Exploring the Cardiotoxicity Spectrum of Anti-Cancer Treatments: Definition, Classification, and Diagnostic Pathways
Abstract
1. Introduction
2. The Spectrum of Cardiotoxicity
2.1. Cancer Therapy Related Cardiac Dysfunction
Specific CTRCD
- Anthracyclines (e.g., doxorubicin, epirubicine, daunorubicin) are frequently employed treatment of solid tumors and hematological tumors but could contribute to the development of left ventricular dysfunction (LVD) (from myocardial cell injury to impaired LVEF and symptomatic HF) [9]. The proposed mechanisms of anthracycline-related cardiomyopathy include the transport of anthracyclines across the cardiomyocyte cell membrane, generation of reactive oxygen species (ROS) through the inhibition of topoisomerase 2β (resulting in activation of cell death pathways and mitochondrial dysfunction), generation of cardiotoxic anthracycline metabolites, and sarcomere disruption [2,10].
- Alkylating agents, such as cyclophosphamide, given in high doses before bone marrow transplantation, may cause HF due to several pathological effects as direct endothelial injury followed by extravasation of toxic metabolites that damage myocytes, interstitial hemorrhage, and edema. Furthermore, an ischemic myocardial injury could be the result of intracapillary microemboli. Cyclophosphamide may also damage the inner mitochondrial membrane of cardiomyocytes, most likely through the induction of oxidative stress [11].
- Cardiotoxicity of cisplatin, used for solid cancers (e.g., testicular, lung, cervical, and ovarian cancers), may result either from direct toxic action on cardiac myocytes or from ROS production, followed by the induction of oxidative stress and the switch to a prothrombotic condition [12]. Of note, platinum-based drugs need an infusion of high intravenous volumes to avoid cardio-toxicity [2,11].
- Immunotherapies and targeted therapies—implying inhibition of human epidermal growth factor receptor 2 (HER2) signaling with either antibodies (trastuzumab, pertuzumab) or TKIs (lapatinib)—have ameliorated survival of patients with HER2-positive breast cancer [2,13]. In addition, 3% to 7% of patients who receive trastuzumab monotherapy develop cardiac dysfunction (from asymptomatic LVEF decline to HF), which is usually reversible with drug interruption and/or HF treatment [2]. This percentage is even higher when trastuzumab is administrated after anthracyclines treatment [2].
- Both antibodies and protein kinase vascular endothelial growth factor (VEGF) signaling pathway inhibitors, used in several solid cancers (e.g., colorectal and lung cancer), induce LVD and HF mainly due to cardiac hypertrophy and mitochondrial abnormalities [2]. In particular, sorafenib-mediated inhibition of RAF1 and BRAF kinase activity will disrupt signaling through the extracellular signal-regulated kinase (ERK) kinase cascade, which is believed to have a role in heart cell survival, especially under conditions of stress [2].
- HF due to TKIs of BCR-ABL (e.g., imatinib, employed in chronic leukemia) has not been uniquely confirmed. However, these drugs could lead to significant mitochondrial dysfunction with loss of membrane potential, the release of cytochrome c, and markedly impaired energy generation with a significant decline in adenosine triphosphate (ATP) concentration, which is crucial to cardiomyocyte contractile function [2].
- Antimicrotubule agents (taxanes, such as docetaxel), frequently used in breast cancer, may be safer than anthracyclines in patients with pre-existing LVD. However, asymptomatic decrease in LVEF as well as overt congestive HF have been observed in patients previously treated with anthracycline and when docetaxel was combined with trastuzumab treatment for HER2-positive disease [2,11,14].
- Several investigators have demonstrated LVD related to proteasome inhibitors (PI), used in multiple myeloma, as a direct consequence of the inability of proteasomes to degrade dysfunctional or unneeded proteins in cardiomyocytes [2].
- ICIs may cause (through not entirely known mechanisms of action) myocarditis as well as non-inflammatory HF syndromes including Takotsubo syndrome [7].
- In addition to chemotherapy, radiation-induced CVD (increased risk for systolic and diastolic (more likely) heart failure) may be observed [16]. The related pathophysiological mechanisms are complex, including deoxyribonucleic acid damage, oxidative stress, and the release of inflammatory and profibrotic cytokines, leading to vascular and myocardial fibrosis, and as a result, the development of stenosis in radiated coronary arteries, subclavian, and carotids [2,16].
