Low-Dose Atropine Induces Changes in Ocular Biometrics in Myopic Children: Exploring Temporal Changes by Linear Mixed Models and Contribution to Treatment Effect by Mediation Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Examinations
2.3. Sample Size and Statistical Analysis
2.4. Approvals
3. Results
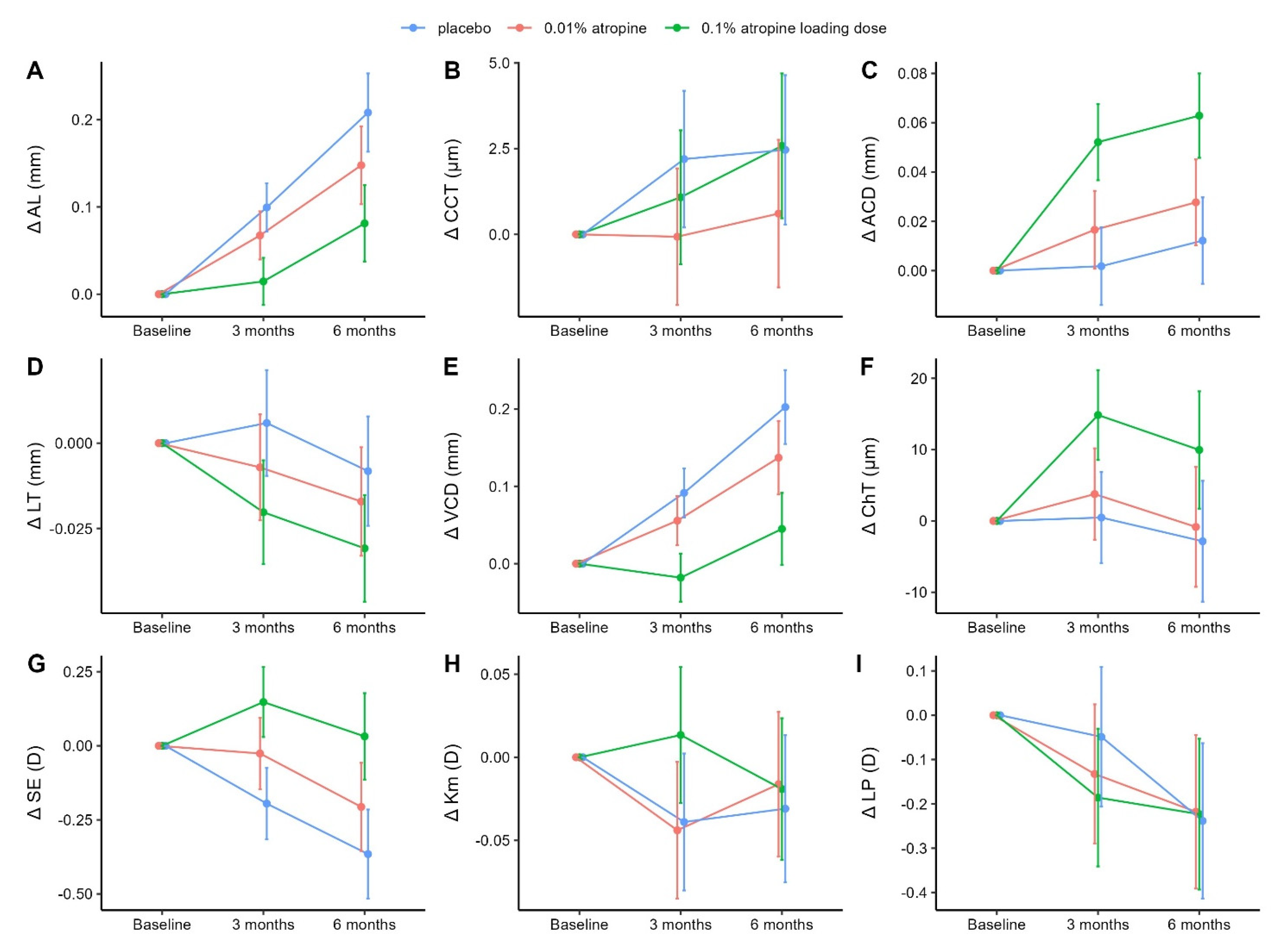
3.1. Changes in Axial Length, Central Corneal Thickness, and Anterior Chamber Depth
3.2. Changes in Lens Thickness, Vitreous Chamber Depth, and Choroidal Thickness
3.3. Changes in Spherical Equivalent, Mean Anterior Corneal Curvature, and Lens Power
3.4. Treatment Effect on SE Progression Mediated by Ocular Biometric Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baird, P.N.; Saw, S.-M.; Lanca, C.; Guggenheim, J.A.; Smith Iii, E.L.; Zhou, X.; Matsui, K.O.; Wu, P.C.; Sankaridurg, P.; Chia, A.; et al. Myopia. Nat. Rev. Dis. Prim. 2020, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.M.; Verhoeven, V.; Cumberland, P.M.; Bertelsen, G.; Wolfram, C.; Buitendijk, G.H.S.; Hofman, A.; Van Duijn, C.M.; Vingerling, J.R.; Kuijpers, R.W.A.M.; et al. Prevalence of refractive error in Europe: The European Eye Epidemiology (E3) Consortium. Eur. J. Epidemiol. 2015, 30, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Saw, S.-M.; Gazzard, G.; Shih-Yen, E.C.; Chua, W.-H. Myopia and associated pathological complications. Ophthalmic Physiol. Opt. 2005, 25, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Kuno, Y. Overview of the Complications of High Myopia. Retina 2017, 37, 2347–2351. [Google Scholar]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Brennan, N.A.; Toubouti, Y.M.; Cheng, X.; Bullimore, M.A. Efficacy in myopia control. Prog. Retin. Eye Res. 2021, 83, 100923. [Google Scholar] [CrossRef]
- Chia, A.; Chua, W.-H.; Cheung, Y.-B.; Wong, W.-L.; Lingham, A.; Fong, A.; Tan, D. Atropine for the Treatment of Childhood Myopia: Safety and Efficacy of 0.5%, 0.1%, and 0.01% Doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012, 119, 347–354. [Google Scholar] [CrossRef]
