Coronary Microcirculation: The Next Frontier in the Management of STEMI
Abstract
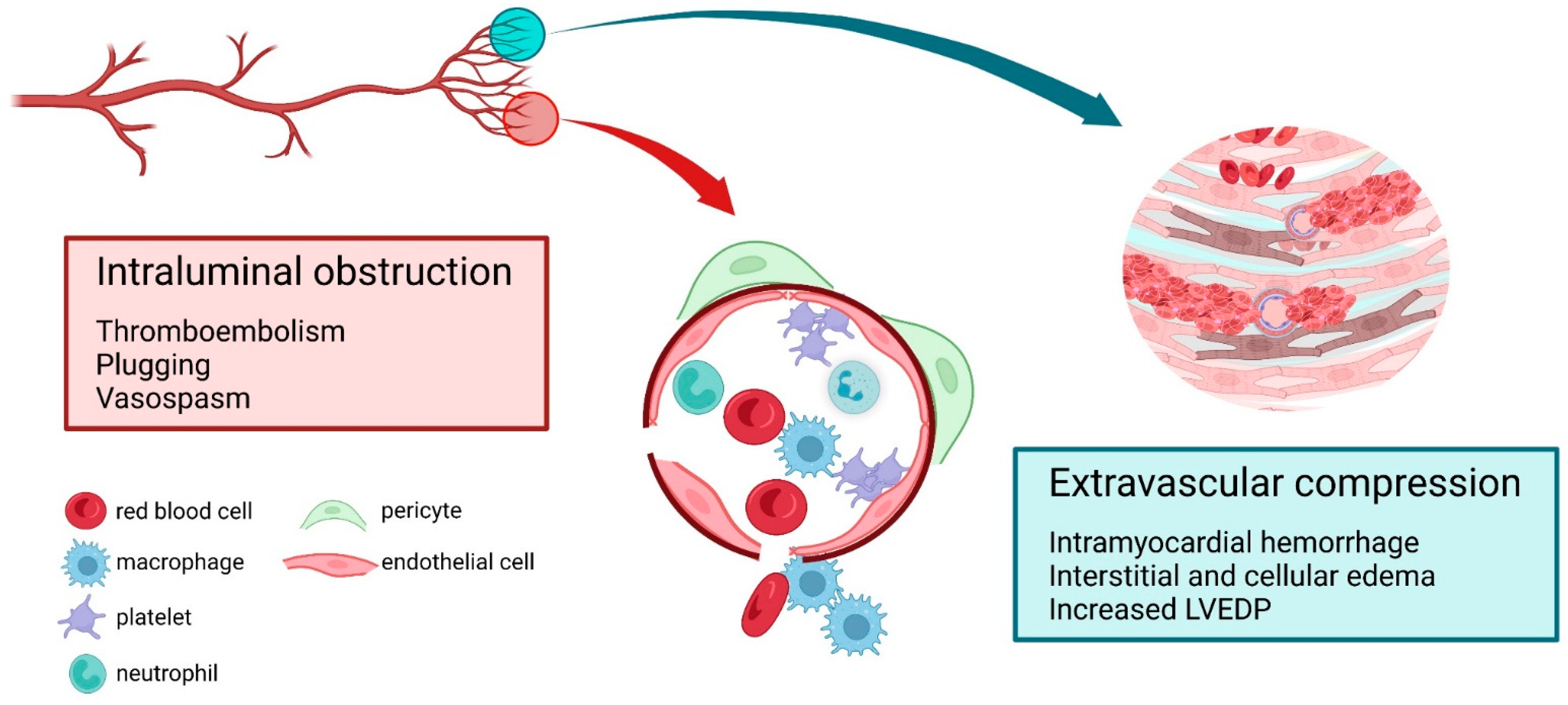
:1. Introduction
2. Frequency and Prognosis of CMD in Patients with STEMI
3. Invasive Assessment of CMD following Primary PCI
3.1. Coronary Flow Reserve
3.2. Thermodilution-Derived Index of Microcirculatory Resistance
3.3. Doppler-Wire-Derived Hyperemic Microvascular Resistance
3.4. Coronary Angiography-Derived Index of Microvascular Resistance
4. Coronary Microcirculation as a Therapeutic Target
5. Gaps in Knowledge and Future Directions
5.1. Heterogeneity and Practicality of The Invasive Assessment of CMD
5.2. Time-Course of Microvascular Damage in STEMI
5.3. Multifactorial Nature of Microvascular Damage in STEMI
6. Conclusions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 Esc Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with St-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with St-Segment Elevation of the European Society of Cardiology (Esc). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed] [Green Version]
- Menees, D.S.; Peterson, E.D.; Wang, Y.; Curtis, J.P.; Messenger, J.C.; Rumsfeld, J.S.; Gurm, H.S. Door-to-Balloon Time and Mortality among Patients Undergoing Primary Pci. N. Engl. J. Med. 2013, 369, 901–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Ito, H. No-Reflow Phenomenon and Prognosis in Patients with Acute Myocardial Infarction. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 499–506. [Google Scholar] [CrossRef]
- Reffelmann, T.; Kloner, R.A. Microvascular Reperfusion Injury: Rapid Expansion of Anatomic No Reflow During Reperfusion in the Rabbit. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1099–H1107. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Chilian, W.; Crea, F.; Davidson, S.M.; Ferdinandy, P.; Garcia-Dorado, D.; van Royen, N.; Schulz, R.; Heusch, G. The Coronary Circulation in Acute Myocardial Ischaemia/Reperfusion Injury: A Target for Cardioprotection. Cardiovasc. Res. 2019, 115, 1143–1155. [Google Scholar] [CrossRef]
- Konijnenberg, L.S.F.; Damman, P.; Duncker, D.J.; Kloner, R.A.; Nijveldt, R.; van Geuns, R.M.; Berry, C.; Riksen, N.P.; Escaned, J.; van Royen, N. Pathophysiology and Diagnosis of Coronary Microvascular Dysfunction in St-Elevation Myocardial Infarction. Cardiovasc. Res. 2020, 116, 787–805. [Google Scholar] [CrossRef]
- Ito, H.; Tomooka, T.; Sakai, N.; Yu, H.; Higashino, Y.; Fujii, K.; Masuyama, T.; Kitabatake, A.; Minamino, T. Lack of Myocardial Perfusion Immediately after Successful Thrombolysis. A Predictor of Poor Recovery of Left Ventricular Function in Anterior Myocardial Infarction. Circulation 1992, 85, 1699–1705. [Google Scholar] [CrossRef] [Green Version]
- van Kranenburg, M.; Magro, M.; Thiele, H.; de Waha, S.; Eitel, I.; Cochet, A.; Cottin, Y.; Atar, D.; Buser, P.; Wu, E.; et al. Prognostic Value of Microvascular Obstruction and Infarct Size, as Measured by Cmr in Stemi Patients. JACC Cardiovasc. Imaging 2014, 7, 930–939. [Google Scholar] [CrossRef]
- Robbers, L.F.; Eerenberg, E.S.; Teunissen, P.F.; Jansen, M.F.; Hollander, M.R.; Horrevoets, A.J.; Knaapen, P.; Nijveldt, R.; Heymans, M.W.; Levi, M.M.; et al. Magnetic Resonance Imaging-Defined Areas of Microvascular Obstruction after Acute Myocardial Infarction Represent Microvascular Destruction and Haemorrhage. Eur. Heart J. 2013, 34, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- de Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between Microvascular Obstruction and Adverse Events Following Primary Percutaneous Coronary Intervention for St-Segment Elevation Myocardial Infarction: An Individual Patient Data Pooled Analysis from Seven Randomized Trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Waha, S.; Desch, S.; Eitel, I.; Fuernau, G.; Zachrau, J.; Leuschner, A.; Gutberlet, M.; Schuler, G.; Thiele, H. Impact of Early Vs. Late Microvascular Obstruction Assessed by Magnetic Resonance Imaging on Long-Term Outcome after St-Elevation Myocardial Infarction: A Comparison with Traditional Prognostic Markers. Eur. Heart J. 2010, 31, 2660–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, R.W.; Aggarwal, A.; Ou, F.S.; Klein, L.W.; Rumsfeld, J.S.; Roe, M.T.; Wang, T.Y.; Registry American College of Cardiology National Cardiovascular Data. Incidence and Outcomes of No-Reflow Phenomenon During Percutaneous Coronary Intervention among Patients with Acute Myocardial Infarction. Am. J. Cardiol. 2013, 111, 178–184. [Google Scholar] [CrossRef]
- Husser, O.; Monmeneu, J.V.; Sanchis, J.; Nunez, J.; Lopez-Lereu, M.P.; Bonanad, C.; Chaustre, F.; Gomez, C.; Bosch, M.J.; Hinarejos, R.; et al. Cardiovascular Magnetic Resonance-Derived Intramyocardial Hemorrhage after Stemi: Influence on Long-Term Prognosis, Adverse Left Ventricular Remodeling and Relationship with Microvascular Obstruction. Int. J. Cardiol. 2013, 167, 2047–2054. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Ahmed, N.; Carberry, J.; Yue May, V.T.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Lindsay, M.; Hood, S.; et al. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients with an Acute St-Segment-Elevation Myocardial Infarction. Circulation 2016, 134, 1833–1847. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Selker, H.P.; Thiele, H.; Patel, M.R.; Udelson, J.E.; Ohman, E.M.; Maehara, A.; Eitel, I.; Granger, C.B.; Jenkins, P.L.; et al. Relationship between Infarct Size and Outcomes Following Primary Pci: Patient-Level Analysis from 10 Randomized Trials. J. Am. Coll. Cardiol. 2016, 67, 1674–1683. [Google Scholar] [CrossRef]
