Survival of Visual Function in Patients with Advanced Glaucoma after Standard Guarded Trabeculectomy with MMC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Clinical Data
2.3. Surgical Technique
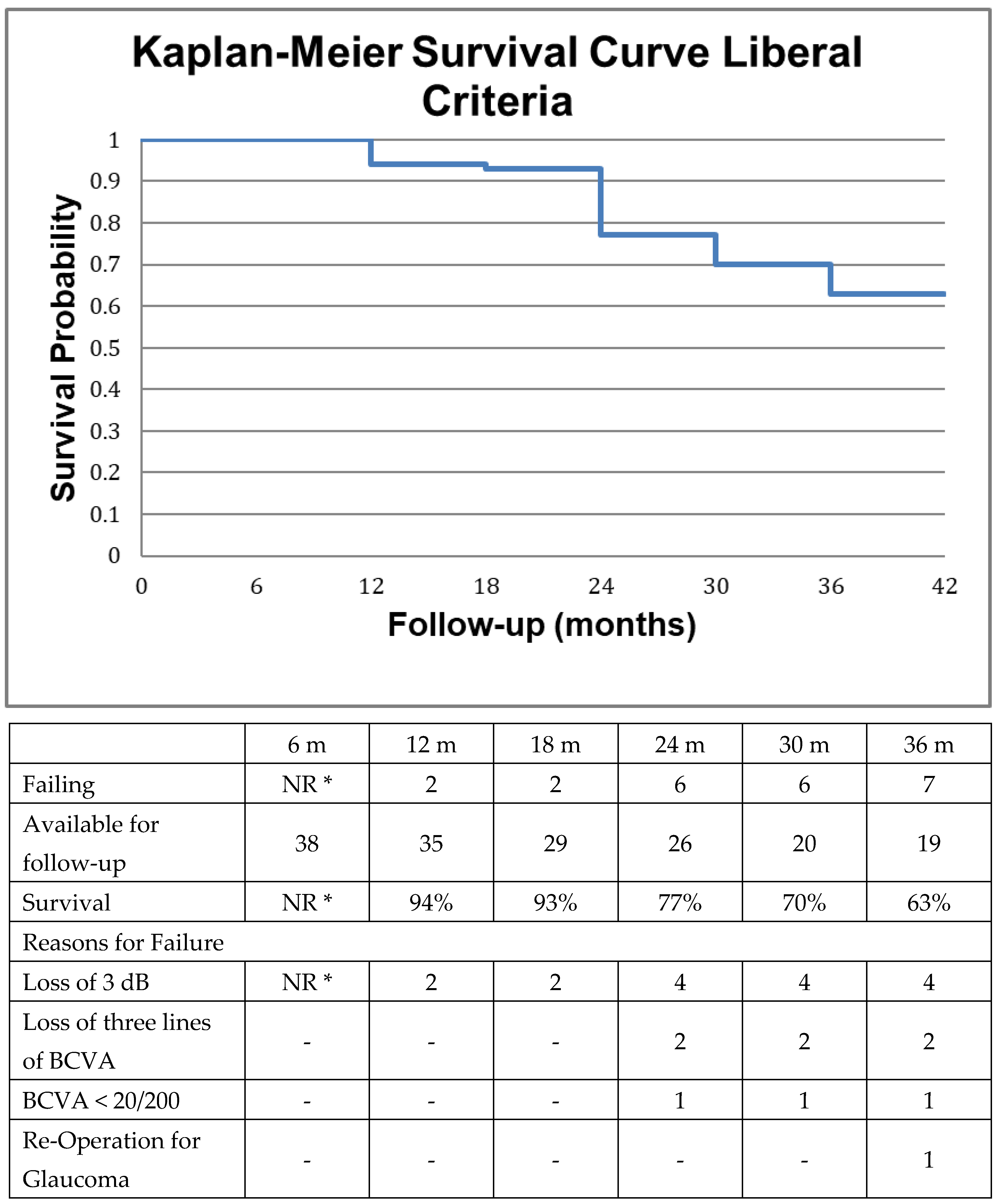
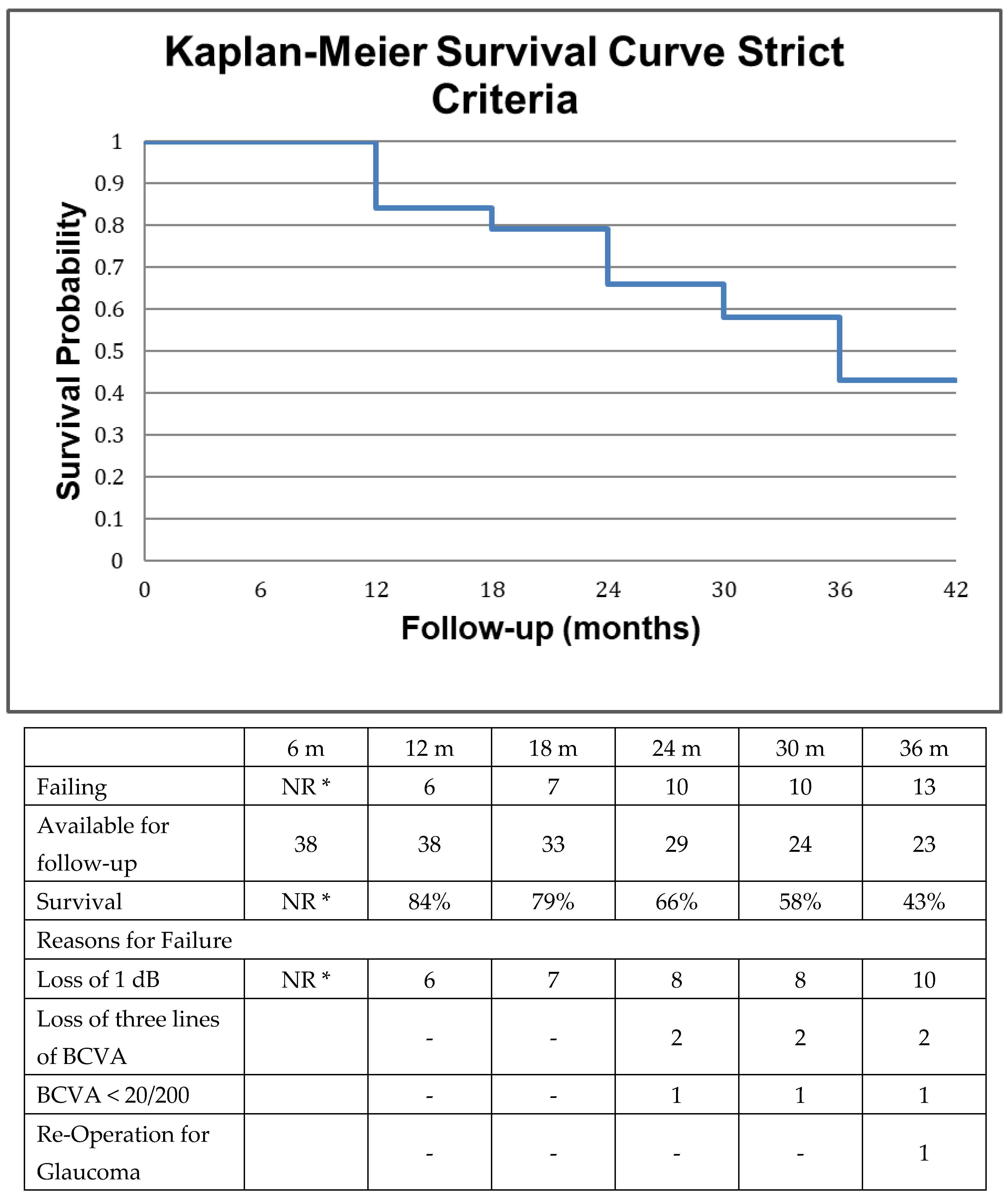
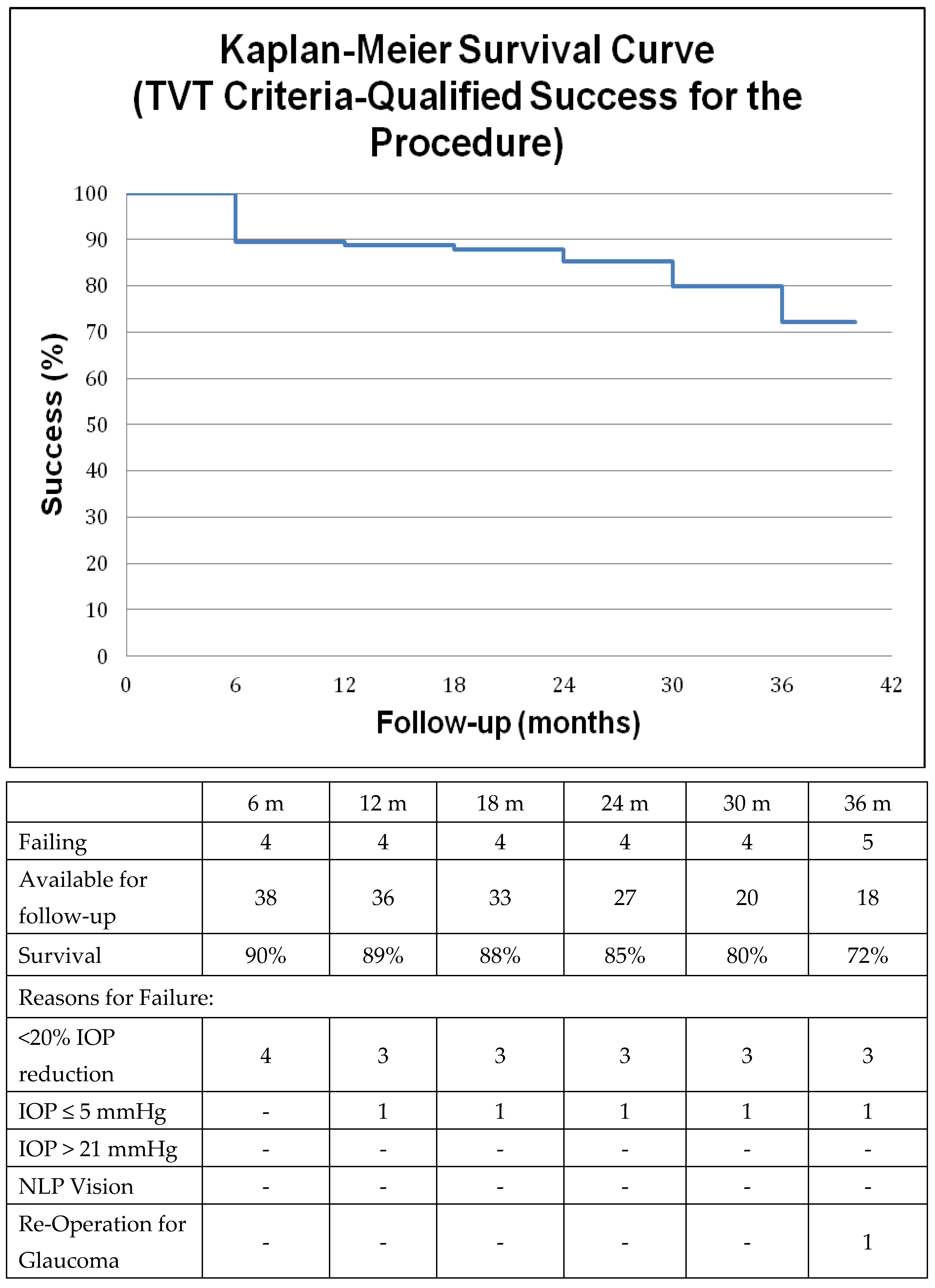
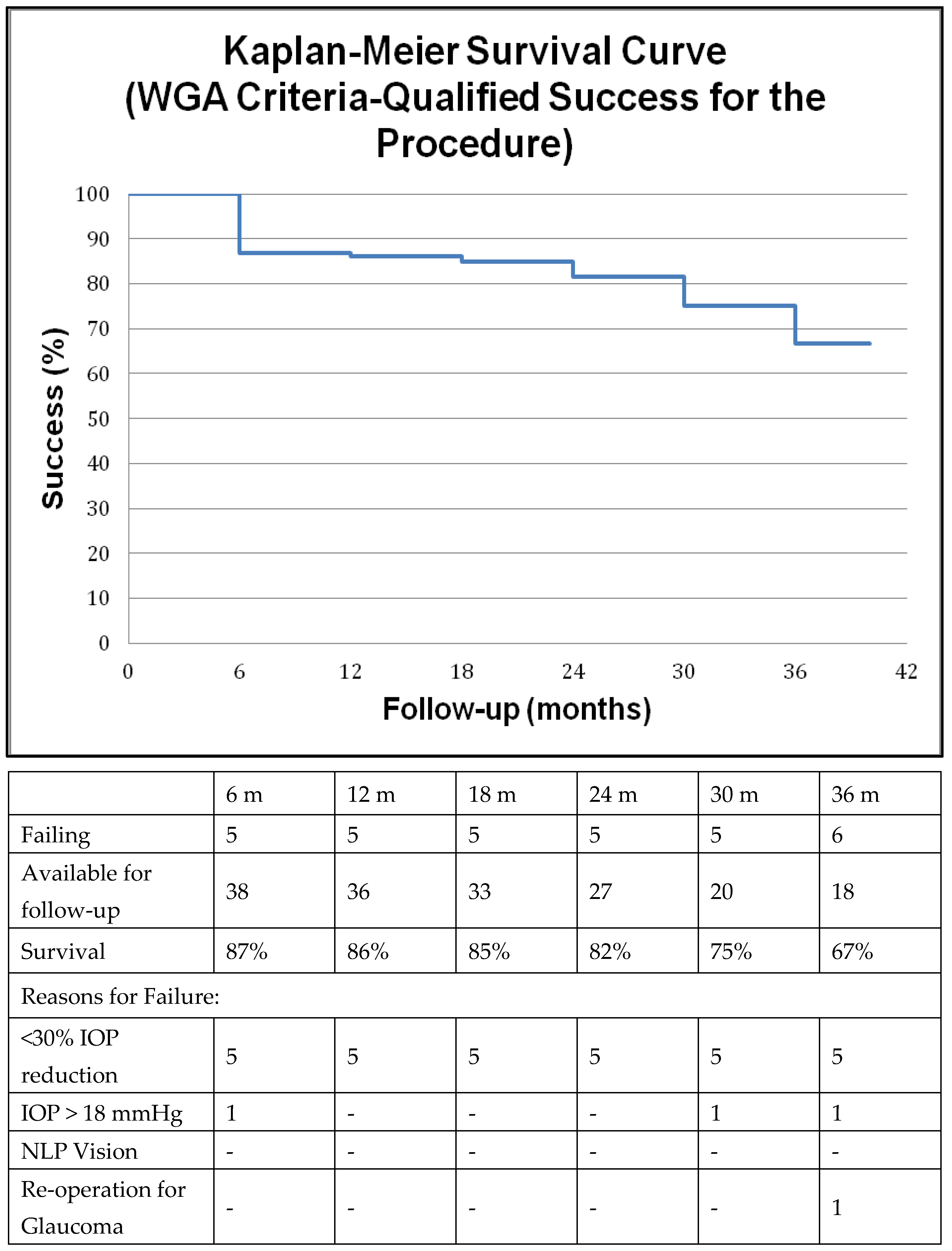
2.4. Survival Analysis
2.5. Statistical Analysis
2.6. Risk Factor Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sotimehin, A.E.; Ramulu, P.Y. Measuring Disability in Glaucoma. Eur. J. Gastroenterol. Hepatol. 2018, 27, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [Green Version]
- Fiscella, R.G.; Lee, J.; Davis, E.J.H.; Walt, J. Cost of Illness of Glaucoma. Pharmacoeconomics 2009, 27, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.C.; Funk, J.; Schwarzer, G.; Antes, G.; Falck-Ytter, Y.T. Treatment of ocular hypertension and open angle glaucoma: Meta-analysis of randomised controlled trials. BMJ 2005, 331, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garway-Heath, D.F.; Crabb, D.P.; Bunce, C.; Lascaratos, G.; Amalfitano, F.; Anand, N.; Azuara-Blanco, A.; Bourne, R.R.; Broadway, D.C.; Cunliffe, I.A.; et al. Latanoprost for open-angle glaucoma (UKGTS): A randomised, multicentre, placebo-controlled trial. Lancet 2015, 385, 1295–1304. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.; Ogston, S.; Cobb, C.; MacEwen, C. Recent trends in glaucoma surgery in Scotland, England and Wales. Br. J. Ophthalmol. 2014, 99, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Strutton, D.R.; Walt, J.G. Trends in Glaucoma Surgery before and after the Introduction of New Topical Glaucoma Pharmacotherapies. Eur. J. Gastroenterol. Hepatol. 2004, 13, 221–226. [Google Scholar] [CrossRef]
- Ramulu, P.Y.; Corcoran, K.; Corcoran, S.L.; Robin, A.L. Utilization of Various Glaucoma Surgeries and Procedures in Medicare Beneficiaries from 1995 to 2004. Ophthalmology 2007, 114, 2265–2270.e1. [Google Scholar] [CrossRef]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L.; Tube versus Trabeculectomy Study Group. Treatment Outcomes in the Tube Versus Trabeculectomy (TVT) Study After Five Years of Follow-up. Am. J. Ophthalmol. 2012, 153, 789–803.e2. [Google Scholar] [CrossRef] [Green Version]
