Abstract
There are relatively few articles on the relationship between serum albumin and acute kidney injury (AKI). Therefore, the objective of this research was to study the relationship between serum albumin and AKI in patients who were undergoing surgery for acute type A aortic dissection. Methods: We retrospectively collected data from 624 patients attending a Chinese hospital between January 2015 and June 2017. The target independent variable was serum albumin measured before surgery after hospital admission, and the dependent variable was AKI, defined in accordance with the Kidney Disease Improving Global Outcomes (KDIGO) criteria. Results: The mean age of these 624 selected patients was 48.5 ± 11.1 years, and almost 73.7% were male. A nonlinear association was detected between serum albumin and AKI; the turning point was 32 g/L. The risk of AKI decreased gradually as the serum albumin level increased up to 32 g/L (adjusted OR = 0.87; 95% CI 0.82–0.92; p < 0.001). When the serum albumin level exceeded 32 g/L, the level of serum albumin was not associated with the risk of AKI (OR = 1.01, 95% CI 0.94–1.08; p = 0.769). Conclusions: The findings suggest that preoperative serum albumin below 32 g/L was an independent risk factor for AKI in patients undergoing surgery for acute type A aortic dissection. Trial registration: A retrospective cohort study.
1. Background
Acute kidney injury (AKI) is not an uncommon postoperative complication of acute type A aortic dissection (ATAAD). Despite improvements in medical management, intensive care unit treatment, and anesthetic and surgical technique, the reported frequency of AKI after surgery for ATAAD remains between 20% and 67%, which is significantly higher than that following other cardiac operations [1,2,3,4,5]. Currently, there are no effective measures for AKI. Therefore, clinicians focus on preventive action and risk factor management [6].
Several studies found that serum albumin may have renal protective effects at cellular and molecular levels [7]. Albumin can effectively promote the reabsorption of interstitial effusion, increase renal flow and urine output, and thus increase circulation volume. In addition, albumin has antioxidant properties, such as scavenging and limiting the production of reactive oxygen species, and providing lysophosphatidic acid with protective effect [8]. Other studies found preoperative hypoalbuminemia may be a potent independent risk factor for AKI after off-pump coronary artery bypass surgery (OPCABG) [9]. However, evidence regarding the relationship between preoperative serum albumin and acute kidney injury following aortic surgery for ATAAD is limited. Due to the complexity of the operation, this kind of procedure is itself an independent risk factor for AKI, including circulatory arrest and longer CPB duration.
Our center is famous for vascular diseases, especially for the treatment of ATAAD patients. We admit ATAAD patients almost every day and are also very concerned about postoperative AKI. Therefore, we want to determine the relationship between serum albumin and postoperative AKI in ATAAD patients through this study, and also provide strong evidence for precise medical treatment. From our own point of view, this will solve the common but difficulty clinical problems.
2. Methods
2.1. Study Design and Participants
A retrospective cohort study was carried out at Beijing Anzhen Hospital, which is one of China’s largest cardiac surgery centers and treats thousands of patients with aortic dissection annually from January 2015 to June 2017. The studies involving human participants were reviewed and approved by the human research and development committees of Beijing Anzhen Hospital (approved no. 2018051X), and they complied with the Declaration of Helsinki and principles of good clinical practice. This study is retrospective and no informed consent can be obtained from the patients, and the ethics committee has also approved this protocol. So, individual consent was waived.
A total of 858 patients who underwent aortic surgery for ATAAD within the aforementioned timeframe were admitted to this study. Of these, 23 patients who underwent renal replacement therapy (RRT) before surgery were excluded because the progression of renal dysfunction could not be evaluated. Another 195 patients who had subacute or chronic aortic dissection were excluded. Five patients were also excluded because they died intraoperatively or within 24 h postoperatively. We cannot define AKI without meaningful data. We also excluded patients without sCr or serum albumin values (n = 11). Clinical data for the remaining 624 patients were obtained and subsequently analyzed, including demographic data, laboratory data, comorbidities, operative techniques, postoperative morbidity, and mortality. The bromocresol green dye-binding method was used to measure serum albumin concentrations. The reference range of the albumin assay is 40–55 g/L at the hospital. A flowchart for study participant screening and enrolment is shown in Figure 1.
