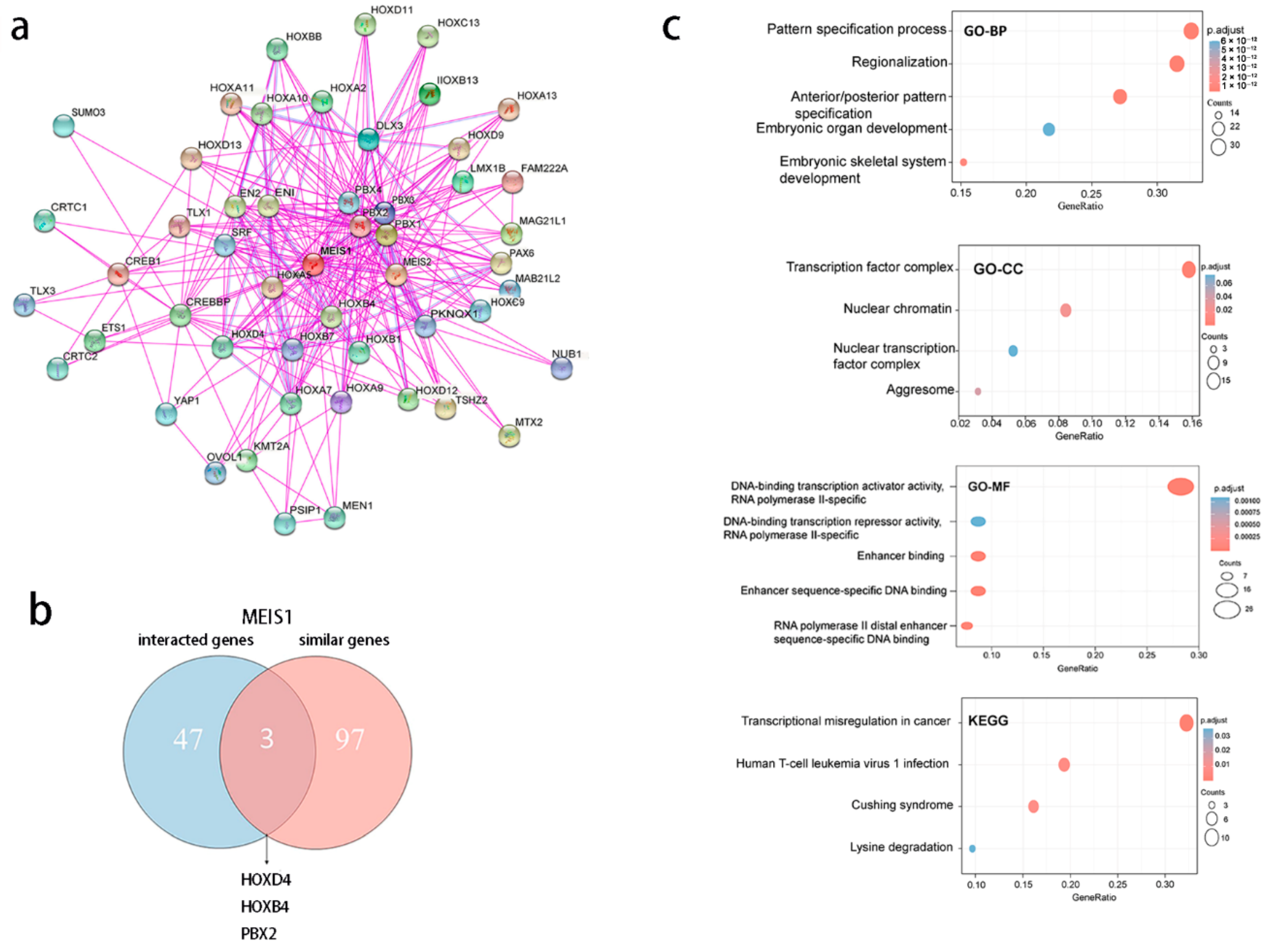
2.4. Gene Enrichment Analysis of MEIS1
2.5. Ethical Approval
3. Results
3.1. Differential Expression of MEIS1 in Cancers
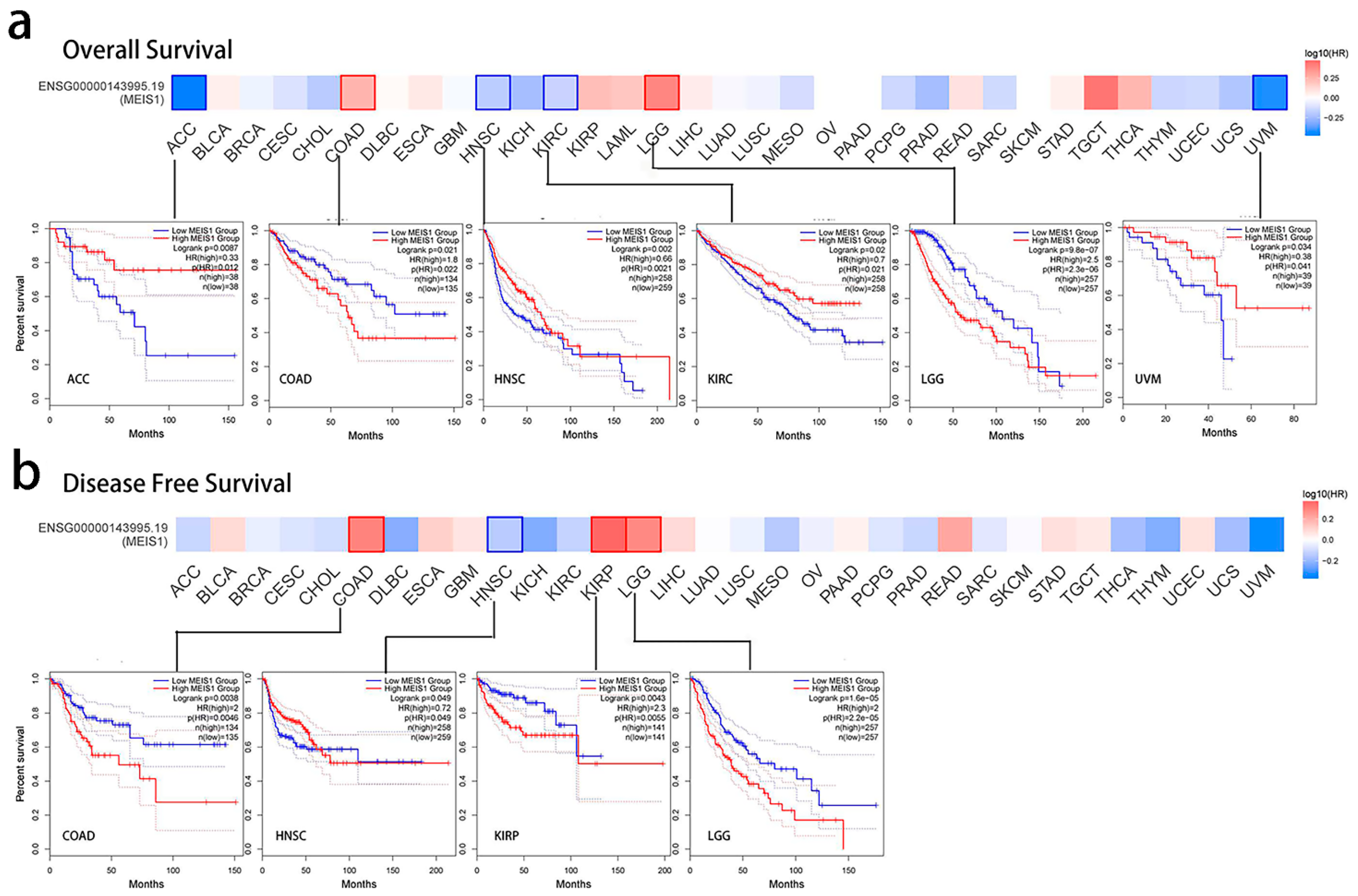
3.2. Prognostic Index of MEIS1 in Different Cancers
3.3. Alteration of MEIS1 Gene Analysis Data
3.4. Phosphorylation and Promoter Methylation Expression of MEIS1 across Different Cancers
3.5. MEIS1 Expression in Immune and Molecular Subtypes across Different Cancers
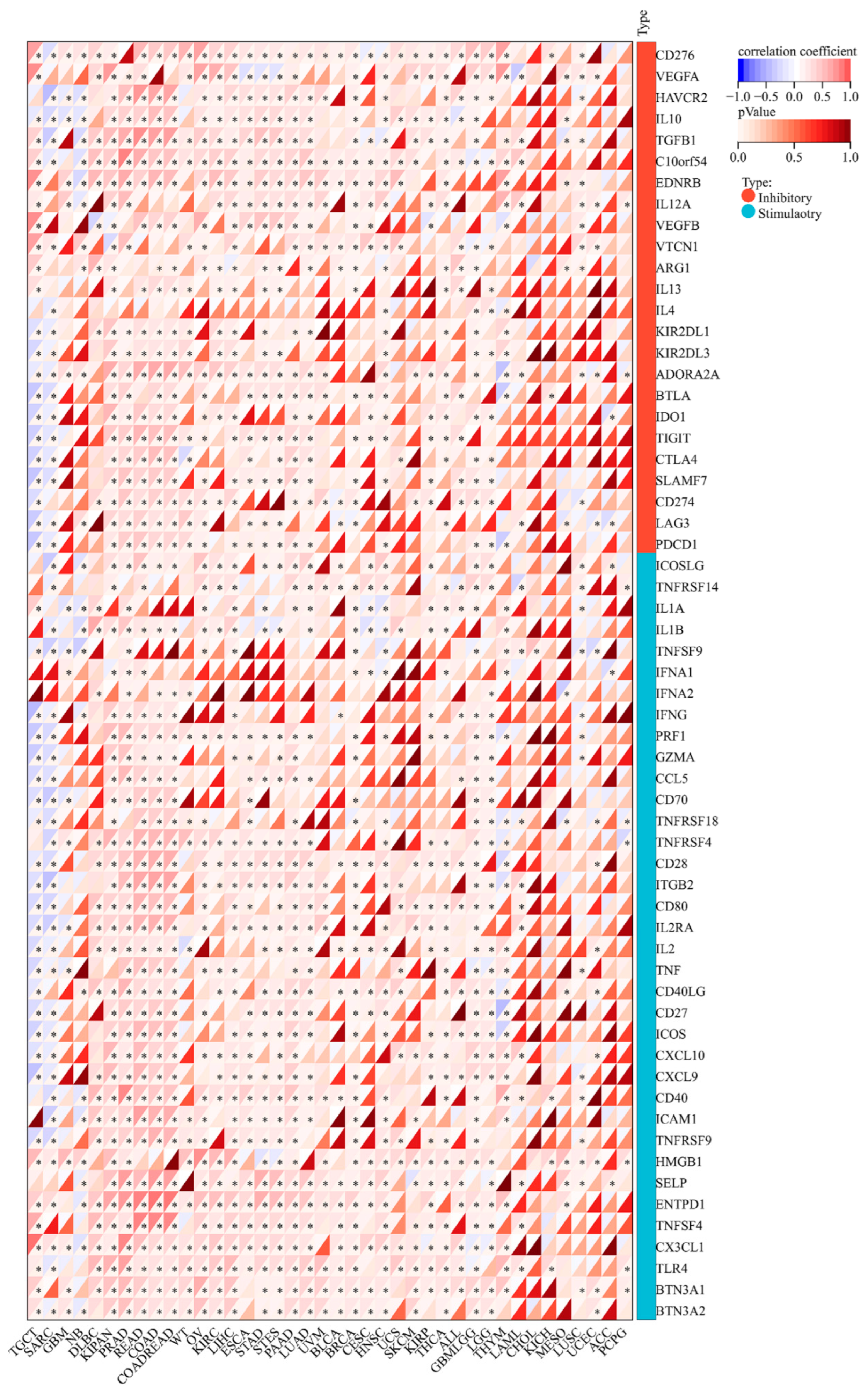
3.6. MEIS1 Expression Is Related to ICPGs Expression in Different Cancers
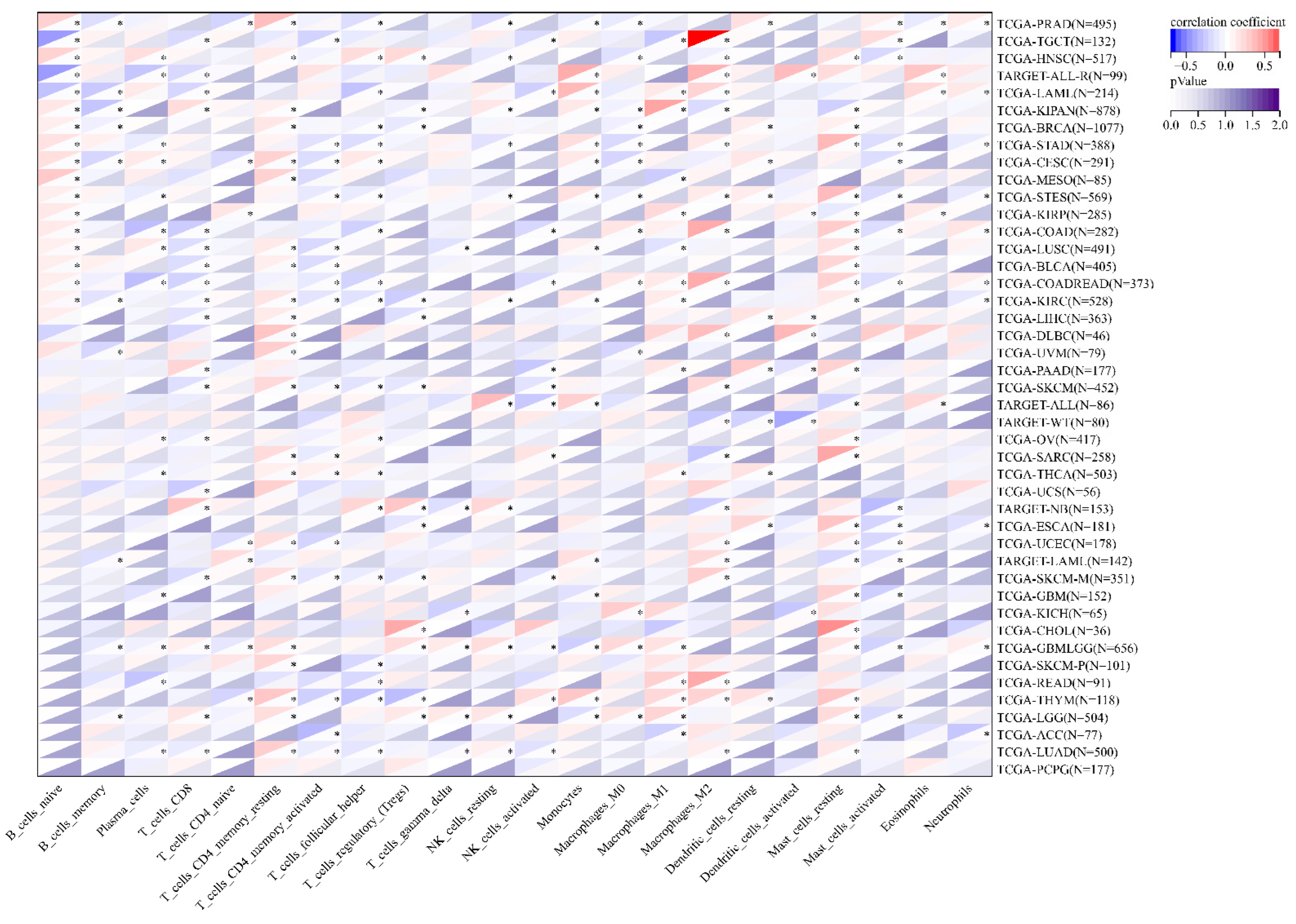
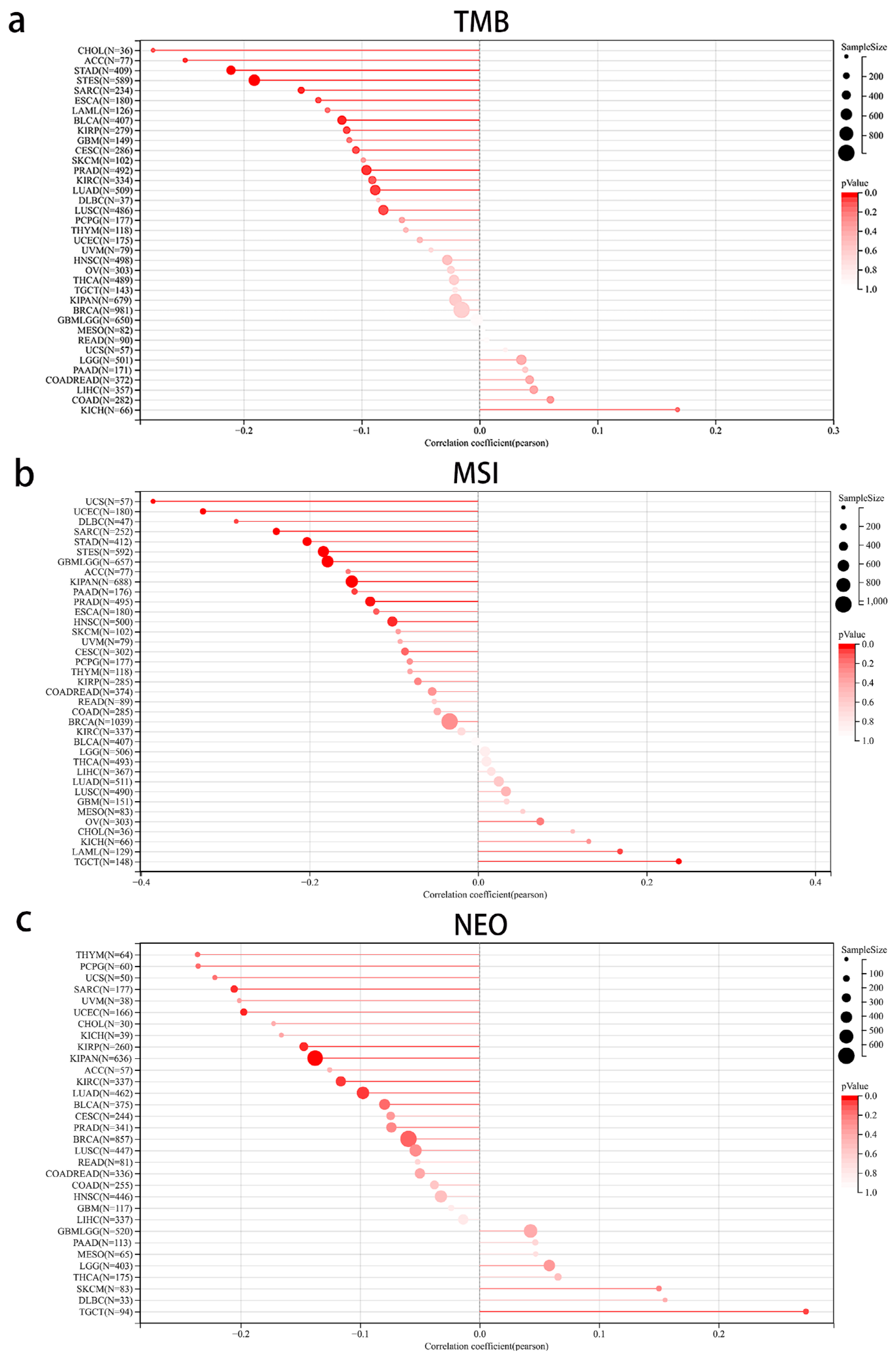
3.7. The Correlation between MEIS1 Expression and Immune Cell Infiltration and TME in Different Cancers
3.8. MEIS1-Related Genes Are Correlated to Cell Proliferation and Differentiation
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, Y.; Huang, Y.; Gao, L. Current situation of immuno-oncology development. Sci. Technol. Rev. 2017, 35, 61–65. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Types of Cancer Treatment. Available online: https://www.cancer.gov/about-cancer/treatment/types (accessed on 19 January 2023).
- De Miguel, M.; Calvo, E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Veeraraghavan, J.; Philip, R. Translating Immuno-oncology Biomarkers to Diagnostic Tests: A Regulatory Perspective. Methods Mol. Biol. 2020, 2055, 701–716. [Google Scholar]