- Jampel, H. Trabeculectomy: More effective at causing cataract surgery than lowering intraocular pressure? Ophthalmology 2009, 116, 173–174. [Google Scholar] [CrossRef]
- Gedde, S.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L.; Feuer, W.J.; Schiffman, J.C. Postoperative Complications in the Tube Versus Trabeculectomy (TVT) Study During Five Years of Follow-up. Am. J. Ophthalmol. 2012, 153, 804–814.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Kerrigan-Baumrind, L.; Quigley, H.A.; Pease, M.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Opthalmology Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Medeiros, F.A.; Gracitelli, C.P.; Boer, E.R.; Weinreb, R.N.; Zangwill, L.M.; Rosen, P.N. Longitudinal Changes in Quality of Life and Rates of Progressive Visual Field Loss in Glaucoma Patients. Ophthalmology 2014, 122, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisboa, R.; Chun, Y.S.; Zangwill, L.M.; Weinreb, R.N.; Rosen, P.N.; Liebmann, J.M.; Girkin, C.; Medeiros, F.A. Association Between Rates of Binocular Visual Field Loss and Vision-Related Quality of Life in Patients With Glaucoma. JAMA Ophthalmol 2013, 131, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Moster, M.R.; Moster, M.L. Wipe-out: A complication of glaucoma surgery or just a blast from the past? Am. J. Ophthalmol. 2005, 140, 705–706. [Google Scholar] [CrossRef]
- Costa, V.P.; Smith, M.; Spaeth, G.L.; Gandham, S.; Markovitz, B. Loss of Visual Acuity after Trabeculectomy. Ophthalmology 1993, 100, 599–612. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Zangwill, L.M.; Bowd, C.; Mansouri, K.; Weinreb, R.N. The Structure and Function Relationship in Glaucoma: Implications for Detection of Progression and Measurement of Rates of Change. Investig. Opthalmology Vis. Sci. 2012, 53, 6939–6946. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, M.F.; Ewing, R.H. Reassessing Split Fixation in Advanced Glaucoma: Preliminary studies with computerized perimetry. In Proceedings of the IX International Perimetric Society Meeting; Mills, R.P., Heijl, A., Eds.; Kugler Publications: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Parrish, R.K.; Heuer, D.K.; Brandt, J.D. The Tube Versus Trabeculectomy Study: Design and Baseline Characteristics of Study Patients. Am. J. Ophthalmol. 2005, 140, 275.e1–275.e14. [Google Scholar] [CrossRef]
- World Glaucoma Association. Reporting of Glaucoma Surgical Trials. In Consensus on the Definition of Success, 1st ed.; Shaarawy, T.M., Sherwood, M.B., Grehn, F., Eds.; Kugler Publications: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Lee, P.P.; Walt, J.G.; Doyle, J.J.; Kotak, S.V.; Evans, S.J.; Budenz, D.L.; Chen, P.P.; Coleman, A.L.; Feldman, R.M.; Jampel, H.D.; et al. A Multicenter, Retrospective Pilot Study of Resource Use and Costs Associated With Severity of Disease in Glaucoma. Arch. Ophthalmol. 2006, 124, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Gieser, D.K.; Williams, R.T.; O’Connell, W.; Pasquale, L.R.; Rosenthal, B.P.; Walt, J.G.; Katz, L.M.; Siegartel, L.R.; Wang, L.; Rosenblatt, L.C.; et al. Costs and Utilization of End-stage Glaucoma Patients Receiving Visual Rehabilitation Care: A US Multisite Retrospective Study. Eur. J. Gastroenterol. Hepatol. 2006, 15, 419–425. [Google Scholar] [CrossRef]
- Koleva, D.; Motterlini, N.; Schiavone, M.; Garattini, L. Medical Costs of Glaucoma and Ocular Hypertension in Italian Referral Centres: A Prospective Study. Ophthalmologica 2007, 221, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, B.; Nordmann, J.-P.; Sellem, E.; Chen, E.; Gold, R.; Polland, W.; Williamson, W.; Buchholz, P.; Walt, J.G.; Groleau, D.; et al. A multicentre, retrospective study of resource utilization and costs associated with glaucoma management in France and Sweden. Acta Ophthalmol. Scand. 