Figure 1.
Flow diagram of the screening and enrolment of study patients. After exclusion criteria were applied, 624 consecutive patients were included in this cohort.
2.2. Outcome Variables
The primary endpoint was the incidence of AKI after aortic surgery. Postoperative AKI was diagnosed in accordance with the Kidney Disease Improving Global Outcomes (KDIGO) criteria [10]: increase in sCr ≥ 0.3 mg/dL (at any time within 48 h following operation) or ≥1.5 times by baseline (at any time within 7 days following operation). Urine output was not used, due to potential errors in volume collection and other uncontrollable variables that may arise with retrospective collection.
Other postoperative outcomes, including reoperation for bleeding, dialysis after surgery, mechanical ventilation time, intensive care unit (ICU) time, length of hospital admission, and in-hospital mortality, were also obtained. Indications for postoperative dialysis were significant biochemical abnormalities, anuresis, uremia, and volume overburden.
2.3. Judgement of Covariates
Based on our previous studies and other risk factors reported in the literature for AKI after surgery, we selected several covariates, such as hypertension, diabetes mellitus, hemoglobin before surgery, estimated glomerular filtration rate (eGFR), baseline sCr before surgery, preoperative malperfusion syndromes, preoperative renal malperfusion, preoperative blood urea nitrogen (BUN), CPB duration, and intraoperative packed red blood cells (PRBCs). Hemoglobin, baseline sCr, and BUN were obtained from the laboratory testing after the patient was admitted to the hospital but before surgery.
2.4. Surgical Technique
All patients received surgery with median sternotomy and CPB. In patients with ascending aorta replacement, femoral artery intubation was used for CPB. Right axillary artery cannulation was performed for CPB in patients with hemi-arch replacement and total arch replacement and selective cerebral perfusion (SCP) (5–15 mL/(kg·min)). The specific surgical procedures have been described in detail in previous articles [11,12,13,14,15,16,17].
2.5. Statistical Analysis
Continuous variables were shown as the mean ± standard deviation or median (quartile), categorical variables were presented as percentages (%) in light of the data dispersion, and the t-test was used for comparison if the continuous variables were normally distributed, while the nonparametric Mann-Whitney U test was used if the data were skewed. Categorical variables were compared with the chi-square test. To identify the risk factors for AKI, we used univariate logistic regression analysis. Multiple logistic regression models were used to assess the associations between preoperative serum albumin and AKI after surgery. We established three models: (1) unadjusted; (2) adjusted for demographics, i.e., age; sex; and (3) adjusted for age; sex; BMI; preoperative red blood cell (RBC); preoperative BUN; eGFR; preoperative uric acid; CPB time; operative time; reoperation for bleeding; low cardiac output syndrome; time interval from diagnosis to operation; preoperative malperfusion syndromes; preoperative renal malperfusion. Based on the suggestions of the STROBE statement [18], the results were analyzed from unadjusted or marginally adjusted and completely adjusted data in parallel. Whether the concomitant variable was adjusted was decided according to the following regulation: an adjustment was made if the variable changed the matching odds ratio by at least 10% when added to the model [19]. Smooth curve fitting was performed to detect any nonlinear relationships between the preoperative serum albumin and the risk of AKI in patients who received aortic surgery for ATAAD; this method of application for smooth curve fitting was detailed by Motulsky [20]. Then, the threshold effect between preoperative serum albumin and AKI was analyzed using piecewise regression models, likelihood ratio tests, and bootstrap resampling [21]. We considered it to be statistically significant when a two-tailed p-value was less than 0.05. All analyses were implemented with the statistical software package R (http://www. Rproject.org, The R Foundation, accessed on 1 September 2022, Beijng, China) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA).