- Masucci, G.V.; Cesano, A.; Hawtin, R.; Janetzki, S.; Zhang, J.; Kirsch, I.; Dobbin, K.K.; Alvarez, J.; Robbins, P.B.; Selvan, S.R.; et al. Validation of biomarkers to predict response to immunotherapy in cancer: Volume I—Pre-analytical and analytical validation. J. Immunother. Cancer 2016, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, X.; Nie, K.; Cheng, L.; Zhang, Z.; Hu, Y.; Peng, W. Systematic Pan-Cancer Analysis Identifies TREM2 as an Immunological and Prognostic Biomarker. Front. Immunol. 2021, 12, 646523. [Google Scholar] [CrossRef]
- Jain, K.K. Personalized Immuno-Oncology. Med. Princ. Pract. 2021, 30, 1–16. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, S.; Bai, M.; Zhang, A. The emerging role of MEIS1 in cell proliferation and differentiation. Am. J. Physiol. Cell Physiol. 2021, 320, C264–C269. [Google Scholar] [CrossRef]
- Aksoz, M.; Turan, R.D.; Albayrak, E.; Kocabas, F. Emerging Roles of Meis1 in Cardiac Regeneration, Stem Cells and Cancer. Curr. Drug Targets 2018, 19, 181–190. [Google Scholar] [CrossRef]
- Zargari, S.; Negahban Khameneh, S.; Rad, A.; Forghanifard, M.M. MEIS1 promotes expression of stem cell markers in esophageal squamous cell carcinoma. BMC Cancer 2020, 20, 789. [Google Scholar] [CrossRef]
- Paul, S.; Zhang, X.; He, J.Q. Homeobox gene Meis1 modulates cardiovascular regeneration. Semin. Cell Dev. Biol. 2020, 100, 52–61. [Google Scholar] [CrossRef]
- Whitlock, N.C.; Trostel, S.Y.; Wilkinson, S.; Terrigino, N.T.; Hennigan, S.T.; Lake, R.; Carrabba, N.V.; Atway, R.; Walton, E.D.; Gryder, B.E.; et al. MEIS1 down-regulation by MYC mediates prostate cancer development through elevated HOXB13 expression and AR activity. Oncogene 2020, 39, 5663–5674. [Google Scholar] [CrossRef]