- Kitabata, H.; Imanishi, T.; Kubo, T.; Takarada, S.; Kashiwagi, M.; Matsumoto, H.; Tsujioka, H.; Ikejima, H.; Arita, Y.; Okochi, K.; et al. Coronary Microvascular Resistance Index Immediately after Primary Percutaneous Coronary Intervention as a Predictor of the Transmural Extent of Infarction in Patients with St-Segment Elevation Anterior Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2009, 2, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Orn, S.; Manhenke, C.; Greve, O.J.; Larsen, A.I.; Bonarjee, V.V.; Edvardsen, T.; Dickstein, K. Microvascular Obstruction Is a Major Determinant of Infarct Healing and Subsequent Left Ventricular Remodelling Following Primary Percutaneous Coronary Intervention. Eur. Heart J. 2009, 30, 1978–1985. [Google Scholar] [CrossRef] [Green Version]
- Caixeta, A.; Lansky, A.J.; Mehran, R.; Brener, S.J.; Claessen, B.; Genereux, P.; Palmerini, T.; Witzenbichler, B.; Guagliumi, G.; Brodie, B.R.; et al. Revascularization Harmonizing Outcomes With, and investigators Stents in Acute Myocardial Infarction trial. “Predictors of Suboptimal Timi Flow after Primary Angioplasty for Acute Myocardial Infarction: Results from the Horizons-Ami Trial. EuroIntervention 2013, 9, 220–227. [Google Scholar] [CrossRef]
- Nijveldt, R.; Beek, A.M.; Hirsch, A.; Stoel, M.G.; Hofman, M.B.; Umans, V.A.; Algra, P.R.; Twisk, J.W.; van Rossum, A.C. Functional Recovery after Acute Myocardial Infarction: Comparison between Angiography, Electrocardiography, and Cardiovascular Magnetic Resonance Measures of Microvascular Injury. J. Am. Coll. Cardiol. 2008, 52, 181–189. [Google Scholar] [CrossRef] [Green Version]
- van ‘t Hof, A.W.; Liem, A.; Suryapranata, H.; Hoorntje, J.C.; de Boer, M.J.; Zijlstra, F. Angiographic Assessment of Myocardial Reperfusion in Patients Treated with Primary Angioplasty for Acute Myocardial Infarction: Myocardial Blush Grade. Zwolle Myocardial Infarction Study Group. Circulation 1998, 97, 2302–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marra, M.P.; Corbetti, F.; Cacciavillani, L.; Tarantini, G.; Ramondo, A.B.; Napodano, M.; Basso, C.; Lacognata, C.; Marzari, A.; Maddalena, F.; et al. Relationship between Myocardial Blush Grades, Staining, and Severe Microvascular Damage after Primary Percutaneous Coronary Intervention a Study Performed with Contrast-Enhanced Magnetic Resonance in a Large Consecutive Series of Patients. Am. Heart J. 2010, 159, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Marc, M.C.; Iancu, A.C.; Ober, C.D.; Homorodean, C.; Balanescu, S.; Sitar, A.V.; Bolboaca, S.; Dregoesc, I.M. Pre-Revascularization Coronary Wedge Pressure as Marker of Adverse Long-Term Left Ventricular Remodelling in Patients with Acute St-Segment Elevation Myocardial Infarction. Sci. Rep. 2018, 8, 1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doucette, J.W.; Corl, P.D.; Payne, H.M.; Flynn, A.E.; Goto, M.; Nassi, M.; Segal, J. Validation of a Doppler Guide Wire for Intravascular Measurement of Coronary Artery Flow Velocity. Circulation 1992, 85, 1899–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijls, N.H.; De Bruyne, B.; Smith, L.; Aarnoudse, W.; Barbato, E.; Bartunek, J.; Bech, G.J.; Van De Vosse, F. Coronary Thermodilution to Assess Flow Reserve: Validation in Humans. Circulation 2002, 105, 2482–2486. [Google Scholar] [CrossRef]
- Barbato, E.; Aarnoudse, W.; Aengevaeren, W.R.; Werner, G.; Klauss, V.; Bojara, W.; Herzfeld, I.; Oldroyd, K.G.; Pijls, N.H.; De Bruyne, B.; et al. Validation of Coronary Flow Reserve Measurements by Thermodilution in Clinical Practice. Eur. Heart J. 2004, 25, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Everaars, H.; de Waard, G.A.; Driessen, R.S.; Danad, I.; van de Ven, P.M.; Raijmakers, P.G.; Lammertsma, A.A.; van Rossum, A.C.; Knaapen, P.; van Royen, N. Doppler Flow Velocity and Thermodilution to Assess Coronary Flow Reserve: A Head-to-Head Comparison with [(15)O]H(2)O Pet. JACC Cardiovasc. Interv. 2018, 11, 2044–2054. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Nakamura, M.; Tsunoda, T.; Toma, H.; Degawa, T.; Oki, T.; Yamaguchi, T. Coronary Flow Velocity Immediately after Primary Coronary Stenting as a Predictor of Ventricular Wall Motion Recovery in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2000, 35, 1835–1841. [Google Scholar] [CrossRef] [Green Version]
- Garot, P.; Pascal, O.; Simon, M.; Monin, J.L.; Teiger, E.; Garot, J.; Gueret, P.; Dubois-Rande, J.L. Impact of Microvascular Integrity and Local Viability on Left Ventricular Remodelling after Reperfused Acute Myocardial Infarction. Heart 2003, 89, 393–397. [Google Scholar] [CrossRef] [Green Version]
- Bax, M.; de Winter, R.J.; Schotborgh, C.E.; Koch, K.T.; Meuwissen, M.; Voskuil, M.; Adams, R.; Mulder, K.J.; Tijssen, J.G.; Piek, J.J. Short- and Long-Term Recovery of Left Ventricular Function Predicted at the Time of Primary Percutaneous Coronary Intervention in Anterior Myocardial Infarction. J. Am. Coll. Cardiol. 2004, 43, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Hiasa, Y.; Ohara, Y.; Miyazaki, S.; Ogura, R.; Miyajima, H.; Yuba, K.; Suzuki, N.; Hosokawa, S.; Kishi, K.; et al. Usefulness of Coronary Flow Reserve Immediately after Primary Coronary Angioplasty for Acute Myocardial Infarction in Predicting Long-Term Adverse Cardiac Events. Am. J. Cardiol. 2007, 100, 806–811. [Google Scholar] [CrossRef] [PubMed]