3. Results
3.1. Characteristics of the Studied Patients
After the exclusion criteria were implemented, 624 consecutive patients with an average age of 48.5 ± 11.1 years were enrolled in this cohort. Of these, 460 (73.7%) were male. The overall incidence rate of AKI was 37.7% (235 patients). Those patients developing AKI were more likely to have advanced age, diabetes, hypertension, lower albumin concentration, lower Hematocrit (%), preoperative malperfusion syndromes, and preoperative renal malperfusion. In-hospital mortality of the AKI group was significantly higher than the Non-AKI group. The characteristics of the 624 study patients are given in Table 1.

Table 1.
Characteristics of study patients at baseline.
3.2. Univariate Analysis of Risk Factors Related to Postoperative AKI in Patients with ATAAD
The results of a univariate analysis are shown in Table 2, which showed that age, male sex, diabetes, hypertension, preoperative acute liver failure, hematocrit, preoperative TP, preoperative serum albumin, uric acid, preoperative BUN, eGFR, sCr, preoperative D-dimer, preoperative TNI, preoperative malperfusion syndromes, preoperative renal malperfusion, intraoperative PRBC transfusion, CPB duration, aortic occlusion time, operative time, minimum rhinopharyngeal temperature, minimum rectal temperature, and reoperation for bleeding were significantly correlated with postoperative AKI.

Table 2.
Univariate analysis of risk factors associated with postoperative acute kidney injury in patients with acute type A aortic dissection.
3.3. The Nonlinear Relationship between Preoperative Serum Albumin and AKI after Adjusting for Covariates
After adjusting for these possible factors related to AKI, including age, sex, BMI, preoperative RBC, preoperative BUN, eGFR, preoperative uric acid, CPB time, operative time, reoperation for bleeding, and low cardiac output syndrome, a nonlinear relationship between age and AKI was observed (Figure 2) in smooth curve fitting. Table 3 shows the threshold influence of preoperative serum albumin on the hazard of AKI from piecewise linear regression. In Model I (unadjusted), when preoperative serum albumin was less than the turning point (32 g/L), it was inversely related to the risk of AKI (OR = 0.86, 95% CI: 0.82–0.91; p < 0.001). When preoperative serum albumin was more than 32 g/L, it was not related to the risk of AKI (OR = 1.00, 95% CI: 0.94–1.07; p = 0.898). In Model II (adjusted for age and sex), when preoperative serum albumin was less than 32 g/L, it was inversely related to the risk of AKI (OR = 0.86, 95% CI: 0.81–0.90; p < 0.001). When preoperative serum albumin was more than 32 g/L, it was not related to the risk of AKI (OR = 1.01, 95% CI: 0.95–1.08; p = 0.796). In Model III (adjusted for: age; sex; BMI; preoperative RBC; preoperative BUN; eGFR; preoperative uric acid; CPB time; operative time; reoperation for bleeding; low cardiac output syndrome; Time interval from diagnosis to operation; preoperative malperfusion syndromes; preoperative renal malperfusion), when preoperative serum albumin was less than 32 g/L, it was inversely related to the risk of AKI (OR = 0.85, 95% CI: 0.79–0.91; p < 0.001). When preoperative serum albumin was more than 32 g/L, it was not related to the risk of AKI (OR = 1.04, 95% CI: 0.95–1.15; p = 0.374). (LRT: p < 0.05 indicates a nonlinear relationship between preoperative serum albumin and AKI.)