- Li, F.F.; Yam, J.C. Low-Concentration Atropine Eye Drops for Myopia Progression. Asia-Pac. J. Ophthalmol. 2019, 8, 360–365. [Google Scholar] [CrossRef]
- Li, F.F.; Kam, K.W.; Zhang, Y.; Tang, S.M.; Young, A.L.; Chen, L.J.; Tham, C.C.; Pang, C.P.; Yam, J.C. Differential Effects on Ocular Biometrics by 0.05%, 0.025%, and 0.01% Atropine: Low-Concentration Atropine for Myopia Progression Study. Ophthalmology 2020, 127, 1603–1611. [Google Scholar] [CrossRef]
- Gao, L.; Zhuo, X.; Kwok, A.K.H.; Yu, N.; Ma, L.; Wang, J. The change in ocular refractive components after cycloplegia in children. Jpn. J. Ophthalmol. 2002, 46, 293–298. [Google Scholar] [CrossRef]
- Bennett, A.G. A method of determining the equivalent powers of the eye and its crystalline lens without resort to phakometry. Ophthalmic. Physiol. Opt. 1988, 8, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Rozema, J.J.; Atchison, D.A.; Tassignon, M.-J. Comparing methods to estimate the human lens power. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7937–7942. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.-H.; Balakrishnan, V.; Chan, Y.-H.; Tong, L.; Ling, Y.; Quah, B.-L.; Tan, D. Atropine for the Treatment of Childhood Myopia. Ophthalmology 2006, 113, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H. Myopia progression in young school children. A prospective study of myopia progression and the effect of a trial with bifocal lenses and beta blocker eye drops. Acta Ophthalmol. Suppl. 1991, 200, 1–79. [Google Scholar]
- Vander Weele, T.J.; Vansteelandt, S. Conceptual issues concerning mediation, interventions and composition. Stat. Its Interface 2009, 2, 457–468. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Ozenne, B.; Forman, J. LMMstar: Repeated Measurement Models for Discrete Times. Available online: https://cran.r-project.org/web/packages/LMMstar/index.html (accessed on 2 January 2023).