2005, 84, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Topouzis, F.; Tranos, P.; Koskosas, A.; Pappas, T.; Anastasopoulos, E.; Dimitrakos, S.; Wilson, M.R. Risk of Sudden Visual Loss Following Filtration Surgery in End-Stage Glaucoma. Am. J. Ophthalmol. 2005, 140, 661.e1–661.e7. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Hudson, J.; Fernie, G.; Kernohan, A.; Azuara-Blanco, A.; Burr, J.; Homer, T.; Shabaninejad, H.; Sparrow, J.M.; Garway-Heath, D.; et al. Primary trabeculectomy for advanced glaucoma: Pragmatic multicentre randomised controlled trial (TAGS). BMJ 2021, 373, n1014. [Google Scholar] [CrossRef] [PubMed]
- Sofi, R.A.; Shafi, S.; Qureshi, W.; Ashraf, S. Merits of trabeculectomy in advanced and end-stage glaucoma. Int. J. Health Sci. 2018, 12, 57–60. [Google Scholar]
- Sethi, H.S.; Naik, M.P.; Saluja, K. Role of trabeculectomy in advanced glaucoma: Whether we stand to consider it a bane or a boon today? Int. Ophthalmol. 2017, 39, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.T.; Khaw, P.T.; Aung, T.; Foster, P.; Htoon, H.M.; Oen, F.T.; Gazzard, G.; Husain, R.; Devereux, J.G.; Minassian, D.; et al. The Singapore 5-Fluorouracil Trabeculectomy Study: Effects on Intraocular Pressure Control and Disease Progression at 3 Years. Ophthalmology 2009, 116, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, M.; Monsellato, G.; Villani, E.; Lizzio, R.A.U.; Cremonesi, E.; Luccarelli, S.; Nucci, P. Intraocular pressure control after combined phacotrabeculectomy versus trabeculectomy alone. Eur. J. Ophthalmol. 2021, 32, 327–335. [Google Scholar] [CrossRef]
- Broadway, D.C.; Chang, L.P. Trabeculectomy, Risk Factors for Failure and the Preoperative State of the Conjunctiva. Eur. J. Gastroenterol. Hepatol. 2001, 10, 237–249. [Google Scholar] [CrossRef]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 3. Baseline characteristics of black and white patients. Ophthalmology 1998, 105, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- The Advanced Glaucoma Intervention Study Investigators. Advanced Glaucoma Intervention Study: 2. Visual Field Test Scoring and Reliability. Ophthalmology 1994, 101, 1445–1455. [Google Scholar]