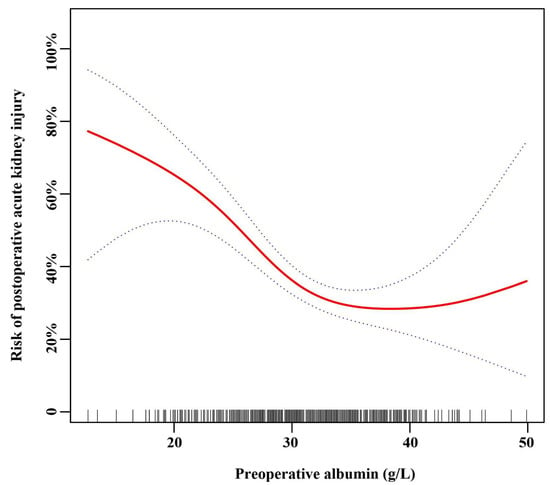
Figure 2.
Spline smoothing was performed using a GAM (generalized additive model) to explore the association between preoperative serum albumin and postoperative acute kidney injury after adjusting for: age; sex; body mass index; preoperative red blood cells; preoperative blood urea nitrogen; estimated glomerular filtration rate; preoperative uric acid; cardiopulmonary bypass time; operative time; reoperation for bleeding; low cardiac output syndrome; time interval from diagnosis to operation; preoperative malperfusion syndromes; preoperative renal malperfusion. The red line indicates the estimated risk of AKI, and the dotted lines represent the pointwise 95% confidence interval. The vertical lines of the X-axis indicate the distribution of the single observation.

Table 3.
Threshold effect of preoperative albumin on postoperative AKI in patients with acute type A aortic dissection.
4. Discussion
The present study analyzed the results of 624 Chinese patients obtained for a retrospective cohort study where each participant underwent aortic surgery for STAAAD. We found, for the first time, that preoperative serum albumin was conversely related to the hazard of AKI in the presence of less than 32 g/L among Chinese patients even after adjustment for significant confounding factors before surgery and perioperatively. In the event that the preoperative serum albumin increased by 1 g/L, the risk of postoperative AKI decreased by 15%. When preoperative serum albumin exceeded 32 g/L, it was not associated with the risk of postoperative AKI.
Numerous clinical studies have identified preoperative serum albumin as a notable risk factor for AKI among patients undergoing cardiac surgery. In addition, a previous meta-analysis indicated that hypoalbuminemia was an independent hazard of AKI [22]. Despite the strong association between hypoalbuminemia and AKI, a limited number of studies have evaluated the influence of serum albumin levels before surgery on AKI following surgery in patients who received aortic surgery for ATAAD. Kim et al. analyzed 702 patients undergoing surgery on the thoracic aorta with CPB, 352 of whom underwent aortic dissection [23]. The study showed that preoperative albumin <4.0 g/dL (OR = 2.50; CI 1.39–4.50; p = 0.008) was an independent risk factor for AKI. Several existing cohort studies have obtained similar results. Lee et al. evaluated the effect of preoperative albumin (<4 g/dL) on postoperative AKI in 1182 patients undergoing OPCABG and observed that hypoalbuminemia was an independent hazard of postoperative AKI after adjustment for confounders by logistic regression and propensity analyses [8]. An additional study assessed the association between preoperative hypoalbuminemia and postoperative adverse events in 5168 patients who underwent coronary artery bypass grafting (CABG) with or without valve surgery [24] and found that patients with hypoalbuminemia (<2.5 g/dL) had a substantial risk of renal failure (OR = 2.0, 95% CI: 1.3–3.2) following surgery after adjustment for confounders with multivariable logistic regression. Moreover, several other studies have shown that preoperative hypoalbuminemia was an independent predictive factor of postoperative AKI in patients undergoing cardiac operation, which further verifies the findings of the present research study [25,26].
Preoperative serum albumin may be causally associated with the progression of AKI following surgery rather than acting merely as a marker for alternative pathophysiological courses. Existing literature shows that serum albumin operates as a renal defense component that influences both cells and molecules. Kaufmann et al. observed that albumin could improve kidney perfusion and glomerular filtration via increased potent renal vasodilation [7]. Iglesias et al. affirmed that albumin could prevent apoptosis of renal tubular cells by scavenging reactive oxygen species and transporting protective lysophosphatidic acid [27]. Furthermore, Dixon et al. discovered that albumin could stimulate the proliferation of renal tubular cells via activation of phosphatidylinositide 3-kinase [28]. All of these findings indicate the significance of albumin in the preservation of the integrity and function of tubular cells.