2.2. Myocarditis
2.3. Vascular Toxicity
2.3.1. Coronary Artery Disease (CAD)
2.3.2. Peripheral Vascular Disease and Stroke
2.3.3. Thromboembolic Disease
2.4. Systemic Arterial Hypertension (HTN)
2.5. Arrhythmias
2.6. Pericardial Disease
2.7. Valvular Heart Disease
2.8. Pulmonary Hypertension
3. Diagnostic Pathways
3.1. Clinical Assessment
3.2. Serum Biomarkers
3.3. Cardiovascular Imaging
3.3.1. Transthoracic Echocardiography
3.3.2. Transephofageal Echocardiography (TEE)
3.3.3. Vascular Ultrasound
3.3.4. Cardiac Magnetic Resonance
3.3.5. Cardiac Nuclear Imaging
3.3.6. Coronary Computed Tomography Angiography and Imaging Stress Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| 5-FU | 5-fluorouracil |
| 18F-FDG PET | Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography |
| ACS | acute coronary syndromes |
| AF | atrial fibrillation |
| ATP | adenosine triphosphate |
| BNP | B-type natriuretic peptide |
| CAD | coronary artery disease |
| CAR-T | Chimeric antigen receptor T cell |
| CCS | chronic coronary syndromes |
| CCT | cardiac computed tomography |
| CCTA | Coronary computed tomography angiography |
| CMR | cardiac magnetic resonance |
| CTA | computed tomography angiography |
| cTn | cardiac troponins |
| CTRCD | cancer therapy related cardiac dysfunction |
| CTR-CTV | cancer therapy-related cardiovascular toxicity |
| CV | cardiovascular |
| CVD | cardiovascular diseases |
| DUS | duplex ultrasound |
| EDV | end-diastolic volume |
| EGFR | Epidermal Growth Factor Receptor |
| ESV | end-systolic volume |
| ERK | extracellular signal-regulated kinase |
| FAC | fractional area change |
| GCS | global circumferential strain |
| GLS | global longitudinal strain |
| HER2 | human epidermal growth factor receptor 2 |
| HER2i | HER2 inhibitors |
| HF | heart failure |
| hs-cTn | high sensitivity cardiac troponin |
| hs-cTnI | high sensitivity cardiac troponin I |
| hs-cTnT | high sensitivity cardiac troponin T |
| HTN | systemic arterial hypertension |
| ICI | immune checkpoint inhibitors |
| IVC | inferior vena cava |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| LVD | left ventricular dysfunction |
| MI | myocardial infarction |
| MUGA | multigated acquisition |
| NPs | natriuretic peptides |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| PAD | peripheral artery disease |
| PE | pulmonary embolism |
| PH | pulmonary hypertension |
| PI | proteasome inhibitors |
| ROS | reactive oxygen species |
| RV | right ventricular |
| RVEF | right ventricular ejection fraction |
| TAPSE | tricuspid annular plane systolic excursion |
| TEE | transesophageal echocardiography |
| TKIs | small molecule tyrosine kinase inhibitors |
| TIL | tumor-infiltrating lymphocytes |
| TR | tricuspid regurgitation |
| TTE | transthoracic echocardiography |
| TTS | Takotsubo syndrome |
| VEGF | vascular endothelial growth factor |
| VEGFi | vascular endothelial growth factor inhibitors |
| VHD | valvular heart disease |
| VTE | venous thrombosis and venous thromboembolism |
References
- Loud, J.; Murphy, J. Cancer Screening and Early Detection in the 21st Century. Semin. Oncol. Nurs. 2017, 33, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Daher, I.N.; Daigle, T.R.; Bhatia, N.; Durand, J.-B. The Prevention of Cardiovascular Disease in Cancer Survivors. Tex. Heart Inst. J. 2012, 39, 190–198. [Google Scholar] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: A Report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.—Cardiovasc. Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef]
- Virani, S.A.; Dent, S.; Brezden-Masley, C.; Clarke, B.; Davis, M.K.; Jassal, D.S.; Johnson, C.; Lemieux, J.; Paterson, I.; Sebag, I.A.; et al. Canadian Cardiovascular Society Guidelines for Evaluation and Management of Cardiovascular Complications of Cancer Therapy. Can. J. Cardiol. 2016, 32, 831–841. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of Cardiac Disease in Cancer Patients throughout Oncological Treatment: ESMO Consensus Recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Omerovic, E.; Citro, R.; Bossone, E.; Redfors, B.; Backs, J.; Bruns, B.; Ciccarelli, M.; Couch, L.S.; Dawson, D.; Grassi, G.; et al. Pathophysiology of Takotsubo Syndrome—A Joint Scientific Statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology—Part 2: Vascular Pathophysiology, Gender and Sex Hormones, Genetics, Chronic Cardiovascular Problems and Clinical Implications. Eur. J. Heart Fail. 2022, 24, 274–286. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Bhatia, S. Genetics of Anthracycline Cardiomyopathy in Cancer Survivors: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2020, 2, 539–552. [Google Scholar] [CrossRef]