- Medeiros, F.A.; Zangwill, L.M.; Anderson, D.R.; Liebmann, J.M.; Girkin, C.; Harwerth, R.S.; Fredette, M.-J.; Weinreb, R.N. Estimating the Rate of Retinal Ganglion Cell Loss in Glaucoma. Am. J. Ophthalmol. 2012, 154, 814–824.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moraes, C.G.; Juthani, V.J.; Liebmann, J.M.; Teng, C.C.; Tello, C.; Susanna, R.; Ritch, R. Risk factors for visual field progression in treated glaucoma. Arch. Ophthalmol. 2011, 129, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; Gordon, M.O.; Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713, discussion 829–830. [Google Scholar] [CrossRef] [Green Version]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Harwerth, R.S.; Quigley, H.A. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch. Ophthalmol. 2006, 124, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Harwerth, R.; Wheat, J.; Fredette, M.; Anderson, D. Linking structure and function in glaucoma. Prog. Retin. Eye Res. 2010, 29, 249–271. [Google Scholar] [CrossRef] [Green Version]
- Heijl, A.; Bengtsson, B.; Hyman, L.; Leske, M.C. Natural History of Open-Angle Glaucoma. Ophthalmology 2009, 116, 2271–2276. [Google Scholar] [CrossRef] [Green Version]
- Francis, B.A.; Hong, B.; Winarko, J.; Kawji, S.; Dustin, L.; Chopra, V. Vision loss and recovery after trabeculectomy: Risk and associated risk factors. Arch. Ophthalmol. 2011, 129, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Law, S.K.; Nguyen, A.M.; Coleman, A.L.; Caprioli, J. Severe loss of central vision in patients with advanced glaucoma undergoing trabeculectomy. Arch. Ophthalmol. 2007, 125, 1044–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Much, J.W.; Liu, C.; Piltz-Seymour, J.R. Long-term Survival of Central Visual Field in End-Stage Glaucoma. Ophthalmology 2008, 115, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.A.; Brown, R.H.; Lynch, M.G.; Caplan, M.B. Risk of Postoperative Visual Loss in Advanced Glaucoma. Am. J. Ophthalmol. 