- Holst, K.K.; Budtz-Jørgensen, E. Linear latent variable models: The lava-package. Comput Stat. 2013, 28, 1385–1452. [Google Scholar] [CrossRef]
- Atchison, D.; Smith, G. Optics of the Human Eye; Butterworth-Heinemann: Edinburgh, UK, 2000. [Google Scholar]
- Kumaran, A.; Htoon, H.M.; Tan, D.; Chia, A. Analysis of Changes in Refraction and Biometry of Atropine- and Placebo-Treated Eyes. Investig. Opthalmology Vis. Sci. 2015, 56, 5650–5655. [Google Scholar] [CrossRef]
- Wang, T.; Liu, L.; Li, Z.; Hu, S.; Yang, W.; Zhu, X. An ultrasound biomicroscopic study on changes of the structure of ocular anterior segment after topical application of cycloplegia. Zhonghua Yan Ke Za Zhi 1998, 34, 137–140. [Google Scholar]
- Li, W.; Jiang, R.; Zhu, Y.; Zhou, J.; Cui, C. Effect of 0.01% atropine eye drops on choroidal thickness in myopic children. J. Fr. Ophtalmol. 2020, 43, 862–868. [Google Scholar] [CrossRef]
- Yam, J.C.; Jiang, Y.; Lee, J.; Li, S.; Zhang, Y.; Sun, W.; Yuan, N.; Wang, Y.M.; Yip, B.H.K.; Kam, K.W.; et al. The Association of Choroidal Thickening by Atropine with Treatment Effects for Myopia: Two-Year Clinical Trial of the Low-concentration Atropine for Myopia Progression (LAMP) Study. Am. J. Ophthalmol. 2022, 237, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Yam, J.C.; Jiang, Y.; Tang, S.M.; Law, A.K.; Chan, J.J.; Wong, E.; Ko, S.T.; Young, A.L.; Tham, C.C.; Chen, L.J.; et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology 2019, 126, 113–124. [Google Scholar] [CrossRef]
- Yam, J.C.; Zhang, X.J.; Zhang, Y.; Wang, Y.M.; Tang, S.M.; Li, F.F.; Kam, K.W.; Ko, S.T.; Yip, B.H.; Young, A.L.; et al. Three-Year Clinical Trial of Low-Concentration Atropine for Myopia Progression (LAMP) Study: Continued Versus Washout: Phase 3 Report. Ophthalmology 2022, 129, 308–321. [Google Scholar] [CrossRef]

| Treatment Groups | ||||
|---|---|---|---|---|
| Characteristic | All | Placebo | Low Dose (0.01% Atropine) | Loading Dose (0.1% to 0.01% Atropine) |
| N (%) | 97 | 32 (33.0) | 32 (33.0) | 33 (34.0) |
| Female/male (%) | 55/42 (57/43) | 18/14 (56.2/43.8) | 18/14 (56.2/43.8) | 19/14 (57.6/42.4) |
| Age, mean (SD), years | 9.4 (1.7) | 9.2 (1.6) | 9.4 (1.9) | 9.5 (1.5) |
| AL, mean (SD), mm | 24.48 (0.84) | 24.41 (0.90) | 24.56 (0.78) | 24.48 (0.86) |
| CCT, mean (SD), µm | 546.8 (30.3) | 546.0 (35.1) | 546.4 (25.7) | 547.8 (30.3) |
| ACD, mean (SD), mm | 3.31 (0.24) | 3.32 (0.27) | 3.33 (0.26) | 3.28 (0.19) |