- Madeddu, C.; Deidda, M.; Piras, A.; Cadeddu, C.; Demurtas, L.; Puzzoni, M.; Piscopo, G.; Scartozzi, M.; Mercuro, G. Pathophysiology of Cardiotoxicity Induced by Nonanthracycline Chemotherapy. J. Cardiovasc. Med. 2016, 17 (Suppl. 1), e12–e18. [Google Scholar] [CrossRef]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin Induces a Mitochondrial-ROS Response That Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef]
- Scott, S.S.; Greenlee, A.N.; Matzko, A.; Stein, M.; Naughton, M.T.; Zaramo, T.Z.; Schwendeman, E.J.; Mohammad, S.J.; Diallo, M.; Revan, R.; et al. Intracellular Signaling Pathways Mediating Tyrosine Kinase Inhibitor Cardiotoxicity. Heart Fail. Clin. 2022, 18, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.; Cognetti, F.; Maraninchi, D.; Snyder, R.; Mauriac, L.; Tubiana-Hulin, M.; Chan, S.; Grimes, D.; Antón, A.; Lluch, A.; et al. Randomized Phase II Trial of the Efficacy and Safety of Trastuzumab Combined with Docetaxel in Patients with Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer Administered as First-Line Treatment: The M77001 Study Group. J. Clin. Oncol. 2005, 23, 4265–4274. [Google Scholar] [CrossRef]
- Alvi, R.M.; Frigault, M.J.; Fradley, M.G.; Jain, M.D.; Mahmood, S.S.; Awadalla, M.; Lee, D.H.; Zlotoff, D.A.; Zhang, L.; Drobni, Z.D.; et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J. Am. Coll. Cardiol. 2019, 74, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.D.; Cehic, D.A.; Morgia, M.; Bergom, C.; Toohey, J.; Guerrero, P.A.; Ferencik, M.; Kikuchi, R.; Carver, J.R.; Zaha, V.G.; et al. Cardiovascular Manifestations From Therapeutic Radiation: A Multidisciplinary Expert Consensus Statement From the International Cardio-Oncology Society. JACC CardioOncol. 2021, 3, 360–380. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse Cardiac Effects of Cancer Therapies: Cardiotoxicity and Arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef] [PubMed]
- Ederhy, S.; Cautela, J.; Ancedy, Y.; Escudier, M.; Thuny, F.; Cohen, A. Takotsubo-Like Syndrome in Cancer Patients Treated With Immune Checkpoint Inhibitors. JACC Cardiovasc. Imaging 2018, 11, 1187–1190. [Google Scholar] [CrossRef]
- Sinagra, G.; Anzini, M.; Pereira, N.L.; Bussani, R.; Finocchiaro, G.; Bartunek, J.; Merlo, M. Myocarditis in Clinical Practice. Mayo. Clin. Proc. 2016, 91, 1256–1266. [Google Scholar] [CrossRef]
- Zito, C.; Manganaro, R.; Carerj, S.; Antonini-Canterin, F.; Benedetto, F. Peripheral Artery Disease and Stroke. J. Cardiovasc. Echogr. 2020, 30, S17–S25. [Google Scholar] [CrossRef] [PubMed]
- Plummer, C.; Henderson, R.D.; O’Sullivan, J.D.; Read, S.J. Ischemic Stroke and Transient Ischemic Attack after Head and Neck Radiotherapy: A Review. Stroke 2011, 42, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, M.L.; Dorresteijn, L.D.A.; van’t Veer, M.B.; Krol, A.D.G.; van der Pal, H.J.; Kappelle, A.C.; Boogerd, W.; Aleman, B.M.P.; van Leeuwen, F.E. Increased Risk of Stroke and Transient Ischemic Attack in 5-Year Survivors of Hodgkin Lymphoma. J. Natl. Cancer. Inst. 2009, 101, 928–937. [Google Scholar] [CrossRef]
- Haddad, T.C.; Greeno, E.W. Chemotherapy-Induced Thrombosis. Thromb. Res. 2006, 118, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Muñoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 International Clinical Practice Guidelines for the Treatment and Prophylaxis of Venous Thromboembolism in Patients with Cancer, Including Patients with COVID-19. Lancet Oncol. 2022, 23, e334–e347. [Google Scholar] [CrossRef]