- van de Hoef, T.P.; Bax, M.; Meuwissen, M.; Damman, P.; Delewi, R.; de Winter, R.J.; Koch, K.T.; Schotborgh, C.; Henriques, J.P.; Tijssen, J.G.; et al. Impact of Coronary Microvascular Function on Long-Term Cardiac Mortality in Patients with Acute St-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2013, 6, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, M.K.; Yeung, A.C.; Fearon, W.F. Invasive Assessment of the Coronary Microcirculation: Superior Reproducibility and Less Hemodynamic Dependence of Index of Microcirculatory Resistance Compared with Coronary Flow Reserve. Circulation 2006, 113, 2054–2061. [Google Scholar] [CrossRef]
- Fearon, W.F.; Aarnoudse, W.; Pijls, N.H.; De Bruyne, B.; Balsam, L.B.; Cooke, D.T.; Robbins, R.C.; Fitzgerald, P.J.; Yeung, A.C.; Yock, P.G. Microvascular Resistance Is Not Influenced by Epicardial Coronary Artery Stenosis Severity: Experimental Validation. Circulation 2004, 109, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Aarnoudse, W.; Fearon, W.F.; Manoharan, G.; Geven, M.; van de Vosse, F.; Rutten, M.; De Bruyne, B.; Pijls, N.H. Epicardial Stenosis Severity Does Not Affect Minimal Microcirculatory Resistance. Circulation 2004, 110, 2137–2142. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.G.; Hung, O.Y.; Lee, J.W.; Lee, J.H.; Youn, Y.J.; Ahn, M.S.; Kim, J.Y.; Yoo, B.S.; Lee, S.H.; Yoon, J.; et al. Combination of the Thermodilution-Derived Index of Microcirculatory Resistance and Coronary Flow Reserve Is Highly Predictive of Microvascular Obstruction on Cardiac Magnetic Resonance Imaging after St-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2016, 9, 793–801. [Google Scholar] [CrossRef]
- Fukunaga, M.; Fujii, K.; Kawasaki, D.; Sawada, H.; Miki, K.; Tamaru, H.; Imanaka, T.; Iwasaku, T.; Nakata, T.; Shibuya, M.; et al. Thermodilution-Derived Coronary Blood Flow Pattern Immediately after Coronary Intervention as a Predictor of Microcirculatory Damage and Midterm Clinical Outcomes in Patients with St-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2014, 7, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Bulluck, H.; Foin, N.; Cabrera-Fuentes, H.A.; Yeo, K.K.; Wong, A.S.; Fam, J.M.; Wong, P.E.; Tan, J.W.; Low, A.F.; Hausenloy, D.J. Index of Microvascular Resistance and Microvascular Obstruction in Patients with Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2016, 9, 2172–2174. [Google Scholar] [CrossRef]
- McAlindon, E.; Pufulete, M.; Harris, J.; Lawton, C.; Johnson, T.; Strange, J.; Baumbach, A.; Bucciarelli-Ducci, C. Microvascular Dysfunction Determines Infarct Characteristics in Patients with Reperfused St-Segment Elevation Myocardial Infarction: The Microcirculation in Acute Myocardial Infarction (Micro-Ami) Study. PLoS ONE 2018, 13, e0203750. [Google Scholar] [CrossRef] [Green Version]
- Maznyczka, A.M.; Oldroyd, K.G.; Greenwood, J.P.; McCartney, P.J.; Cotton, J.; Lindsay, M.; McEntegart, M.; Rocchiccioli, J.P.; Good, R.; Robertson, K.; et al. Comparative Significance of Invasive Measures of Microvascular Injury in Acute Myocardial Infarction. Circ. Cardiovasc. Interv. 2020, 13, e008505. [Google Scholar] [CrossRef]
- Scarsini, R.; De Maria, G.L.; Borlotti, A.; Kotronias, R.A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Ferreira, V.M.; Ribichini, F.; Channon, K.M.; et al. Incremental Value of Coronary Microcirculation Resistive Reserve Ratio in Predicting the Extent of Myocardial Infarction in Patients with Stemi. Insights from the Oxford Acute Myocardial Infarction (Oxami) Study. Cardiovasc. Revasc. Med. 2019, 20, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Shah, M.; Ng, M.; Brinton, T.; Wilson, A.; Tremmel, J.A.; Schnittger, I.; Lee, D.P.; Vagelos, R.H.; Fitzgerald, P.J.; et al. Predictive Value of the Index of Microcirculatory Resistance in Patients with St-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 51, 560–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.S.; Yoon, M.H.; Tahk, S.J.; Yang, H.M.; Choi, B.J.; Choi, S.Y.; Sheen, S.S.; Hwang, G.S.; Kang, S.J.; Shin, J.H. Usefulness of the Index of Microcirculatory Resistance for Invasively Assessing Myocardial Viability Immediately after Primary Angioplasty for Anterior Myocardial Infarction. Eur. Heart J. 2009, 30, 2854–2860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance as a Tool to Characterize Microvascular Obstruction and to Predict Infarct Size Regression in Patients with Stemi Undergoing Primary Pci. JACC Cardiovasc. Imaging 2019, 12, 837–848. [Google Scholar] [CrossRef]
- Bello, D.; Einhorn, A.; Kaushal, R.; Kenchaiah, S.; Raney, A.; Fieno, D.; Narula, J.; Goldberger, J.; Shivkumar, K.; Subacius, H.; et al. Cardiac Magnetic Resonance Imaging: Infarct Size Is an Independent Predictor of Mortality in Patients with Coronary Artery Disease. Magn. Reson. Imaging 2011, 29, 50–56. [Google Scholar] [CrossRef]