| LT, mean (SD), mm | 3.36 (0.17) | 3.36 (0.13) | 3.36 (0.17) | 3.36 (0.20) |
| VCD, mean (SD), mm | 17.27 (0.86) | 17.18 (0.89) | 17.33 (0.76) | 17.30 (0.95) |
| ChT, mean (SD), µm | 248 (66.2) | 244 (65.1) | 260 (66.7) | 240 (67.2) |
| SE, mean (SD), D | −3.02 (1.27) | −3.07 (1.04) | −2.97 (1.13) | −3.0 (1.59) |
| Corneal power | ||||
| K1, mean (SD), D | 43.2 (1.5) | 43.4 (1.2) | 43.0 (1.2) | 43.3 (1.9) |
| K2, mean (SD), D | 44.1 (1.5) | 44.3 (1.3) | 43.9 (1.2) | 44.1 (2.0) |
| Km, mean (SD), D | 43.7 (1.5) | 43.8 (1.2) | 43.5 (1.2) | 43.7 (2.0) |
| LP, mean (SD), D | 22.5 (1.46) | 22.6 (1.44) | 22.4 (1.28) | 22.4 (1.66) |
| Treatment Groups | |||
|---|---|---|---|
| Measurement | Placebo † | Low Dose (0.01% Atropine) ‡ | Loading Dose (0.1% to 0.01% Atropine) ‡ |
| AL, mm | |||
| Baseline § | 24.60 (24.35 to 24.86) | ― | ― |
| 3-month change | 0.10 (0.07 to 0.13) | −0.03 (−0.06 to 0.00) | −0.08 (−0.12 to −0.05) |
| p value/adj-p value || | ― | 0.054/0.114 | <0.001/<0.001 |
| 6-month change | 0.21 (0.16 to 0.25) | −0.06 (−0.11 to −0.01) | −0.13 (−0.18 to −0.07) |
| p value/adj-p value || | ― | 0.025/0.060 | <0.001/<0.001 |
| CCT, µm | |||
| Baseline § | 551.2 (542.0 to 560.4) | ― | ― |
| 3-month change | 2.2 (0.2 to 4.2) | −2.3 (−4.6 to 0.1) | −1.1 (−3.5 to 1.2) |
| p value/adj-p value || | ― | 0.060/0.115 | 0.348/0.464 |
| 6-month change | 2.5 (0.3 to 4.6) | −1.9 (−4.4 to 0.7) | 0.1 (−2.4 to 2.7) |
| p value/adj-p value || | ― | 0.152/0.225 | 0.928/0.928 |
| ACD, mm | |||
| Baseline § | 3.30 (3.23 to 3.37) | ― | ― |
| 3-month change | 0.00 (−0.01 to 0.02) | 0.01 (0.00 to 0.03) | 0.05 (0.03 to 0.07) |
| p value/adj-p value || | ― | 0.117/0.201 | <0.001/<0.001 |
| 6-month change | 0.01 (−0.01 to 0.03) | 0.02 (−0.01 to 0.04) | 0.05 (0.03 to 0.07) |
| p value/adj-p value || | ― | 0.137/0.224 | <0.001/<0.001 |
| LT, mm | |||
| Baseline § | 3.33 (3.28 to 3.38) | ― | ― |
| 3-month change | 0.01 (−0.01 to 0.02) | −0.01 (−0.03 to 0.01) | −0.03 (−0.04 to −0.01) |
| p value/adj-p value || | ― | 0.156/0.225 | 0.005/0.016 |
| 6-month change | −0.01 (−0.02 to 0.01) | −0.01 (−0.03 to 0.01) | −0.02 (−0.04 to 0.00) |
| p value/adj-p value || | ― | 0.338/0.464 | 0.016/0.048 |
| VCD, mm | |||
| Baseline § | 17.43 (17.17 to 17.69) | ― | ― |
| 3-month change | 0.09 (0.06 to 0.12) | −0.04 (−0.07 to 0.00) | −0.11 (−0.15 to −0.07) |
| p value/adj-p value || | ― | 0.061/0.115 | <0.001/<0.001 |