1993, 115, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Langerhorst, C.T.; De Clercq, B.; Berg, T.J.T.P.V.D. Visual field behavior after intra-ocular surgery in glaucoma patients with advanced defects. Doc. Ophthalmol. 1990, 75, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.P.; Hendeles, S. Risk of sudden visual loss following trabeculectomy in advanced primary open-angle glaucoma. Br. J. Ophthalmol. 1986, 70, 97–99. [Google Scholar] [CrossRef] [Green Version]
- Jampel, H.D.; Solus, J.F.; Tracey, P.A.; Gilbert, D.L.; Loyd, T.L.; Jefferys, J.L.; Quigley, H.A. Outcomes and Bleb-Related Complications of Trabeculectomy. Ophthalmology 2012, 119, 712–722. [Google Scholar] [CrossRef]
- Musch, D.C.; Gillespie, B.W.; Niziol, L.M.; Janz, N.K.; Wren, P.A.; Rockwood, E.J.; Lichter, P.R. Cataract extraction in the collaborative initial glaucoma treatment study: Incidence, risk factors, and the effect of cataract progression and extraction on clinical and quality-of-life outcomes. Arch. Ophthalmol. 2006, 124, 1694–1700. [Google Scholar] [CrossRef]
- Balekudaru, S.; George, R.; Panday, M.; Singh, M.; Neog, A.; Lingam, V. Prospective Evaluation of Early Visual Loss Following Glaucoma-filtering Surgery in Eyes With Split Fixation. Eur. J. Gastroenterol. Hepatol. 2014, 23, 211–218. [Google Scholar] [CrossRef]
- Fogagnolo, P.; Sangermani, C.; Oddone, F.; Frezzotti, P.; Iester, M.; Figus, M.; Ferreras, A.; Romano, S.; Gandolfi, S.; Centofanti, M.; et al. Long-term perimetric fluctuation in patients with different stages of glaucoma. Br. J. Ophthalmol. 2010, 95, 189–193. [Google Scholar] [CrossRef]
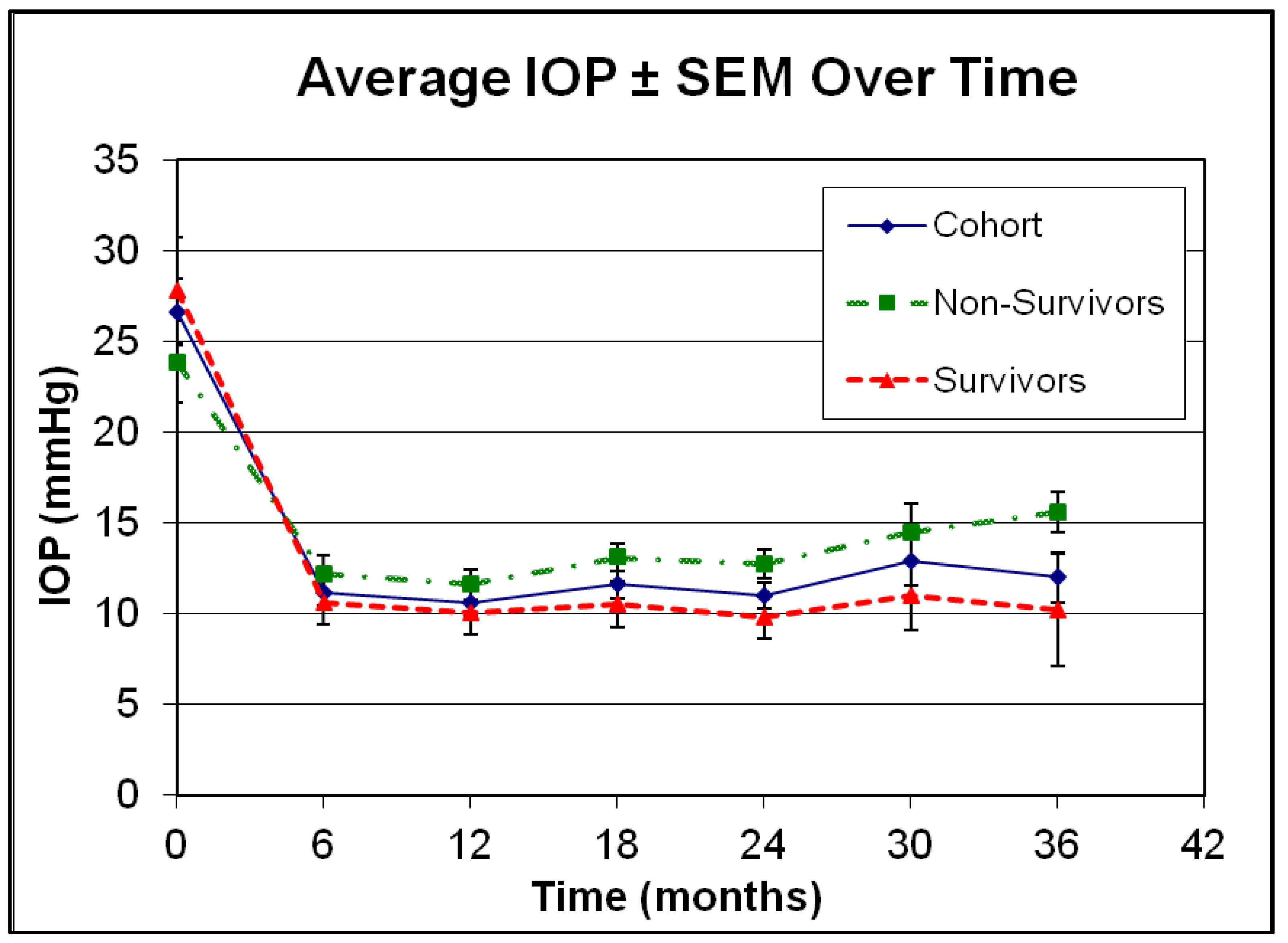

| Demographic Characteristics | |
|---|---|
| No. of Eyes | 40 |
| Average Age ± SD, range (Years) | 71.1 ± 12.8, 33–87 |
| Gender N, % (Male) | 22, 55% |
| Race N, % (Caucasian) | 40, 100% |
| Average Length of Follow-up ± SD, range (months) | 23.3 ± 15.5, 1–55 |
| Average Pre-op IOP ± SD (mmHg) | 26.5 ± 11.4 |
| Average Pre-op Medications ± SD (substances) | 3.8 ± 1 |