- Chiorescu, R.M.; Mocan, M.; Stoia, M.A.; Barta, A.; Goidescu, C.M.; Chiorescu, S.; Farcaş, A.D. Arguments for Using Direct Oral Anticoagulants in Cancer-Related Venous Thromboembolism. Healthcare 2021, 9, 1287. [Google Scholar] [CrossRef]
- Di Nisio, M.; Ferrante, N.; Feragalli, B.; De Tursi, M.; Iacobelli, S.; Cuccurullo, F.; Porreca, E. Arterial Thrombosis in Ambulatory Cancer Patients Treated with Chemotherapy. Thromb. Res. 2011, 127, 382–383. [Google Scholar] [CrossRef]
- Izzedine, H.; Ederhy, S.; Goldwasser, F.; Soria, J.C.; Milano, G.; Cohen, A.; Khayat, D.; Spano, J.P. Management of Hypertension in Angiogenesis Inhibitor-Treated Patients. Ann. Oncol. 2009, 20, 807–815. [Google Scholar] [CrossRef]
- Wu, S.; Chen, J.J.; Kudelka, A.; Lu, J.; Zhu, X. Incidence and Risk of Hypertension with Sorafenib in Patients with Cancer: A Systematic Review and Meta-Analysis. Lancet Oncol. 2008, 9, 117–123. [Google Scholar] [CrossRef]
- Eremina, V.; Jefferson, J.A.; Kowalewska, J.; Hochster, H.; Haas, M.; Weisstuch, J.; Richardson, C.; Kopp, J.B.; Kabir, M.G.; Backx, P.H.; et al. VEGF Inhibition and Renal Thrombotic Microangiopathy. N. Engl. J. Med. 2008, 358, 1129–1136. [Google Scholar] [CrossRef]
- Pothineni, N.V.K.; Van Besien, H.; Fradley, M.G. Arrhythmic Complications Associated with Cancer Therapies. Heart Fail. Clin. 2022, 18, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into Onco-Cardiology: Atrial Fibrillation in Cancer. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Hothi, S.S.; Thompson, A.; Malik, N. Negative Chronotropic Effects and Coronary Ischaemic Abnormalities Following Thalidomide Therapy. Cardiology 2013, 125, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, J.G.; Bonaventura, A.; Vecchié, A.; Wohlford, G.F.; Mauro, A.G.; Jordan, J.H.; Grizzard, J.D.; Montecucco, F.; Berrocal, D.H.; Brucato, A.; et al. Management of Acute and Recurrent Pericarditis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 76–92. [Google Scholar] [CrossRef]
- Hu, J.-R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.N.; Khoobchandani, M.; Brenneman, R.; Mitchell, J.D.; Bergom, C. Radiation-Induced Cardiac Dysfunction: Optimizing Radiation Delivery and Postradiation Care. Heart Fail. Clin. 2022, 18, 403–413. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the Management of Infective Endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Cutter, D.J.; Schaapveld, M.; Darby, S.C.; Hauptmann, M.; van Nimwegen, F.A.; Krol, A.D.G.; Janus, C.P.M.; van Leeuwen, F.E.; Aleman, B.M.P. Risk of Valvular Heart Disease after Treatment for Hodgkin Lymphoma. J. Natl. Cancer Inst. 2015, 107, djv008. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Hancock, S.L.; Lee, B.K.; Mariscal, C.S.; Schnittger, I. Asymptomatic Cardiac Disease Following Mediastinal Irradiation. J. Am. Coll. Cardiol. 2003, 42, 743–749. [Google Scholar] [CrossRef]
- Hering, D.; Faber, L.; Horstkotte, D. Echocardiographic Features of Radiation-Associated Valvular Disease. Am. J. Cardiol. 2003, 92, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Bergot, E.; Günther, S.; Savale, L.; Bergeron, A.; Bourdin, A.; Bouvaist, H.; Canuet, M.; Pison, C.; Macro, M.; et al. Pulmonary Arterial Hypertension in Patients Treated by Dasatinib. Circulation 2012, 125, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of Serum Biomarkers in Cancer Patients Receiving Cardiotoxic Cancer Therapies: A Position Statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef] [PubMed]
- Kwietniak, M.; Al-Amawi, T.; Błaszkowski, T.; Sulżyc-Bielicka, V.; Kładny, J. The Usefulness of D-Dimer in Diagnosis and Prediction of Venous Thromboembolism in Patients with Abdominal Malignancy. Pol. Przegl. Chir. 2017, 89, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology Practical Guidance on the Use of Natriuretic Peptide Concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Neumann, J.T.; Sörensen, N.A.; Blankenberg, S. High-Sensitivity Assays for Troponin in Patients with Cardiac Disease. Nat. Rev. Cardiol. 2017, 14, 472–483. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Mahler, S.A.; Christenson, R.H.; Rymer, J.; Newby, L.K.; Body, R.; Morrow, D.A.; Jaffe, A.S. Recommendations for Institutions Transitioning to High-Sensitivity Troponin Testing: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 1059–1077. [Google Scholar] [CrossRef]