| 6-month change | 0.20 (0.15 to 0.25) | −0.07 (−0.12 to −0.01) | −0.16 (−0.21 to −0.10) |
| p value/adj-p value || | ― | 0.022/0.058 | <0.001/<0.001 |
| ChT, µm | |||
| Baseline § | 242.1 (221.9 to 262.3) | ― | ― |
| 3-month change | 0.5 (−5.9 to 6.9) | 3.3 (−4.3 to 10.9) | 14.4 (6.8 to 21.9) |
| p value/adj-p value || | ― | 0.392/0.486 | <0.001/0.001 |
| 6-month change | −2.8 (−11.3 to 5.6) | 2.0 (−7.9 to 12.0) | 12.8 (2.9 to 22.7) |
| p value/adj-p value || | ― | 0.689/0.775 | 0.012/0.039 |
| SE, D | |||
| Baseline § | −2.99 (−3.37 to −2.60) | ― | ― |
| 3-month change | −0.20 (−0.32 to −0.07) | 0.17 (0.03 to 0.31) | 0.34 (0.20 to 0.48) |
| p value/adj-p value || | ― | 0.021/0.057 | <0.001/<0.001 |
| 6-month change | −0.37 (−0.52 to −0.21) | 0.16 (−0.02 to 0.34) | 0.40 (0.22 to 0.57) |
| p value/adj-p value || | ― | 0.077/0.138 | <0.001/<0.001 |
| Km, D | |||
| Baseline § | 43.46 (43.02 to 43.91) | ― | ― |
| 3-month change | −0.04 (−0.08 to 0.00) | 0.00 (−0.05 to 0.04) | 0.05 (0.00 to 0.10) |
| p value/adj-p value || | ― | 0.840/0.893 | 0.036/0.080 |
| 6-month change | −0.03 (−0.08 to 0.01) | 0.01 (−0.04 to 0.07) | 0.01 (−0.04 to 0.06) |
| p value/adj-p value || | ― | 0.574/0.689 | 0.648/0.753 |
| LP, D | |||
| Baseline § | 22.24 (21.80 to 22.67) | ― | ― |
| 3-month change | −0.05 (−0.21 to 0.11) | −0.08 (−0.27 to 0.10) | −0.14 (−0.32 to 0.05) |
| p value/adj-p value || | ― | 0.376/0.483 | 0.146/0.225 |
| 6-month change | −0.24 (−0.41 to −0.06) | 0.02 (−0.19 to 0.23) | 0.02 (−0.19 to 0.22) |
| p value/adj-p value || | ― | 0.843/0.893 | 0.883/0.909 |
| Estimates | Differences | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo vs. Low Dose (0.01% Atropine) | Placebo vs. Loading Dose (0.1% to 0.01% Atropine) | Effect Size | Differences in Effect Size | ||||||
| Estim. | (95% CI) | % † | Estim. | (95% CI) | % † | Estim. | (95% CI) | p/adj-p ‡ | |
| 3 months (N = 92) | |||||||||
| Direct (δD,3) | 0.02 | (−0.04; +0.08) | 11.5 | −0.01 | (−0.08; +0.06) | −4.1 | −0.03 | (−0.10; +0.03) | 0.336/0.537 |
| Indirect (δI,3) | 0.14 | (−0.01; +0.29) | 88.5 | 0.33 | (0.17; 0.48) | 104.1 | 0.18 | (0.03; 0.34) | 0.020/― |
| AL, mm (δAL,3) | 0.08 | (−0.01; +0.17) | 52.0 | 0.22 | (0.13; 0.32) | 70.9 | 0.14 | (0.05; 0.23) | 0.003/0.023 |
| LP, D (δLP,3) | 0.06 | (−0.07; +0.18) | 35.1 | 0.09 | (−0.03; +0.21) | 29.1 | 0.03 | (−0.09; +0.16) | 0.583/0.777 |