The present research advances evidence from existing studies in two aspects. First, this investigation adopts the widely accepted AKI definition after cardiac surgery as the outcome (KDIGO criteria), which represents the currently recognized diagnostic criteria. Second, the study cohort comprised consecutive patients with different risks undergoing various aortic surgical procedures, so our study encompasses a broader population.
In comparison to the literature, the present study included a large number of participants, particularly ATAAD patients undergoing aortic surgery. The data were obtained by trained nurses who were blinded to the study. During data collection, some nurses only collected the preoperative data without knowing outcomes, and others only collected outcome data without knowing preoperative data. In addition, the present study applied KDIGO recommendations for AKI in place of the earlier two guidelines, as the KDIGO recommendations were recently modified and offer clarity and succinctness in clinical application. Thus, smooth curve fitting was introduced to determine nonlinear correlations between preoperative serum albumin and the risks of AKI in patients who were receiving aortic surgery. There was an adjustment for confounding factors, and the axis turning point was 32 g/L. Finally, this study was observational and therefore easily affected by underlying confounders. There was rigorous statistical adjustment to minimize underlying confounding factors and enhance the validity of these consequences.
The findings may have significant clinical implications. It is important to deepen our understanding of hazardous factors related to AKI progression, and our emphasis on preoperative albumin levels (<32 g/L) as a hazardous factor may direct clinical treatment tactics. Early recognition of high-risk patients for postoperative AKI allows clinical application specialists to strictly observe these patients and to implement preventive and therapeutic methods to decrease the occurrence of AKI. Such methods include avoidance of specific medication that can damage the renal system [29], reduction in time allocated to CPB, rapid identification of AKI, and prompt intervention [30]. Indeed, as we can see, a lot of factors influence the development of this complication. It does not mean that in patients with serum albumin over 32 g/L we do not need to avoid any specific medication that can damage the renal system, or limit time allocated to CPB, etc. Furthermore, if the association between preoperative serum albumin and AKI following aortic surgery is definitely causal, serum albumin before surgery may be a changeable risk variable. Lee discovered that in patients with preoperative serum albumin levels below 4.0 g/dL, prompt administration of 20% exogenous albumin before cardiac surgery increases urine output during surgery and decreases the risk of AKI after OPCABG [9]. Whether exogenous albumin supplementation is safe and beneficial for use in deep hypothermic circulatory arrest cases requires further verification. Moreover, the results of this research will be helpful for future studies to establish diagnostic or predictive models for AKI [31].
The present study had a number of limitations. First, the patients in our study were undergoing aortic surgery for ATAAD. Therefore, there are some deficiencies in the extrapolation and universality of the research. Second, there were several exclusion factors that removed the data of specific participants, namely, those who required RRT for kidney injury preoperatively and patients who died intraoperatively or within 24 h of the operation, which means that the results of this study are only applicable to these patients. Third, the majority of patients in the sample were male. More caution is needed when applying the findings to female patients. Fourth, the study was observational and nonrandomized. Fifth, albumin can change over time, and only using a single time point for albumin may lead to a difference in results. We chose the results that were closest to the time before the operation and tried our best to eliminate the differences in the results caused by changes in albumin. Furthermore, intravenous albumin before surgery may also confound the current finding. However, no patients in our center received intravenous albumin before surgery. Therefore, more caution is needed when applying the findings to patients who have already been received with albumin.