- Finkelman, B.S.; Putt, M.; Wang, T.; Wang, L.; Narayan, H.; Domchek, S.; DeMichele, A.; Fox, K.; Matro, J.; Shah, P.; et al. Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction in Patients With Breast Cancer. J. Am. Coll. Cardiol. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Demissei, B.G.; Freedman, G.; Feigenberg, S.J.; Plastaras, J.P.; Maity, A.; Smith, A.M.; McDonald, C.; Sheline, K.; Simone, C.B.; Lin, L.L.; et al. Early Changes in Cardiovascular Biomarkers with Contemporary Thoracic Radiation Therapy for Breast Cancer, Lung Cancer, and Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 851–860. [Google Scholar] [CrossRef]
- Beer, L.A.; Kossenkov, A.V.; Liu, Q.; Luning Prak, E.; Domchek, S.; Speicher, D.W.; Ky, B. Baseline Immunoglobulin E Levels as a Marker of Doxorubicin- and Trastuzumab-Associated Cardiac Dysfunction. Circ. Res. 2016, 119, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, V.O.-C.; Ferreira, L.R.P.; Ayub-Ferreira, S.M.; Ávila, M.S.; Brandão, S.M.G.; Cruz, F.D.; Santos, M.H.H.; Cruz, C.B.B.V.; Alves, M.S.L.; Issa, V.S.; et al. Circulating MiR-1 as a Potential Biomarker of Doxorubicin-Induced Cardiotoxicity in Breast Cancer Patients. Oncotarget 2017, 8, 6994–7002. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Bossone, E.; Sawaki, D.; Jánosi, R.A.; Erbel, R.; Eagle, K.; Nagai, R. Biomarkers of Aortic Diseases. Am. Heart J. 2013, 165, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Lyon, A.; Saggar, R.; Heaney, L.M.; Aizawa, K.; Cittadini, A.; Mauro, C.; Citro, R.; Limongelli, G.; Ferrara, F.; et al. Editor’s Choice-Biomarkers of Acute Cardiovascular and Pulmonary Diseases. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 416–433. [Google Scholar] [CrossRef]
- Dobric, M.; Ostojic, M.; Giga, V.; Djordjevic-Dikic, A.; Stepanovic, J.; Radovanovic, N.; Beleslin, B. Glycogen Phosphorylase BB in Myocardial Infarction. Clin. Chim. Acta 2015, 438, 107–111. [Google Scholar] [CrossRef]
- Popolo, A.; Adesso, S.; Pinto, A.; Autore, G.; Marzocco, S. L-Arginine and Its Metabolites in Kidney and Cardiovascular Disease. Amino Acids 2014, 46, 2271–2286. [Google Scholar] [CrossRef]
- Otaki, Y.; Watanabe, T.; Kubota, I. Heart-Type Fatty Acid-Binding Protein in Cardiovascular Disease: A Systemic Review. Clin. Chim. Acta 2017, 474, 44–53. [Google Scholar] [CrossRef]
- Chen, Y.; Nilsson, A.H.; Goncalves, I.; Edsfeldt, A.; Engström, G.; Melander, O.; Orho-Melander, M.; Rauch, U.; Tengryd, C.; Venuraju, S.M.; et al. Evidence for a Protective Role of Placental Growth Factor in Cardiovascular Disease. Sci. Transl. Med. 2020, 12, eabc8587. [Google Scholar] [CrossRef]
- Mauricio, R.; Singh, K.; Sanghavi, M.; Ayers, C.R.; Rohatgi, A.; Vongpatanasin, W.; de Lemos, J.A.; Khera, A. Soluble Fms-like Tyrosine Kinase-1 (SFlt-1) Is Associated with Subclinical and Clinical Atherosclerotic Cardiovascular Disease: The Dallas Heart Study. Atherosclerosis 2022, 346, 46–52. [Google Scholar] [CrossRef]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating MicroRNAs Are New and Sensitive Biomarkers of Myocardial Infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef]
- Čelutkienė, J.; Pudil, R.; López-Fernández, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; Haehling, S.; et al. Role of Cardiovascular Imaging in Cancer Patients Receiving Cardiotoxic Therapies: A Position Statement on Behalf of the H Eart F Ailure A Ssociation (HFA), the E Uropean A Ssociation of C Ardiovascular I Maging (EACVI) and the Cardio-Oncology C Ouncil of the E Uropean S Ociety of C Ardiology (ESC). Eur. J. Heart Fail. 2020, 22, 1504–1524. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S.; Khandheria, B.; Stainback, R.F.; Weissman, N.J.; Brindis, R.G.; Patel, M.R.; Khandheria, B.; Alpert, J.S.; Fitzgerald, D.; Heidenreich, P.; et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 Appropriateness Criteria for Transthoracic and Transesophageal Echocardiography: A Report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J. Am. Coll. Cardiol. 