- Echavarria-Pinto, M.; van de Hoef, T.P.; Nijjer, S.; Gonzalo, N.; Nombela-Franco, L.; Ibanez, B.; Sen, S.; Petraco, R.; Jimenez-Quevedo, P.; Nunez-Gil, I.J.; et al. Influence of the Amount of Myocardium Subtended to a Coronary Stenosis on the Index of Microcirculatory Resistance. Implications for the Invasive Assessment of Microcirculatory Function in Ischaemic Heart Disease. EuroIntervention 2017, 13, 944–952. [Google Scholar] [CrossRef]
- Layland, J.; Carrick, D.; McEntegart, M.; Ahmed, N.; Payne, A.; McClure, J.; Sood, A.; McGeoch, R.; MacIsaac, A.; Whitbourn, R.; et al. Vasodilatory Capacity of the Coronary Microcirculation Is Preserved in Selected Patients with Non-St-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2013, 6, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Maznyczka, A.M.; Carrick, D.; Oldroyd, K.G.; James-Rae, G.; McCartney, P.; Greenwood, J.P.; Good, R.; McEntegart, M.; Eteiba, H.; Lindsay, M.M.; et al. Thermodilution-Derived Temperature Recovery Time: A Novel Predictor of Microvascular Reperfusion and Prognosis after Myocardial Infarction. EuroIntervention 2021, 17, 220–228. [Google Scholar] [CrossRef]
- Yew, S.N.; Carrick, D.; Corcoran, D.; Ahmed, N.; Carberry, J.; Teng Yue May, V.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Lindsay, M.; et al. Coronary Thermodilution Waveforms after Acute Reperfused St-Segment-Elevation Myocardial Infarction: Relation to Microvascular Obstruction and Prognosis. J. Am. Heart Assoc. 2018, 7, e008957. [Google Scholar] [CrossRef] [Green Version]
- Fahrni, G.; Wolfrum, M.; De Maria, G.L.; Cuculi, F.; Dawkins, S.; Alkhalil, M.; Patel, N.; Forfar, J.C.; Prendergast, B.D.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance at the Time of Primary Percutaneous Coronary Intervention Predicts Early Cardiac Complications: Insights from the Oxami (Oxford Study in Acute Myocardial Infarction) Cohort. J. Am. Heart Assoc. 2017, 6, e005409. [Google Scholar] [CrossRef] [Green Version]
- Fearon, W.F.; Low, A.F.; Yong, A.S.; McGeoch, R.; Berry, C.; Shah, M.G.; Ho, M.Y.; Kim, H.S.; Loh, J.P.; Oldroyd, K.G. Prognostic Value of the Index of Microcirculatory Resistance Measured after Primary Percutaneous Coronary Intervention. Circulation 2013, 127, 2436–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, G.S.; Ahn, S.G.; Woo, S.I.; Yoon, M.H.; Lee, M.J.; Choi, S.H.; Seo, J.Y.; Kwon, S.W.; Park, S.D.; Seo, K.W. The Index of Microcirculatory Resistance after Primary Percutaneous Coronary Intervention Predicts Long-Term Clinical Outcomes in Patients with St-Segment Elevation Myocardial Infarction. J. Clin. Med. 2021, 10, 4752. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yoon, M.H.; Seo, K.W.; Tahk, S.J.; Lim, H.S.; Yang, H.M.; Choi, B.J.; Choi, S.Y.; Hwang, G.S.; Shin, J.H.; et al. Usefulness of Hyperemic Microvascular Resistance Index as a Predictor of Clinical Outcomes in Patients with St-Segment Elevation Myocardial Infarction. Korean Circ. J. 2015, 45, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Waard, G.A.; Fahrni, G.; de Wit, D.; Kitabata, H.; Williams, R.; Patel, N.; Teunissen, P.F.; van de Ven, P.M.; Umman, S.; Knaapen, P.; et al. Hyperaemic Microvascular Resistance Predicts Clinical Outcome and Microvascular Injury after Myocardial Infarction. Heart 2018, 104, 127–134. [Google Scholar] [CrossRef]
- Scarsini, R.; Shanmuganathan, M.; De Maria, G.L.; Borlotti, A.; Kotronias, R.A.; Burrage, M.K.; Terentes-Printzios, D.; Langrish, J.; Lucking, A.; Fahrni, G.; et al. Coronary Microvascular Dysfunction Assessed by Pressure Wire and Cmr after Stemi Predicts Long-Term Outcomes. JACC Cardiovasc. Imaging 2021, 14, 1948–1959. [Google Scholar] [CrossRef]
- Choi, K.H.; Dai, N.; Li, Y.; Kim, J.; Shin, D.; Lee, S.H.; Joh, H.S.; Kim, H.K.; Jeon, K.H.; Ha, S.J.; et al. Functional Coronary Angiography-Derived Index of Microcirculatory Resistance in Patients with St-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2021, 14, 1670–1684. [Google Scholar] [CrossRef]
- Williams, R.P.; de Waard, G.A.; De Silva, K.; Lumley, M.; Asrress, K.; Arri, S.; Ellis, H.; Mir, A.; Clapp, B.; Chiribiri, A.; et al. Doppler Versus Thermodilution-Derived Coronary Microvascular Resistance to Predict Coronary Microvascular Dysfunction in Patients with Acute Myocardial Infarction or Stable Angina Pectoris. Am. J. Cardiol. 2018, 121, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Teunissen, P.F.; de Waard, G.A.; Hollander, M.R.; Robbers, L.F.; Danad, I.; Biesbroek, P.S.; Amier, R.P.; Echavarria-Pinto, M.; Quiros, A.; Broyd, C.; et al. Doppler-Derived Intracoronary Physiology Indices Predict the Occurrence of Microvascular Injury and Microvascular Perfusion Deficits after Angiographically Successful Primary Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2015, 8, e001786. [Google Scholar] [CrossRef] [Green Version]
- Escaned, J.; Flores, A.; Garcia-Pavia, P.; Segovia, J.; Jimenez, J.; Aragoncillo, P.; Salas, C.; Alfonso, F.; Hernandez, R.; Angiolillo, D.J.; et al. Assessment of Microcirculatory Remodeling with Intracoronary Flow Velocity and Pressure Measurements: Validation with Endomyocardial Sampling in Cardiac Allografts. Circulation 2009, 120, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Shimada, K.; Sakanoue, Y.; Kobayashi, Y.; Ehara, S.; Hirose, M.; Nakamura, Y.; Fukuda, D.; Yamagishi, H.; Yoshiyama, M.; Takeuchi, K.; et al. Assessment of Myocardial Viability Using Coronary Zero Flow Pressure after Successful Angioplasty in Patients with Acute Anterior Myocardial Infarction. Heart 2003, 89, 71–76. [Google Scholar] [CrossRef]