- Yokoyama, T.; Nakatake, M.; Kuwata, T.; Couzinet, A.; Goitsuka, R.; Tsutsumi, S.; Aburatani, H.; Valk, P.J.; Delwel, R.; Nakamura, T. MEIS1-mediated transactivation of synaptotagmin-like, 1 promotes CXCL12/CXCR4 signaling and leukemogenesis. J. Clin. Invest. 2016, 126, 1664–1678. [Google Scholar] [CrossRef]
- Li, Y.; Gan, Y.; Liu, J.; Li, J.; Zhou, Z.; Tian, R.; Sun, R.; Liu, J.; Xiao, Q.; Li, Y.; et al. Downregulation of MEIS1 mediated by ELFN1-AS1/EZH2/DNMT3a axis promotes tumorigenesis and oxaliplatin resistance in colorectal cancer. Signal Transduct. Target. Ther. 2022, 7, 87. [Google Scholar] [CrossRef]
- Eagle, K.; Harada, T.; Kalfon, J.; Perez, M.W.; Heshmati, Y.; Ewers, J.; Koren, J.V.; Dempster, J.M.; Kugener, G.; Paralkar, V.R.; et al. Transcriptional Plasticity Drives Leukemia Immune Escape. Blood Cancer Discov. 2022, 3, 394–409. [Google Scholar] [CrossRef]
- Walker, A.R.; Byrd, J.C.; Blachly, J.S.; Bhatnagar, B.; Mims, A.S.; Orwick, S.; Lin, T.L.; Crosswell, H.E.; Zhang, D.; Minden, M.D.; et al. Entospletinib in Combination with Induction Chemotherapy in Previously Untreated Acute Myeloid Leukemia: Response and Predictive Significance of HOXA9 and MEIS1 Expression. Clin. Cancer Res. 2020, 26, 5852–5859. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.V.; Chaney, K.E.; Choi, K.; Largaespada, D.A.; Kumar, A.R.; Ratner, N. An ShRNA Screen Identifies MEIS1 as a Driver of Malignant Peripheral Nerve Sheath Tumors. EBioMedicine 2016, 9, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.J.; Heyn, H.; Vizoso, M.; Moutinho, C.; Vidal, E.; Gomez, A.; Martínez-Cardús, A.; Simó-Riudalbas, L.; Moran, S.; Jost, E.; et al. DNMT3A mutations mediate the epigenetic reactivation of the leukemogenic factor MEIS1 in acute myeloid leukemia. Oncogene 2017, 36, 4233. [Google Scholar] [CrossRef]