| Average Central Corneal Thickness ± SD (μm) | 524.5 ± 41.2 |
| Average Mean Deviation on SAP ± SD, range (dB) | −26.3 ± 4.1, −20.1 to −33.29 |
| −20.01 to −24.00 | 39% |
| −24.01 to −28.00 | 17% |
| >28.01 | 44% |
| Eyes with Split Fixation N, % | 32 (80%) |
| Eyes with Previous Laser Glaucoma Surgery N, % | 2 (5%) |
| Pseudophakia N, % | 8 (20%) |
| Diagnosis N, % | |
| Primary Open Angle Glaucoma (POAG) | 14 (35%) |
| Exfoliative Glaucoma (XFG) | 16 (40%) |
| *Other: | 10 (25%) |
| Outcomes and Operative Characteristics | p-Value | |
|---|---|---|
| Average Pre-op IOP ± SD (mmHg) | 26.5 ± 11.4 | 1.3 × 10−9 |
| Average Post-op IOP ± SD (mmHg) | 11.4 ± 4.0 | |
| Average Pre-op Medications ± SD (substances) | 3.7 ± 1.0 | 7.7 × 10−14 |
| Average Post-op Medications ± SD (substances) | 0.9 ± 1.1 | |
| Average Pre-op logMAR Visual Acuity ± SD | 0.5 ± 0.4 | 0.9 |
| Average Post-op logMAR Visual Acuity ± SD | 0.4 ± 0.5 | |
| Number of Combined Cases N, % (+ uncomplicated phacoemulsification | 6, 15% | |
| Independent Variables | Survival of Visual Function Strict Criteria Risk Factor Analysis (ANOVA) | Survival of Visual Function Liberal Criteria Risk Factor Analysis (ANOVA) |
|---|---|---|
| Pre-op Characteristics | ||
| p-value | p-value | |
| Age per decade | 0.22 | 0.44 |
| Gender | 0.21 | 0.16 |
| Diagnosis other than POAG | 0.55 | 0.52 |
| Mean Deviation on SAP (per 4 dB) | 0.44 | 0.86 |
| IOP (mmHg) | 0.032 * | 0.38 |
| Central Corneal Thickness (per 40 μm) | 0.61 | 0.27 |
| Glaucoma Medication Requirements | 0.71 | 0.97 |
| Phakic Status | 0.17 | 0.068 # |
| logMAR BCVA | 0.71 | 0.63 |
| Operative Characteristics | ||
| Surgeon | 0.58 | 0.14 |
| Combined Cases | 0.46 | 0.25 |
| Post-operative Characteristics | ||
| IOP at 3 months (mmHg) | 0.19 | 0.36 |
| IOP at 24 months (mmHg) | 0.045 * | <0.0001 * |
| IOP fluctuations (mmHg) | 0.74 | 0.82 |
| Glaucoma Medication Requirements at 24 months | 0.08 # | 0.87 |
| Length of Follow-up (per year) | 0.50 | 0.77 |