- Van Herck, P.L.; Carlier, S.G.; Claeys, M.J.; Haine, S.E.; Gorissen, P.; Miljoen, H.; Bosmans, J.M.; Vrints, C.J. Coronary Microvascular Dysfunction after Myocardial Infarction: Increased Coronary Zero Flow Pressure Both in the Infarcted and in the Remote Myocardium Is Mainly Related to Left Ventricular Filling Pressure. Heart 2007, 93, 1231–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.; Petraco, R.; Dall’Armellina, E.; Kassimis, G.; De Maria, G.L.; Dawkins, S.; Lee, R.; Prendergast, B.D.; Choudhury, R.P.; Forfar, J.C.; et al. Zero-Flow Pressure Measured Immediately after Primary Percutaneous Coronary Intervention for St-Segment Elevation Myocardial Infarction Provides the Best Invasive Index for Predicting the Extent of Myocardial Infarction at 6 Months: An Oxami Study (Oxford Acute Myocardial Infarction). JACC Cardiovasc. Interv. 2015, 8, 1410–1421. [Google Scholar] [PubMed] [Green Version]
- Li, J.; Gong, Y.; Wang, W.; Yang, Q.; Liu, B.; Lu, Y.; Xu, Y.; Huo, Y.; Yi, T.; Liu, J.; et al. Accuracy of Computational Pressure-Fluid Dynamics Applied to Coronary Angiography to Derive Fractional Flow Reserve: Flash Ffr. Cardiovasc. Res. 2020, 116, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.; et al. Angiography-Derived Index of Microcirculatory Resistance as a Novel, Pressure-Wire-Free Tool to Assess Coronary Microcirculation in St Elevation Myocardial Infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1395–1406. [Google Scholar] [CrossRef]
- Mejia-Renteria, H.; Wang, L.; Chipayo-Gonzales, D.; van de Hoef, T.P.; Travieso, A.; Espejo, C.; Nunez-Gil, I.J.; Macaya, F.; Gonzalo, N.; Escaned, J. Angiography-Derived Assessment of Coronary Microcirculatory Resistance in Patients with Suspected Myocardial Ischaemia and Non-Obstructive Coronary Arteries. EuroIntervention 2022. [Google Scholar] [CrossRef]
- Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Ox, A.M.I.S.I.; Ribichini, F.; Ferreira, V.M.; et al. Angiography-Derived Index of Microcirculatory Resistance (Imr(Angio)) as a Novel Pressure-Wire-Free Tool to Assess Coronary Microvascular Dysfunction in Acute Coronary Syndromes and Stable Coronary Artery Disease. Int. J. Cardiovasc. Imaging 2021, 37, 1801–1813. [Google Scholar] [CrossRef]
- Maznyczka, A.M.; Oldroyd, K.G.; McCartney, P.; McEntegart, M.; Berry, C. The Potential Use of the Index of Microcirculatory Resistance to Guide Stratification of Patients for Adjunctive Therapy in Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2019, 12, 951–966. [Google Scholar] [CrossRef]
- Sezer, M.; Oflaz, H.; Goren, T.; Okcular, I.; Umman, B.; Nisanci, Y.; Bilge, A.K.; Sanli, Y.; Meric, M.; Umman, S. Intracoronary Streptokinase after Primary Percutaneous Coronary Intervention. N. Engl. J. Med. 2007, 356, 1823–1834. [Google Scholar] [CrossRef] [Green Version]
- McCartney, P.J.; Eteiba, H.; Maznyczka, A.M.; McEntegart, M.; Greenwood, J.P.; Muir, D.F.; Chowdhary, S.; Gershlick, A.H.; Appleby, C.; Cotton, J.M.; et al. Effect of Low-Dose Intracoronary Alteplase During Primary Percutaneous Coronary Intervention on Microvascular Obstruction in Patients with Acute Myocardial Infarction: A Randomized Clinical Trial. JAMA 2019, 321, 56–68. [Google Scholar] [CrossRef]
- McCartney, P.J.; Maznyczka, A.M.; Eteiba, H.; McEntegart, M.; Oldroyd, K.G.; Greenwood, J.P.; Maredia, N.; Schmitt, M.; McCann, G.P.; Fairbairn, T.; et al. Low-Dose Alteplase During Primary Percutaneous Coronary Intervention According to Ischemic Time. J. Am. Coll. Cardiol. 2020, 75, 1406–1421. [Google Scholar] [CrossRef]
- De Maria, G.L.; Cuculi, F.; Patel, N.; Dawkins, S.; Fahrni, G.; Kassimis, G.; Choudhury, R.P.; Forfar, J.C.; Prendergast, B.D.; Channon, K.M.; et al. How Does Coronary Stent Implantation Impact on the Status of the Microcirculation During Primary Percutaneous Coronary Intervention in Patients with St-Elevation Myocardial Infarction? Eur. Heart J. 2015, 36, 3165–3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezer, M.; Escaned, J.; Broyd, C.J.; Umman, B.; Bugra, Z.; Ozcan, I.; Sonsoz, M.R.; Ozcan, A.; Atici, A.; Aslanger, E.; et al. Gradual Versus Abrupt Reperfusion During Primary Percutaneous Coronary Interventions in St-Segment-Elevation Myocardial Infarction (Guard). J. Am. Heart Assoc. 2022, 11, e024172. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Nanto, S.; Doi, Y.; Kurozumi, Y.; Natsukawa, T.; Shibata, H.; Morita, M.; Kawata, A.; Tsuruoka, A.; Sawano, H.; et al. Beneficial Effects of Intracoronary Nicorandil on Microvascular Dysfunction after Primary Percutaneous Coronary Intervention: Demonstration of Its Superiority to Nitroglycerin in a Cross-over Study. Cardiovasc. Drugs Ther. 2013, 27, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Zhang, Y.; Dong, W.; Jiang, Z.C.; Li, T.; Cheng, L.Q.; Zou, Y.T.; Jiang, X.S.; Zhou, H.; A, X.; et al. Effects of Nicorandil Administration on Infarct Size in Patients with St-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: The Change Trial. J. Am. Heart Assoc. 2022, 11, e026232. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Ichimiya, S.; Kanashiro, M.; Amano, T.; Imai, K.; Murohara, T.; Matsubara, T. Impact of a Single Intravenous Administration of Nicorandil before Reperfusion in Patients with St-Segment-Elevation Myocardial Infarction. Circulation 2005, 112, 1284–1288. [Google Scholar] [CrossRef] [Green Version]