- Geerts, D.; Schilderink, N.; Jorritsma, G.; Versteeg, R. The role of the MEIS homeobox genes in neuroblastoma. Cancer Lett. 2003, 197, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Saito, M.; Isogai, E.; Aoto, Y.; Hachiya, T.; Sakakibara, Y.; Katsuragi, Y.; Hirose, S.; Kominami, R.; Goitsuka, R.; et al. Meis1 regulates epidermal stem cells and is required for skin tumorigenesis. PLoS ONE 2014, 9, e102111. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Chen, H.; Han, L.; Ji, X.; Wang, Q.; Wei, L.; Miao, Y.; Wang, J.; Mao, J.; et al. Decreased HLF Expression Predicts Poor Survival in Lung Adenocarcinoma. Med. Sci. Monit. 2021, 27, e929333. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Gu, Y.; Yang, Z.; Zhong, K.; Chen, Z. MEIS1 and its potential as a cancer therapeutic target. Int. J. Mol. Med. 2021, 48, 181. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, K.; Guo, H.; Cui, G. Meis1 regulates proliferation of non-small-cell lung cancer cells. J. Thorac. Dis. 2014, 6, 850–855. [Google Scholar] [PubMed]
- Rad, A.; Farshchian, M.; Forghanifard, M.M.; Matin, M.M.; Bahrami, A.R.; Geerts, D.; A’rabi, A.; Memar, B.; Abbaszadegan, M.R. Predicting the molecular role of MEIS1 in esophageal squamous cell carcinoma. Tumour. Biol. 2016, 37, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Huang, M.; Shi, X.; Mayakonda, A.; Hu, K.; Jiang, Y.Y.; Guo, X.; Chen, L.; Pang, B.; Doan, N.; et al. Super-enhancer-associated MEIS1 promotes