2007, 50, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Flachskampf, F.A.; Decoodt, P.; Fraser, A.G.; Daniel, W.G.; Roelandt, J.R.; Subgroup on Transesophageal Echocardiography and Valvular Heart Disease; Working Group on Echocardiography of the European Society of Cardiology Guidelines from the Working Group. Recommendations for Performing Transesophageal Echocardiography. Eur. J. Echocardiogr. 2001, 2, 8–21. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS): Document Covering Atherosclerotic Disease of Extracranial Carotid and Vertebral, Mesenteric, Renal, Upper and Lower Extremity ArteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Grothues, F.; Smith, G.C.; Moon, J.C.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of Interstudy Reproducibility of Cardiovascular Magnetic Resonance with Two-Dimensional Echocardiography in Normal Subjects and in Patients with Heart Failure or Left Ventricular Hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
- Contaldi, C.; Dellegrottaglie, S.; Mauro, C.; Ferrara, F.; Romano, L.; Marra, A.M.; Ranieri, B.; Salzano, A.; Rega, S.; Scatteia, A.; et al. Role of Cardiac Magnetic Resonance Imaging in Heart Failure. Heart Fail. Clin. 2021, 17, 207–221. [Google Scholar] [CrossRef]
- Jordan, J.H.; Todd, R.M.; Vasu, S.; Hundley, W.G. Cardiovascular Magnetic Resonance in the Oncology Patient. JACC Cardiovasc. Imaging 2018, 11, 1150–1172. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Wintersperger, B.J.; Flamm, S.D.; Marwick, T.H. Cardiac MRI in the Assessment of Cardiac Injury and Toxicity from Cancer Chemotherapy: A Systematic Review. Circ. Cardiovasc. Imaging 2013, 6, 1080–1091. [Google Scholar] [CrossRef]
- Galán-Arriola, C.; Lobo, M.; Vílchez-Tschischke, J.P.; López, G.J.; de Molina-Iracheta, A.; Pérez-Martínez, C.; Agüero, J.; Fernández-Jiménez, R.; Martín-García, A.; Oliver, E.; et al. Serial Magnetic Resonance Imaging to Identify Early Stages of Anthracycline-Induced Cardiotoxicity. J. Am. Coll. Cardiol. 2019, 73, 779–791. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Olenchock, B.A.; Salem, J.-E.; Wiviott, S.D.; Ederhy, S.; Cohen, A.; Stewart, G.C.; Choueiri, T.K.; Di Carli, M.; Allenbach, Y.; et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation 2019, 140, 80–91. [Google Scholar] [CrossRef]
- Lee, S.P.; Park, J.B.; Kim, H.K.; Kim, Y.J.; Grogan, M.; Sohn, D.W. Contemporary Imaging Diagnosis of Cardiac Amyloidosis. J. Cardiovasc. Imaging 2019, 27, 1–10. [Google Scholar] [CrossRef]
- Mousavi, N.; Cheezum, M.K.; Aghayev, A.; Padera, R.; Vita, T.; Steigner, M.; Hulten, E.; Bittencourt, M.S.; Dorbala, S.; Di Carli, M.F.; et al. Assessment of Cardiac Masses by Cardiac Magnetic Resonance Imaging: Histological Correlation and Clinical Outcomes. J. Am. Heart Assoc. 2019, 8, e007829. [Google Scholar] [CrossRef]
- Harries, I.; Liang, K.; Williams, M.; Berlot, B.; Biglino, G.; Lancellotti, P.; Plana, J.C.; Bucciarelli-Ducci, C. Magnetic Resonance Imaging to Detect Cardiovascular Effects of Cancer Therapy: JACC CardioOncology State-of-the-Art Review. Cardio Oncol. 2020, 2, 270–292. [Google Scholar] [CrossRef]
- Gambril, J.A.; Chum, A.; Goyal, A.; Ruz, P.; Mikrut, K.; Simonetti, O.; Dholiya, H.; Patel, B.; Addison, D. Cardiovascular Imaging in Cardio-Oncology: The Role of Echocardiography and Cardiac MRI in Modern Cardio-Oncology. Heart Fail. Clin. 2022, 18, 455–478. [Google Scholar] [CrossRef]
- Soufer, A.; Liu, C.; Henry, M.L.; Baldassarre, L.A. Nuclear Cardiology in the Context of Multimodality Imaging to Detect Cardiac Toxicity from Cancer Therapeutics: Established and Emerging Methods. J. Nucl. Cardiol. 2020, 27, 1210–1224. [Google Scholar] [CrossRef]
- van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.M.; Krol, A.D.G.; Petersen, E.J.; Raemaekers, J.M.M.; Kok, W.E.M.; Aleman, B.M.P.; van Leeuwen, F.E. Cardiovascular Disease after Hodgkin Lymphoma Treatment: 40-Year Disease Risk. JAMA Intern. Med. 2015, 175, 1007–1017. [Google Scholar] [CrossRef]