- Kitakaze, M.; Asakura, M.; Kim, J.; Shintani, Y.; Asanuma, H.; Hamasaki, T.; Seguchi, O.; Myoishi, M.; Minamino, T.; Ohara, T.; et al. Human Atrial Natriuretic Peptide and Nicorandil as Adjuncts to Reperfusion Treatment for Acute Myocardial Infarction (J-Wind): Two Randomised Trials. Lancet 2007, 370, 1483–1493. [Google Scholar] [CrossRef]
- Nazir, S.A.; McCann, G.P.; Greenwood, J.P.; Kunadian, V.; Khan, J.N.; Mahmoud, I.Z.; Blackman, D.J.; Been, M.; Abrams, K.R.; Shipley, L.; et al. Strategies to Attenuate Micro-Vascular Obstruction During P-Pci: The Randomized Reperfusion Facilitated by Local Adjunctive Therapy in St-Elevation Myocardial Infarction Trial. Eur. Heart J. 2016, 37, 1910–1919. [Google Scholar] [CrossRef] [Green Version]
- Van de Hoef, T.P.; Nolte, F.; Delewi, R.; Henriques, J.P.; Spaan, J.A.; Tijssen, J.G.; Siebes, M.; Wykrzykowska, J.J.; Stone, G.W.; Piek, J.J. Intracoronary Hemodynamic Effects of Pressure-Controlled Intermittent Coronary Sinus Occlusion (Picso): Results from the First-in-Man Prepare Picso Study. J. Interv. Cardiol. 2012, 25, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Egred, M.; Bagnall, A.; Spyridopoulos, I.; Purcell, I.F.; Das, R.; Palmer, N.; Grech, E.D.; Jain, A.; Stone, G.W.; Nijveldt, R.; et al. Effect of Pressure-Controlled Intermittent Coronary Sinus Occlusion (Picso) on Infarct Size in Anterior Stemi: Picso in Acs Study. Int. J. Cardiol. Heart Vasc. 2020, 28, 100526. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Borlotti, A.; Wolfrum, M.; Gaughran, L.; Dall’Armellina, E.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.K.; et al. Index of Microcirculatory Resistance-Guided Therapy with Pressure-Controlled Intermittent Coronary Sinus Occlusion Improves Coronary Microvascular Function and Reduces Infarct Size in Patients with St-Elevation Myocardial Infarction: The Oxford Acute Myocardial Infarction-Pressure-Controlled Intermittent Coronary Sinus Occlusion Study (Oxami-Picso Study). EuroIntervention 2018, 14, e352–e359. [Google Scholar]
- Botker, H.E.; Kharbanda, R.; Schmidt, M.R.; Bottcher, M.; Kaltoft, A.K.; Terkelsen, C.J.; Munk, K.; Andersen, N.H.; Hansen, T.M.; Trautner, S.; et al. Remote Ischaemic Conditioning before Hospital Admission, as a Complement to Angioplasty, and Effect on Myocardial Salvage in Patients with Acute Myocardial Infarction: A Randomised Trial. Lancet 2010, 375, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Kharbanda, R.K.; Moller, U.K.; Ramlall, M.; Aaroe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of Remote Ischaemic Conditioning on Clinical Outcomes in Patients with Acute Myocardial Infarction (Condi-2/Eric-Ppci): A Single-Blind Randomised Controlled Trial. Lancet 2019, 394, 1415–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirma, C.; Erkol, A.; Pala, S.; Oduncu, V.; Dundar, C.; Izgi, A.; Tigen, K.; Gibson, C.M. Intracoronary Bolus-Only Compared with Intravenous Bolus Plus Infusion of Tirofiban Application in Patients with St-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Catheter. Cardiovasc. Interv. 2012, 79, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Witzenbichler, B.; Godlewski, J.; Parise, H.; Dambrink, J.H.; Ochala, A.; Carlton, T.W.; Cristea, E.; Wolff, S.D.; et al. Intracoronary Abciximab and Aspiration Thrombectomy in Patients with Large Anterior Myocardial Infarction: The Infuse-Ami Randomized Trial. JAMA 2012, 307, 1817–1826. [Google Scholar] [CrossRef] [Green Version]
- Thiele, H.; Wohrle, J.; Hambrecht, R.; Rittger, H.; Birkemeyer, R.; Lauer, B.; Neuhaus, P.; Brosteanu, O.; Sick, P.; Wiemer, M.; et al. Intracoronary Versus Intravenous Bolus Abciximab During Primary Percutaneous Coronary Intervention in Patients with Acute St-Elevation Myocardial Infarction: A Randomised Trial. Lancet 2012, 379, 923–931. [Google Scholar] [CrossRef]
- Collison, D.; Didagelos, M.; Aetesam-Ur-Rahman, M.; Copt, S.; McDade, R.; McCartney, P.; Ford, T.J.; McClure, J.; Lindsay, M.; Shaukat, A.; et al. Post-Stenting Fractional Flow Reserve Vs Coronary Angiography for Optimization of Percutaneous Coronary Intervention (Target-Ffr). Eur. Heart J. 2021, 42, 4656–4668. [Google Scholar] [CrossRef]
- Jeremias, A.; Davies, J.E.; Maehara, A.; Matsumura, M.; Schneider, J.; Tang, K.; Talwar, S.; Marques, K.; Shammas, N.W.; Gruberg, L.; et al. Blinded Physiological Assessment of Residual Ischemia after Successful Angiographic Percutaneous Coronary Intervention: The Define Pci Study. JACC Cardiovasc. Interv. 2019, 12, 1991–2001. [Google Scholar] [CrossRef]
- Silva, M.; Paiva, L.; Teixeira, R.; Ferreira, M.J.; Goncalves, L. Microcirculation Function Assessment in Acute Myocardial Infarction: A Systematic Review of Microcirculatory Resistance Indices. Front. Cardiovasc. Med 2022, 9, 1041444. [Google Scholar] [CrossRef]
- Bulluck, H.; Foin, N.; Tan, J.W.; Low, A.F.; Sezer, M.; Hausenloy, D.J. Invasive Assessment of the Coronary Microcirculation in Reperfused St-Segment-Elevation Myocardial Infarction Patients: Where Do We Stand? Circ. Cardiovasc. Interv. 2017, 10, e004373. [Google Scholar] [CrossRef]