transcriptional dysregulation in Ewing sarcoma in co-operation with EWS-FLI1. Nucleic Acids Res. 2019, 47, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Johng, D.; Torga, G.; Ewing, C.M.; Jin, K.; Norris, J.D.; Mcdonnell, D.P.; Isaacs, W.B. HOXB13 interaction with MEIS1 modifies proliferation and gene expression in prostate cancer. Prostate 2019, 79, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Vanopstall, C.; Perike, S.; Brechka, H.; Gillard, M.; Lamperis, S.; Zhu, B.; Brown, R.; Bhanvadia, R.; Vander Griend, D.J. MEIS-mediated suppression of human prostate cancer growth and metastasis through HOXB13-dependent regulation of proteoglycans. Elife 2020, 9, e53600. [Google Scholar] [CrossRef]
- Meng, L.; Tian, Z.; Wang, J.; Liu, X.; Zhang, W.; Hu, M.; Wang, M.; Zhang, Y. Effect of myeloid ecotropic viral integration site (MEIS) family genes on tumor microenvironment remodeling and its potential therapeutic effect. Transl. Androl. Urol. 2021, 10, 594–608. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Chen, F.; Fan, Y.; Cao, P.; Liu, B.; Hou, J.; Zhang, B.; Tan, K. Pan-Cancer Analysis of the Prognostic and Immunological Role of HSF1: A Potential Target for Survival and Immunotherapy. Oxid. Med. Cell. Longev. 2021, 2021, 5551036. [Google Scholar] [CrossRef]
- Schulte, D.; Geerts, D. MEIS transcription factors in development and disease. Development 2019, 146, dev174706. [Google Scholar] [CrossRef] [PubMed]