| ChT, mm (δChT,3) | 0.00 | (−0.01; +0.01) | 1.4 | 0.01 | (−0.01; +0.04) | 4.1 | 0.01 | (−0.01; +0.03) | 0.306/0.537 |
| Total (δT,3) | 0.16 | (0.00; 0.32) | 100.0 | 0.31 | (0.15; 0.48) | 100.0 | 0.15 | (−0.01; +0.31) | 0.068/― |
| 6 months (N = 94) | |||||||||
| Direct (δD,6) | 0.02 | (−0.04; +0.09) | 15.2 | 0.08 | (0.01; 0.14) | 20.1 | 0.05 | (−0.01; +0.12) | 0.121/0.322 |
| Indirect (δI,6) | 0.13 | (−0.06; +0.33) | 84.8 | 0.30 | (0.10; 0.50) | 79.9 | 0.16 | (−0.03; +0.36) | 0.101/― |
| AL, mm (δAL,6) | 0.15 | (0.01; +0.29) | 93.7 | 0.32 | (0.18; 0.46) | 86.0 | 0.17 | (0.03; 0.31) | 0.015/0.061 |
| LP, D (δLP,6) | −0.01 | (−0.15; +0.12) | −8.9 | −0.02 | (−0.16; +0.12) | −6.1 | −0.01 | (−0.15; +0.13) | 0.901/0.995 |
| ChT, mm (δChT,6) | 0.00 | (−0.00; +0.00) | 0.0 | 0.00 | (−0.02; +0.02) | 0.0 | 0.00 | (−0.01; 0.01) | 0.995/0.995 |
| Total (δT,6) | 0.16 | (−0.05; +0.36) | 100.0 | 0.37 | (0.17; 0.58) | 100.0 | 0.22 | (0.01; 0.42) | 0.039/― |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hvid-Hansen, A.; Jacobsen, N.; Hjortdal, J.; Møller, F.; Ozenne, B.; Kessel, L. Low-Dose Atropine Induces Changes in Ocular Biometrics in Myopic Children: Exploring Temporal Changes by Linear Mixed Models and Contribution to Treatment Effect by Mediation Analyses. J. Clin. Med. 2023, 12, 1605. https://doi.org/10.3390/jcm12041605
Hvid-Hansen A, Jacobsen N, Hjortdal J, Møller F, Ozenne B, Kessel L. Low-Dose Atropine Induces Changes in Ocular Biometrics in Myopic Children: Exploring Temporal Changes by Linear Mixed Models and Contribution to Treatment Effect by Mediation Analyses. Journal of Clinical Medicine. 2023; 12(4):1605. https://doi.org/10.3390/jcm12041605
Chicago/Turabian StyleHvid-Hansen, Anders, Nina Jacobsen, Jesper Hjortdal, Flemming Møller, Brice Ozenne, and Line Kessel. 2023. "Low-Dose Atropine Induces Changes in Ocular Biometrics in Myopic Children: Exploring Temporal Changes by Linear Mixed Models and Contribution to Treatment Effect by Mediation Analyses" Journal of Clinical Medicine 12, no. 4: 1605. https://doi.org/10.3390/jcm12041605
APA StyleHvid-Hansen, A., Jacobsen, N., Hjortdal, J., Møller, F., Ozenne, B., & Kessel, L. (2023). Low-Dose Atropine Induces Changes in Ocular Biometrics in Myopic Children: Exploring Temporal Changes by Linear Mixed Models and Contribution to Treatment Effect by Mediation Analyses. Journal of Clinical Medicine, 12(4), 1605. https://doi.org/10.3390/jcm12041605