- Chamuleau, S.A.; Siebes, M.; Meuwissen, M.; Koch, K.T.; Spaan, J.A.; Piek, J.J. Association between Coronary Lesion Severity and Distal Microvascular Resistance in Patients with Coronary Artery Disease. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2194–H2200. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Sato, H.; Tateishi, H.; Kawagoe, T.; Shimatani, Y.; Kurisu, S.; Sakai, K. Time Course of Impaired Coronary Flow Reserve after Reperfusion in Patients with Acute Myocardial Infarction. Am. J. Cardiol. 1996, 78, 1103–1108. [Google Scholar] [CrossRef]
- Neumann, F.J.; Kosa, I.; Dickfeld, T.; Blasini, R.; Gawaz, M.; Hausleiter, J.; Schwaiger, M.; Schomig, A. Recovery of Myocardial Perfusion in Acute Myocardial Infarction after Successful Balloon Angioplasty and Stent Placement in the Infarct-Related Coronary Artery. J. Am. Coll. Cardiol. 1997, 30, 1270–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.C.; Kim, R.J.; Bluemke, D.A.; Rochitte, C.E.; Zerhouni, E.A.; Becker, L.C.; Lima, J.A. Quantification and Time Course of Microvascular Obstruction by Contrast-Enhanced Echocardiography and Magnetic Resonance Imaging Following Acute Myocardial Infarction and Reperfusion. J. Am. Coll. Cardiol. 1998, 32, 1756–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrick, D.; Haig, C.; Ahmed, N.; Rauhalammi, S.; Clerfond, G.; Carberry, J.; Mordi, I.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; et al. Temporal Evolution of Myocardial Hemorrhage and Edema in Patients after Acute St-Segment Elevation Myocardial Infarction: Pathophysiological Insights and Clinical Implications. J. Am. Heart Assoc. 2016, 5, e002834. [Google Scholar] [CrossRef] [Green Version]
- Cuculi, F.; Dall’Armellina, E.; Manlhiot, C.; De Caterina, A.R.; Colyer, S.; Ferreira, V.; Morovat, A.; Prendergast, B.D.; Forfar, J.C.; Alp, N.J.; et al. Early Change in Invasive Measures of Microvascular Function Can Predict Myocardial Recovery Following Pci for St-Elevation Myocardial Infarction. Eur. Heart J. 2014, 35, 1971–1980. [Google Scholar] [CrossRef] [Green Version]
- Cuculi, F.; De Maria, G.L.; Meier, P.; Dall’Armellina, E.; de Caterina, A.R.; Channon, K.M.; Prendergast, B.D.; Choudhury, R.P.; Forfar, J.C.; Kharbanda, R.K.; et al. Impact of Microvascular Obstruction on the Assessment of Coronary Flow Reserve, Index of Microcirculatory Resistance, and Fractional Flow Reserve after St-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2014, 64, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Sezer, M.; Aslanger, E.K.; Cimen, A.O.; Yormaz, E.; Turkmen, C.; Umman, B.; Nisanci, Y.; Bugra, Z.; Adalet, K.; Umman, S. Concurrent Microvascular and Infarct Remodeling after Successful Reperfusion of St-Elevation Acute Myocardial Infarction. Circ. Cardiovasc. Interv. 2010, 3, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Bax, M.; de Winter, R.J.; Koch, K.T.; Schotborgh, C.E.; Tijssen, J.G.; Piek, J.J. Time Course of Microvascular Resistance of the Infarct and Noninfarct Coronary Artery Following an Anterior Wall Acute Myocardial Infarction. Am. J. Cardiol. 2006, 97, 1131–1136. [Google Scholar] [CrossRef]
- van der Hoeven, N.W.; Janssens, G.N.; de Waard, G.A.; Everaars, H.; Broyd, C.J.; Beijnink, C.W.H.; van de Ven, P.M.; Nijveldt, R.; Cook, C.M.; Petraco, R.; et al. Temporal Changes in Coronary Hyperemic and Resting Hemodynamic Indices in Nonculprit Vessels of Patients with St-Segment Elevation Myocardial Infarction. JAMA Cardiol. 2019, 4, 736–744. [Google Scholar] [CrossRef]
- Niccoli, G.; Montone, R.A.; Ibanez, B.; Thiele, H.; Crea, F.; Heusch, G.; Bulluck, H.; Hausenloy, D.J.; Berry, C.; Stiermaier, T.; et al. Optimized Treatment of St-Elevation Myocardial Infarction. Circ. Res. 2019, 125, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.; Taylor, A.J.; Ellims, A.H.; Lefkovits, L.; Wong, C.; Kingwell, B.A.; Natoli, A.; Croft, K.D.; Mori, T.; Kaye, D.M.; et al. Effect of Iron Chelation on Myocardial Infarct Size and Oxidative Stress in St-Elevation-Myocardial Infarction. Circ. Cardiovasc. Interv. 2012, 5, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | Year | Sample Size | Index Cutoff | Primary (Composite) Clinical Endpoint | Event Rate vs. No CMD Group | Adjusted HR (95% CI) | Follow-Up Duration |
|---|---|---|---|---|---|---|---|
| Takahashi et al. [31] | 2007 | 118 | CFR ≤ 1.3 | Cardiac death, HF or recurrent MI | 40% vs. 4% | - | 62 months |
| Fearon et al. [51] | 2013 | 253 | IMR > 40 | Death or HF hospitalization | 20.0% vs. 11.0% | 2.2 (1.1–4.5) | 33 months |
| Van de Hoef [32] | 2013 | 100 | CFR < 1.5 | Cardiac mortality | 20.0% vs. 9.0% | 1.6 (0.5–5.0) | 120 months |
| Fukunaga et al. [37] | 2014 | 88 | Bimodal pattern (thermodilution) | Cardiac death, nonfatal MI and HF rehospitalization | 73.3% vs. 7.3% | 27.8 (2.4–320.5) | 6 months |
| Jin et al. [53] | 2015 | 145 | HMR > 2.8 | Cardiac death or HF hospitalization | 17.2% overall | 1.7 (1.3–2.3) | 85 months |
| Carrick et al. [15] | 2016 | 283 | IMR > 40 | All-cause death or first HF event after index hospitalization | 3.5% overall | 4.4 (1.9–10.1) † | 28 months |
| Fahrni et al. [50] | 2017 | 261 | IMR > 40 | Major cardiac complications • | 16.7% vs. 0% | - | 1 month |
| De Waard et al. [54] | 2018 | 176 | HMR ≥ 3.0 CFR < 1.5 | All-cause death or HF hospitalization | 20.3% vs. 3.6% 14.9% vs. 4.5% | 7.0 (1.5–33.7) 3.5 (1.1–10.8) | 38 months |
| Yew et al. [49] | 2018 | 278 | Bimodal pattern (thermodilution) | All-cause death or HF hospitalization | 14.4% overall | 2.3 (0.9–6.1) | 48 months |