| Independent Variables | Survival of Visual Function Strict Criteria Risk Factor Analysis (Logistic Regression) | Survival of Visual Function Liberal Criteria Risk Factor Analysis (Logistic Regression) | ||||
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| p-value | Regression Coefficient | Standard Error | p-value | Regression Coefficient | Standard Error | |
| Pre-op IOP (mmHg) | 0.51 | 2.7 × 10−2 | 4 × 10−2 | 0.79 | 1.2 × 10−2 | 4.7 × 10−2 |
| Phakic Status | 0.21 | 1.1 | 0.9 | 0.097 # | 1.6 | 1 |
| IOP at 24 months (mmHg) | 0.92 | −1.5 × 10−2 | 0.2 | 0.45 | −0.14 | 0.2 |
| Glaucoma Medication Requirements at 24 months | 0.36 | −0.4 | 0.4 | 0.53 | 0.3 | 0.5 |
| Model 2 | ||||||
| Phakic Status | 0.18 | 1.1 | 0.8 | 0.088 # | 1.6 | 1 |
| IOP at 24 months (mmHg) | 0.48 | −8 × 10−2 | 0.1 | 0.63 | −7 × 10−2 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippopoulos, T.; Tsoukanas, D.; Kandarakis, S.A.; Salonikiou, A.; Georgiou, M.; Topouzis, F. Survival of Visual Function in Patients with Advanced Glaucoma after Standard Guarded Trabeculectomy with MMC. J. Clin. Med. 2023, 12, 1639. https://doi.org/10.3390/jcm12041639
Filippopoulos T, Tsoukanas D, Kandarakis SA, Salonikiou A, Georgiou M, Topouzis F. Survival of Visual Function in Patients with Advanced Glaucoma after Standard Guarded Trabeculectomy with MMC. Journal of Clinical Medicine. 2023; 12(4):1639. https://doi.org/10.3390/jcm12041639
Chicago/Turabian StyleFilippopoulos, Theodoros, Dimitrios Tsoukanas, Stylianos A. Kandarakis, Angeliki Salonikiou, Michalis Georgiou, and Fotis Topouzis. 2023. "Survival of Visual Function in Patients with Advanced Glaucoma after Standard Guarded Trabeculectomy with MMC" Journal of Clinical Medicine 12, no. 4: 1639. https://doi.org/10.3390/jcm12041639
APA StyleFilippopoulos, T., Tsoukanas, D., Kandarakis, S. A., Salonikiou, A., Georgiou, M., & Topouzis, F. (2023). Survival of Visual Function in Patients with Advanced Glaucoma after Standard Guarded Trabeculectomy with MMC. Journal of Clinical Medicine, 12(4), 1639. https://doi.org/10.3390/jcm12041639