- Daniëls, L.A.; Krol, A.D.G.; de Graaf, M.A.; Scholte, A.J.H.A.; Van’t Veer, M.B.; Putter, H.; de Roos, A.; Schalij, M.J.; Creutzberg, C.L. Screening for Coronary Artery Disease after Mediastinal Irradiation in Hodgkin Lymphoma Survivors: Phase II Study of Indication and Acceptance†. Ann. Oncol. 2014, 25, 1198–1203. [Google Scholar] [CrossRef]
- Meijboom, W.B.; van Mieghem, C.A.G.; Mollet, N.R.; Pugliese, F.; Weustink, A.C.; van Pelt, N.; Cademartiri, F.; Nieman, K.; Boersma, E.; de Jaegere, P.; et al. 64-Slice Computed Tomography Coronary Angiography in Patients with High, Intermediate, or Low Pretest Probability of Significant Coronary Artery Disease. J. Am. Coll. Cardiol. 2007, 50, 1469–1475. [Google Scholar] [CrossRef]
- Plana, J.C.; Thavendiranathan, P.; Bucciarelli-Ducci, C.; Lancellotti, P. Multi-Modality Imaging in the Assessment of Cardiovascular Toxicity in the Cancer Patient. JACC Cardiovasc. Imaging 2018, 11, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the Diagnosis and Management of Pericardial Diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. [2018 ESC/ESH Guidelines for the management of arterial hypertension]. Kardiol. Pol. 2019, 77, 71–159. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Rev. Esp. Cardiol. 2021, 74, 544. [Google Scholar] [CrossRef]

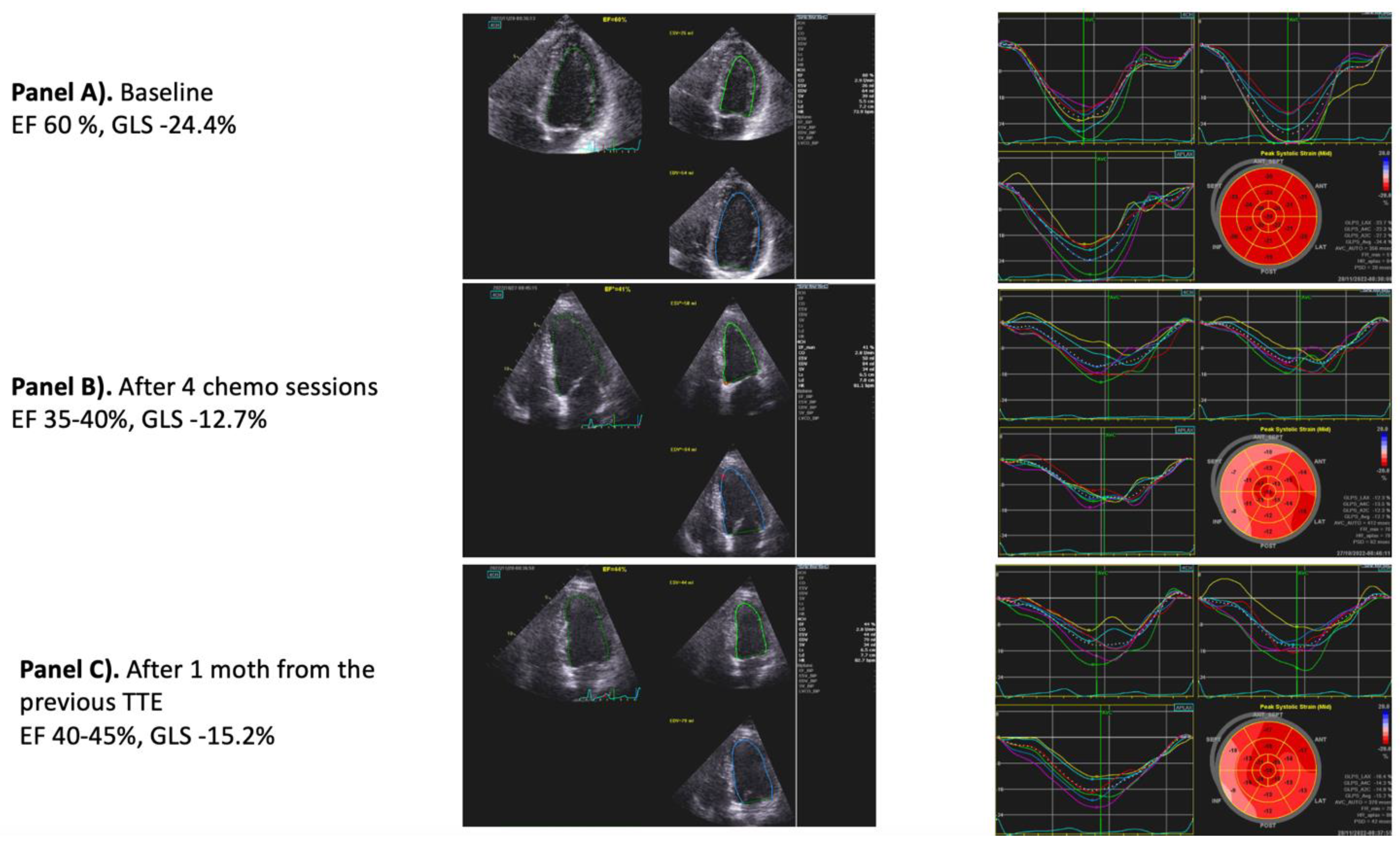

| Chemotherapy | CTRCD | Myocarditis | Vascular Toxicities | Arterial Hypertension | Cardiac Arrhythmias | Pericardial Diseases | PH | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stroke, TIA | MI, ACS, CCS, ATS | PAD | Vasospastic/ Microvascular Angina, Abnormal Vasoreactivity, Raynaud’s Phenomenon | VTE, Arterial Thrombosis | |||||||
| Anthracycline | ++ | ||||||||||
| HER2-targeted therapies | ++ | ||||||||||
| Fluoropyrimidines | ++ * | + | + | ++ | + | ++ | + § | ||||
| VEGFi | + | + | + | + | ++ | + § | |||||
| 1st generation BCR-ABL TKI (Imatinib) | + | + | |||||||||
| 2nd generation BCR-ABL TKI (Nilotinib, Dasatinib, Bosutinib) | + | + | + | + | + | + §# | + | + | |||
| 3rd generation BCR-ABL TKI (Ponatinib) | + | + | + | + | ++ | + # | + | + | |||
| Alkylating agents (Cyclophosphamide, Melphalan) | + | ++ # | |||||||||
| Immunomodulatory drugs (Lenalidomide, Pomalidomide, Thalidomide) | + | + | ++ | + | + # | ||||||
| Proteasome inhibitors (Bortezomib, Carfilzomib) | + | + | ++ | + # | + | ||||||
| Monoclonal antibodies (Daratumumab, Elotuzumab, Isatuxmab) | + | + | ++ | + # | |||||||
| RAF inhibitors | + | ++ | ++ | + § | |||||||
| MEK inhibitors | ++ | ++ | |||||||||
| Androgen deprivation therapy | + | + | ++ | + # | |||||||
| ALK inhibitors | ++ | + § | |||||||||
| EGFR inhibitors | + | + | |||||||||
| CAR-T therapy | + | + # | + | ||||||||
| TIL-therapy | + | + | + | + | |||||||
| ICI | + | ++ | |||||||||
| Biomarkers | Disease | Characteristics |
|---|---|---|
| cTn | ACS, HF, PE | Cardiac-specific structural proteins composing the contractile apparatus of cardiomyocytes |
| BNP and NT-proBNP | HF, PE | Inactive form (prohormone) of BNP secreted by cardiomyocytes from increased transmural tension and neurohormonal stimulation (notably by noradrenaline and angiotensin II) |
| Myeloperoxidase | ACS | Released into extracellular fluid in response to inflammatory processes |
| High-sensitivity C-reactive protein | Aortic dissection, ACS | Marker of evolution of false lumen thrombosis |
| sFlt-1 | Atherosclerotic cardiovascular disease | Markers of inflammation, endothelial function, and myocardial stress or injury. |
| Placental growth factor | ACS | Mitogen for endothelial cells; it can also act as a proinflammatory cytokine |
| Growth differentiation factor-15 | Myocarditis | Marker of extracellular matrix degradation |
| Galectin-3 | HF | Marker of cardiac and vascular fibrosis |
| Arginine | HTN, PH, atherosclerosis, and vasospasm | Endothelial dysfunction as Arg is the main source for the generation of NO via NOS. |
| H-FABP | ACS, HF, arrhythmia, PE | A dominant isoform present in the heart and skeletal muscles acting as marker of ongoing myocardial damage |
| Glycogen phosphorylase BB | ACS | Provide the fuel for the energy supply required for myocardial contraction |