Although numerous variables regarded as affecting AKI following aortic surgery were taken into consideration, the influences of hidden or non-investigated risk factors cannot be completely excluded. The method of albumin measurement (bromocresol green) has been shown to be inferior to the bromocresol purple method in some studies, including those with inflammation or acute-phase globulins. Finally, given the observational nature of the research, we could not obtain a causal correlation between preoperative serum albumin and the risk of AKI following aortic surgery.
5. Conclusions
These findings suggest a nonlinear relationship between preoperative serum albumin and AKI in patients who underwent aortic surgery for ATAAD. Serum albumin less than 32 g/L before surgery was independently associated with an increased risk of AKI after aortic surgery for ATAAD. We need to design new studies to understand the molecular mechanisms of this connection so that preventative therapies can be developed accordingly.
Author Contributions
Data curation, Z.W.; funding acquisition, H.Z.; investigation, S.X. and M.G.; project administration, J.Z.; resources, L.S.; supervision, Y.L. and D.R.; validation, D.R.; writing—original draft, S.X.; Writing—review and editing, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key R&D Program of China (2017YFC1308000), the Capital Health Research and Development of Special (2018-2-2-66), and the Beijing Lab for Cardiovascular Precision Medicine, Beijing, China. PXM2017_014226_000037.
Institutional Review Board Statement
The study protocol was approved by the human research and development committees of Beijing Anzhen Hospital (approved no. 2018051X). Individual consent was waived because of the nature of the retrospective study.
Informed Consent Statement
Patient consent was waived due to retrospective study and no informed consent can be obtained from the patients, and the ethics committee has also approved this protocol.
Data Availability Statement
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Jie Liu (Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital) and Changzhong Chen, Xinglin Chen, and Chi Chen (Department of Epidemiology and Biostatistics, X&Y Solutions Inc.) for their contribution to the statistical support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AKI | acute kidney injury |
| ACT | activated clotting time |
| BMI | body mass index |
| BUN | blood urea nitrogen |
| CABG | coronary artery bypass grafting |
| CPB | cardiopulmonary bypass |
| eGFR | estimated glomerular filtration rate |
| ICU | intensive care unit |
| KDIGO | Kidney Disease Improving Global Outcomes |
| LRT | log likelihood ratio |
| OPCABG | off-pump coronary artery bypass surgery |
| OR | odds ratio |
| PRBCs | packed red blood cells |
| RBC | red blood cell |
| RRT | renal replacement therapy |
| SCP | selective cerebral perfusion |
| sCr | serum creatinine |
| SD | standard deviation |
| ATAAD | acute type A aortic dissection |
References
- Collins, J.S.; Evangelista, A.; Nienaber, C.A.; Bossone, E.; Fang, J.; Cooper, J.V.; Smith, D.E.; O’Gara, P.T.; Myrmel, T.; Gilon, D.; et al. Differences in Clinical Presentation, Management, and Outcomes of Acute Type A Aortic Dissection in Patients With and Without Previous Cardiac Surgery. Circulation 2004, 110, I237–I242. [Google Scholar] [CrossRef] [PubMed]