| Maznyczka et al. [40] | 2020 | 144 | IMR > 140 RRR ≤ 1.7 | Cardiac death, nonfatal MI, HF hospiltalization | 28.1% vs. 8.0% 20.8% vs. 10.4% | 4.4 (1.7–11.7) ◊ 2.2 (0.8–5.8) ◊ | 12 months |
| Scarsini et al. [55] | 2021 | 198 | IMR > 40 | All-cause mortality, HF, resuscitated cardiac arrest, malignant ventricular arrhythmias or the need for a primary prevention ICD | 25.5% * vs. 5.7% 22.2% ° vs. 5.7% | 4.6 (1.4–16.1) * 6.8 (1.8–25.2) ° | 40 months |
| Maznyczka et al. [48] | 2021 | 271 ‡ 144 ‖ | TRT > 0.5 | All-cause death or HF hospitalization | 19.2% overall 15.0% overall | 5.4 (2.0–14.4) 5.8 (1.4–23.9) | 60 months 12 months |
| Yoon et al. [52] | 2021 | 326 | IMR > 29 | All-cause death or HF hospitalization | 10.3% vs. 2.1% | 4.0 (1.2–12.9) | 65 months |
| Choi et al. [56] | 2021 | 309 | Angio-IMR > 40 | Cardiac death or HF hospitalization | 46.7% vs. 16.6% | 2.2 (1.2–4.1) | 120 months |
| Therapeutic Strategy | Study | Year | Design | Sample Size | Comparison | Primary Endpoint Intervention vs. Control | Overall Outcome |
|---|---|---|---|---|---|---|---|
| Intracoronary fibrinolytics | Sezer et al. [68] | 2007 | RCT | 41 | IC streptokinase (250 kU) vs. placebo | CFR: 2.0 vs. 1.4, p = 0.002 IMR: 16.3 vs. 32.5, p < 0.01 | Improvement in IMR and CFR, but no effect on infarct size |
| McCartney et al. [69] | 2019 | RCT | 440 | IC alteplase (20 mg) * vs. placebo | MVO on CMR as %LV 3.5% vs. 2.3%, p = NS | No reduction in MVO, even harmful in patients presenting >4 h of symptom onset [70] | |
| Antiplatelets | Kirma et al. [83] | 2012 | RCT | 49 | IC vs. IV tirofiban | IMR/CFR at day 5 IMR: 27 vs. 35, p = NS CFR: 2.2 vs. 1.9, p = NS | No difference in the effect on microcirculation |
| Stone et al. [84] | 2012 | RCT | 353 ° | IC abciximab vs. placebo | Infarct size 15.1% vs. 17.9%, p = 0.03 | Reduction in infarct size with IC abciximab | |
| Thiele et al. [85] | 2012 | RCT | 2065 | IC vs. IV bolus + infusion abciximab | Death, MI or HF 7.0 vs. 7.6%, p = NS | No difference in clinical outcomes between IV and IC abciximab | |
| Deferred stenting | Sezer et al. [72] | 2022 | RCT | 20 | Delayed vs. immediate stenting | Pressure at zero flow 41.5 vs. 76.9, p = 0.001 | Delaying stenting for 30 min after IRA recanalization was associated with less microvascular injury |
| NCT03581513 | 2022 | RCT | 880 | Deferred vs. immediate stenting | All-cause death, HF, MI and target vessel revascularization | Prematurely terminated due to safety concerns upon inclusion of 629 patients | |
| Vasodilating agents | Ito et al. [73] | 2013 | RCT | 60 | IC nicorandil vs. nitroglycerine | Reduction in IMR 10 vs. 2, p = 0.0002 | Nicorandil IC reduces CMD assessed by IMR |
| Qian et al. [74] | 2022 | RCT | 238 | IV nicorandil vs. placebo | Infarct size on CMR 19.5 g vs. 25.7 g, p = 0.008 | Nicorandil IV given prior to reperfusion reduced infarct size | |
| Kitakaze et al. [76] | 2007 | RCT | 1216 | IV ANP vs. placebo IV nicorandil vs. placebo | Infarct size (CK IU/mL) 66.45 vs. 77.78, p = 0.016 70.52 vs. 70.85, p = NS | IV ANP but not nicorandil decreased CK release and improved LVEF in the follow-up | |
| Nazir et al. [77] | 2016 | RCT | 247 | IC adenosine vs. SNP vs. control | Infarct size on CMR 10% vs. 10% vs. 8%, p = NS | Neither adenosine nor SNP reduced infarct size or MVO | |
| Device therapy | De Maria et al. [80] | 2018 | Obs. | 105 | PICSO vs. control | IMR/infarct size on CMR IMR: 24.8 vs. 45.0, p < 0.01 IS: 26% vs. 33%, p = 0.006 | PICSO reduced IMR and IS in patients with IMR > 40 at the end of primary PCI |
| Botker et al. [81] | 2010 | RCT | 333 | RIC vs. control | Myocardial salvage index 0.75 vs. 0.55, p = 0.03 | Remote conditioning by intermittent arm ischemia increased the proportion of salvaged myocardial area at risk | |
| Hausenloy [82] | 2019 | RCT | 5401 | RIC vs. control | Cardiac death or HF 9.4% vs. 8.6% | Remote conditioning by intermittent arm ischemia did not impact clinical outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milasinovic, D.; Nedeljkovic, O.; Maksimovic, R.; Sobic-Saranovic, D.; Dukic, D.; Zobenica, V.; Jelic, D.; Zivkovic, M.; Dedovic, V.; Stankovic, S.; et al. Coronary Microcirculation: The Next Frontier in the Management of STEMI. J. Clin. Med. 2023, 12, 1602. https://doi.org/10.3390/jcm12041602
Milasinovic D, Nedeljkovic O, Maksimovic R, Sobic-Saranovic D, Dukic D, Zobenica V, Jelic D, Zivkovic M, Dedovic V, Stankovic S, et al. Coronary Microcirculation: The Next Frontier in the Management of STEMI. Journal of Clinical Medicine. 2023; 12(4):1602. https://doi.org/10.3390/jcm12041602
Chicago/Turabian StyleMilasinovic, Dejan, Olga Nedeljkovic, Ruzica Maksimovic, Dragana Sobic-Saranovic, Djordje Dukic, Vladimir Zobenica, Dario Jelic, Milorad Zivkovic, Vladimir Dedovic, Sanja Stankovic, and et al. 2023. "Coronary Microcirculation: The Next Frontier in the Management of STEMI" Journal of Clinical Medicine 12, no. 4: 1602. https://doi.org/10.3390/jcm12041602
APA StyleMilasinovic, D., Nedeljkovic, O., Maksimovic, R., Sobic-Saranovic, D., Dukic, D., Zobenica, V., Jelic, D., Zivkovic, M., Dedovic, V., Stankovic, S., Asanin, M., & Vukcevic, V. (2023). Coronary Microcirculation: The Next Frontier in the Management of STEMI. Journal of Clinical Medicine, 12(4), 1602. https://doi.org/10.3390/jcm12041602