| Immunoglobulin E | Cardiac dysfunction, HF | Dysregulation of the inflammatory response due could worse cardiac remodeling to cardiac injury |
| microRNAs | ACS | Non-coding RNAs which inhibit mRNA translation or induce its degradation; involved in all cardiac functions, including the conductance of electrical signals, heart muscle contraction, and growth |
| Parameters | Clinically Significant Changes |
|---|---|
| LV size and function | |
| LVEF by Simpson’s 2D, or (semi)automatic 3D | Drop >10% (percentage points) for 2D, >5% for 3D from pre-treatment value |
| 2D/3D GLS, GCS | Relative reduction by >10–15% from pre-treatment value and to below lower limit of normal |
| LV 2D/3D systolic and diastolic volumes | Increase by 15 mL for ESV, 30–35 mL for EDV |
| RV function, pulmonary artery pressure, and volemia | |
| Markers of systolic RV function | TAPSE < 1.7 cm, FAC < 35%, RV free wall strain < 20%, 3D RVEF < 45% |
| Velocity of TR | Peak systolic TR velocity > 2.8 m/s |
| IVC diameter, collapse on inspiration | Dilatation > 2.1 cm or narrowing < 1.3 cm |
| VHD | Valvular calcification; valve regurgitation/stenosis |
| Pericardium | Pericardial effusion, cardiac tamponade, constrictive physiology |
| Topic | Guidelines | Ref. |
|---|---|---|
| Cardio-oncology | 2022 ESC Guidelines on cardio-oncology. Eur. Heart J. | [5,7] |
| Canadian Cardiovascular Society Guidelines for Cardiovascular Complications of Cancer Therapy. Can. J. Cardiol. | ||
| HF | 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. | [36] |
| Infective endocarditis | 2015 ESC Guidelines for the management of infective endocarditis. Eur. Heart J. | [37] |
| VHD | 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. | [38] |
| PH | 2022 ESC/ERS Guidelines for the diagnosis and treatment of PH. Eur. Heart J. | [42] |
| PAD | 2017 ESC Guidelines on the Diagnosis and Treatment of PAD. Eur. Heart J. | [65] |
| Aortic Disease | 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease. Circulation. | [66] |
| CCS | 2019 ESC Guidelines for the diagnosis and management of CCS. Eur. Heart J. | [82] |
| ACS | 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. | [83,87] |
| 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. | ||
| Pericardial diseases | 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur. Heart J. | [84] |
| HTN | 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. | [85] |
| AF | 2020 ESC Guidelines for the diagnosis and management of AF. Eur. Heart J. | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauro, C.; Capone, V.; Cocchia, R.; Cademartiri, F.; Riccardi, F.; Arcopinto, M.; Alshahid, M.; Anwar, K.; Carafa, M.; Carbone, A.; et al. Exploring the Cardiotoxicity Spectrum of Anti-Cancer Treatments: Definition, Classification, and Diagnostic Pathways. J. Clin. Med. 2023, 12, 1612. https://doi.org/10.3390/jcm12041612
Mauro C, Capone V, Cocchia R, Cademartiri F, Riccardi F, Arcopinto M, Alshahid M, Anwar K, Carafa M, Carbone A, et al. Exploring the Cardiotoxicity Spectrum of Anti-Cancer Treatments: Definition, Classification, and Diagnostic Pathways. Journal of Clinical Medicine. 2023; 12(4):1612. https://doi.org/10.3390/jcm12041612
Chicago/Turabian StyleMauro, Ciro, Valentina Capone, Rosangela Cocchia, Filippo Cademartiri, Ferdinando Riccardi, Michele Arcopinto, Maie Alshahid, Kashif Anwar, Mariano Carafa, Andreina Carbone, and et al. 2023. "Exploring the Cardiotoxicity Spectrum of Anti-Cancer Treatments: Definition, Classification, and Diagnostic Pathways" Journal of Clinical Medicine 12, no. 4: 1612. https://doi.org/10.3390/jcm12041612
APA StyleMauro, C., Capone, V., Cocchia, R., Cademartiri, F., Riccardi, F., Arcopinto, M., Alshahid, M., Anwar, K., Carafa, M., Carbone, A., Castaldo, R., Chianese, S., Crisci, G., D’Assante, R., De Luca, M., Franzese, M., Galzerano, D., Maffei, V., Marra, A. M., ... Salzano, A. (2023). Exploring the Cardiotoxicity Spectrum of Anti-Cancer Treatments: Definition, Classification, and Diagnostic Pathways. Journal of Clinical Medicine, 12(4), 1612. https://doi.org/10.3390/jcm12041612