- Adamaki, M.; Lambrou, G.I.; Athanasiadou, A.; Vlahopoulos, S.; Papavassiliou, A.G.; Moschovi, M. HOXA9 and MEIS1 gene overexpression in the diagnosis of childhood acute leukemias: Significant correlation with relapse and overall survival. Leuk. Res. 2015, 39, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Li, D.; Li, J. Technologies for targeting DNA methylation modifications: Basic mechanism and potential application in cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188454. [Google Scholar] [CrossRef]
- Koch, A.; Joosten, S.C.; Feng, Z.; De Ruijter, T.C.; Draht, M.X.; Melotte, V.; Smits, K.M.; Veeck, J.; Herman, J.G.; Van Neste, L.; et al. Analysis of DNA methylation in cancer: Location revisited. Nat. Rev. Clin. Oncol. 2018, 15, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Saghafinia, S.; Mina, M.; Riggi, N.; Hanahan, D.; Ciriello, G. Pan-Cancer Landscape of Aberrant DNA Methylation across Human Tumors. Cell Rep. 2018, 25, 1066–1080.e8. [Google Scholar] [CrossRef]
- Bi, K.W.; Wei, X.G.; Qin, X.X.; Li, B. BTK Has Potential to Be a Prognostic Factor for Lung Adenocarcinoma and an Indicator for Tumor Microenvironment Remodeling: A Study Based on TCGA Data Mining. Front. Oncol. 2020, 10, 424. [Google Scholar] [CrossRef]
- Tamborero, D.; Rubio-Perez, C.; Muiños, F.; Sabarinathan, R.; Piulats, J.M.; Muntasell, A.; Dienstmann, R.; Lopez-Bigas, N.; Gonzalez-Perez, A. A Pan-cancer Landscape of Interactions between Solid Tumors and Infiltrating Immune Cell Populations. Clin. Cancer Res. 2018, 24, 3717–3728. [Google Scholar] [CrossRef]