- Sinatra, R.; Melina, G.; Pulitani, I.; Fiorani, B.; Ruvolo, G.; Marino, B. Emergency operation for acute type A aortic dissection: Neurologic complications and early mortality. Ann. Thorac. Surg. 2001, 71, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-S.; Tsai, F.-C.; Chen, Y.-C.; Wu, L.-S.; Chen, S.-W.; Chu, J.-J.; Lin, P.-J.; Chu, P.-H. Impact of Acute Kidney Injury on One-Year Survival After Surgery for Aortic Dissection. Ann. Thorac. Surg. 2012, 94, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Roh, G.U.; Lee, J.W.; Nam, S.B.; Lee, J.; Choi, J.-R.; Shim, Y.H. Incidence and Risk Factors of Acute Kidney Injury After Thoracic Aortic Surgery for Acute Dissection. Ann. Thorac. Surg. 2012, 94, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Pan, X.; Gong, Z.; Zheng, J.; Liu, Y.; Zhu, J.; Sun, L. Risk factors for acute kidney injury in overweight patients with acute type A aortic dissection: A retrospective study. J. Thorac. Dis. 2015, 7, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.-M.; Wen, H.; Huang, J.-W.; Hsieh, C.-C.; Chen, H.-M.; Chiu, C.-C.; Chen, Y.-F. Significance of preoperative acute kidney injury in patients with acute type A aortic dissection. J. Formos. Med. Assoc. 2019, 118, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.; Castelli, I.; Pargger, H.; Drop, L. Nitric oxide dose-response study in the isolated perfused rat kidney after inhibition of endothelium-derived relaxing factor synthesis: The role of serum albumin. J. Pharmacol. Exp. Ther. 1995, 273, 855. [Google Scholar]
- Lee, E.-H.; Baek, S.-H.; Chin, J.-H.; Choi, D.-K.; Son, H.-J.; Kim, W.-J.; Hahm, K.-D.; Sim, J.-Y.; Choi, I.-C. Preoperative hypoalbuminemia is a major risk factor for acute kidney injury following off-pump coronary artery bypass surgery. Intensive Care Med. 2012, 38, 1478–1486. [Google Scholar] [CrossRef]
- Lee, E.-H.; Kim, W.-J.; Kim, J.-Y.; Chin, J.-H.; Choi, D.-K.; Choo, S.-J.; Chung, C.-H.; Lee, J.-W.; Choi, I.-C. Effect of Exogenous Albumin on the Incidence of Postoperative Acute Kidney Injury in Patients Undergoing Off-pump Coronary Artery Bypass Surgery with a Preoperative Albumin Level of Less Than 4.0 g/dL. Anesthesiology 2016, 124, 1001–1011. [Google Scholar] [CrossRef]
- Lameire, N.; Levin, A.; Kellum, J.; Cheung, M.; Jadoul, M.; Winkelmayer, W.; Stevens, P. Harmonizing acute and chronic kidney disease definition and classification: Report of a kidney disease: Improving global outcomes (kdigo) consensus conference. Kidney Int. 2021, 100, 516–526. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Sun, L.-Z.; Chang, Q.; Zhu, J.-M.; Dong, C.; Yu, C.-T.; Liu, Y.-M.; Zhang, H.-T. Should the “elephant trunk” be skeletonized? Total arch replacement combined with stented elephant trunk implantation for Stanford type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2006, 131, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-Z.; Qi, R.-D.; Chang, Q.; Zhu, J.-M.; Liu, Y.-M.; Yu, C.-T.; Lv, B.; Zheng, J.; Tian, L.-X.; Lu, J.-G. Is total arch replacement combined with stented elephant trunk implantation justified for patients with chronic Stanford type A aortic dissection? J. Thorac. Cardiovasc. Surg. 2009, 138, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qi, R.; Zhu, J.; Liu, Y.; Chang, Q.; Zheng, J. Repair of Acute Type A Dissection: Our Experiences and Results. Ann. Thorac. Surg. 2011, 91, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qi, R.; Zhu, J.-M.; Liu, Y.; Zheng, J. Total arch replacement combined with stented elephant trunk implantation: A new “standard” therapy for type a dissection involving repair of the aortic arch? Circulation 2011, 123, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-Z.; Qi, R.-D.; Chang, Q.; Zhu, J.-M.; Liu, Y.-M.; Yu, C.-T.; Lv, B.; Zheng, J.; Tian, L.-X.; Lu, J.-G. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: Experience with 107 patients. J. Thorac. Cardiovasc. Surg. 2009, 138, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qi, R.; Chang, Q.; Zhu, J.; Liu, Y.; Yu, C.; Zhang, H.; Lv, B.; Zheng, J.; Tian, L.; et al. Surgery for Acute Type A Dissection with the Tear in the Descending Aorta Using a Stented Elephant Trunk Procedure. Ann. Thorac. Surg. 2009, 87, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qi, R.; Chang, Q.; Zhu, J.; Liu, Y.; Yu, C.; Zhang, H.; Lv, B.; Zheng, J.; Tian, L.; et al. Surgery for Marfan Patients with Acute Type A Dissection Using a Stented Elephant Trunk Procedure. Ann. Thorac. Surg. 2008, 86, 1821–1825. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Kernan, W.N.; Viscoli, C.M.; Brass, L.M.; Broderick, J.P.; Brott, T.; Feldmann, E.; Morgenstern, L.B.; Wilterdink, J.L.; Horwitz, R.I. Phenylpropanolamine and the Risk of Hemorrhagic Stroke. New Engl. J. Med. 2000, 343, 1826–1832. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef]
- Li, W.; Shi, D.; Song, W.; Xu, L.; Zhang, L.; Feng, X.; Lu, R.; Wang, X.; Meng, H. A novel U-shaped relationship between BMI and risk of generalized aggressive periodontitis in Chinese: A cross-sectional study. J. Periodontol. 2018, 90, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J.; Wiedermann, W.; Joannidis, M. Correction to: Hypoalbuminemia and acute kidney injury: A meta-analysis of observational clinical studies. Intensive Care Med. 2020, 47, 262. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Park, M.H.; Kim, H.-J.; Lim, H.-Y.; Shim, H.S.; Sohn, J.-T.; Kim, C.S.; Lee, S.M. Potentially modifiable risk factors for acute kidney injury after surgery on the thoracic aorta: A propensity score matched case-control study. Medicine 2015, 94, e273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xue, F. Assessing the Effect of Exogenous Albumin on the Incidence of Postoperative Acute Kidney Injury in Patients Undergoing Off-pump Coronary Artery Bypass Surgery. Anesthesiology 2016, 125, 1251–1252. [Google Scholar] [CrossRef] [PubMed]
- Gassa, A.; Borghardt, J.H.; Maier, J.; Kuhr, K.; Michel, M.; Ney, S.; Eghbalzadeh, K.; Sabashnikov, A.; Rudolph, T.; Baldus, S.; et al. Effect of preoperative low serum albumin on postoperative complications and early mortality in patients undergoing transcatheter aortic valve replacement. J. Thorac. Dis. 2018, 10, 6763–6770. [Google Scholar] [CrossRef] [PubMed]
- Karas, P.L.; Goh, S.L.; Dhital, K. Is low serum albumin associated with postoperative complications in patients undergoing cardiac surgery? Interact. Cardiovasc. Thorac. Surg. 2015, 21, 777. [Google Scholar] [CrossRef]
- Iglesias, J.; Abernethy, V.E.; Wang, Z.; Lieberthal, W.; Koh, J.S.; Levine, J.S. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am. J. Physiol. Physiol. 1999, 277, F711–F722. [Google Scholar] [CrossRef]
- Dixon, R.; Brunskill, N.J. Activation of mitogenic pathways by albumin in kidney proximal tubule epithelial cells: Implications for the pathophysiology of proteinuric states. J. Am. Soc. Nephrol. 1999, 10, 1487–1497. [Google Scholar] [CrossRef]
- Wiedermann, C.J.; Dunzendorfer, S.; Gaioni, L.U.; Zaraca, F.; Joannidis, M. Hyperoncotic colloids and acute kidney injury: A meta-analysis of randomized trials. Crit. Care 2010, 14, R191–R199. [Google Scholar] [CrossRef]
- Gaffney, A.M.; Sladen, R.N. Acute kidney injury in cardiac surgery. Curr. Opin. Anaesthesiol. 2015, 28, 50–59. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Choi, J.; Kim, E.; Lee, J.; Jung, J.; Ahn, J.; Sung, K.; Kim, C.; Cho, H. Simplified clinical risk score to predict acute kidney injury after aortic surgery. J. Cardiothorac. Vasc. Anesth. 2013, 27, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).