- Lehrer, S.; Rheinstein, P.H. Expression of the Vesicular Monoamine Transporter Gene Solute Carrier Family 18 Member 1 (SLC18A1) in Lung Cancer. Cancer Genom. Proteom. 2018, 15, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Moghbeli, M.; Rad, A.; Farshchian, M.; Taghehchian, N.; Gholamin, M.; Abbaszadegan, M.R. Correlation Between Meis1 and Msi1 in Esophageal Squamous Cell Carcinoma. J. Gastrointest. Cancer 2016, 47, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Dimartino, J.F.; Selleri, L.; Traver, D.; Firpo, M.T.; Rhee, J.; Warnke, R.; O’gorman, S.; Weissman, I.L.; Cleary, M.L. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood 2001, 98, 618–626. [Google Scholar] [CrossRef]
- Pillay, L.M.; Forrester, A.M.; Erickson, T.; Berman, J.N.; Waskiewicz, A.J. The Hox cofactors Meis1 and Pbx act upstream of gata1 to regulate primitive hematopoiesis. Dev. Biol. 2010, 340, 306–317. [Google Scholar] [CrossRef]
- Nakamura, T.; Largaespada, D.A.; Shaughnessy, J.D., Jr.; Jenkins, N.A.; Copeland, N.G. Cooperative activation of Hoxa and Pbx1-related genes in murine myeloid leukaemias. Nat. Genet. 1996, 12, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. HOX genes and their role in the development of human cancers. J. Mol. Med. 2014, 92, 811–823. [Google Scholar] [CrossRef]
- Koller, K.; Pichler, M.; Koch, K.; Zandl, M.; Stiegelbauer, V.; Leuschner, I.; Hoefler, G.; Guertl, B. Nephroblastomas show low expression of microR-204 and high expression of its target, the oncogenic transcription factor MEIS1. Pediatr. Dev. Pathol. 2014, 17, 169–175. [Google Scholar] [CrossRef]
- Bradner, J.E.; Hnisz, D.; Young, R.A. Transcriptional Addiction in Cancer. Cell 2017, 168, 629–643. [Google Scholar] [CrossRef]
- Zhu, J.; Cui, L.; Xu, A.; Yin, X.; Li, F.; Gao, J. MEIS1 inhibits clear cell renal cell carcinoma cells proliferation and in vitro invasion or migration. BMC Cancer 2017, 17, 176. [Google Scholar] [CrossRef]
- Song, F.; Wang, H.; Wang, Y. Myeloid ecotropic viral integration site, 1 inhibits cell proliferation, invasion or migration in human gastric cancer. Oncotarget 2017, 8, 90050–90060. [Google Scholar] [CrossRef] [PubMed]
- Crist, R.C.; Roth, J.J.; Waldman, S.A.; Buchberg, A.M. A conserved tissue-specific homeodomain-less isoform of MEIS1 is downregulated in colorectal cancer. PLoS ONE 2011, 6, e23665. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Tang, Y.; Hua, L.; Wang, Z.; Du, G.; Wang, S.; Lu, S.; Li, W. A Systematic Pan-Cancer Analysis of MEIS1 in Human Tumors as Prognostic Biomarker and Immunotherapy Target. J. Clin. Med. 2023, 12, 1646. https://doi.org/10.3390/jcm12041646
Li H, Tang Y, Hua L, Wang Z, Du G, Wang S, Lu S, Li W. A Systematic Pan-Cancer Analysis of MEIS1 in Human Tumors as Prognostic Biomarker and Immunotherapy Target. Journal of Clinical Medicine. 2023; 12(4):1646. https://doi.org/10.3390/jcm12041646
Chicago/Turabian StyleLi, Han, Ying Tang, Lichun Hua, Zemin Wang, Guoping Du, Shuai Wang, Shifeng Lu, and Wei Li. 2023. "A Systematic Pan-Cancer Analysis of MEIS1 in Human Tumors as Prognostic Biomarker and Immunotherapy Target" Journal of Clinical Medicine 12, no. 4: 1646. https://doi.org/10.3390/jcm12041646
APA StyleLi, H., Tang, Y., Hua, L., Wang, Z., Du, G., Wang, S., Lu, S., & Li, W. (2023). A Systematic Pan-Cancer Analysis of MEIS1 in Human Tumors as Prognostic Biomarker and Immunotherapy Target. Journal of Clinical Medicine, 12(4), 1646. https://doi.org/10.3390/jcm12041646




