Effects of Craniotomy and Endoscopic Endonasal Transsphenoidal Surgery on Bodyweight in Adult-Onset Craniopharyngioma: A Single-Center Retrospective Study
Abstract
1. Introduction
2. Patients and Methods
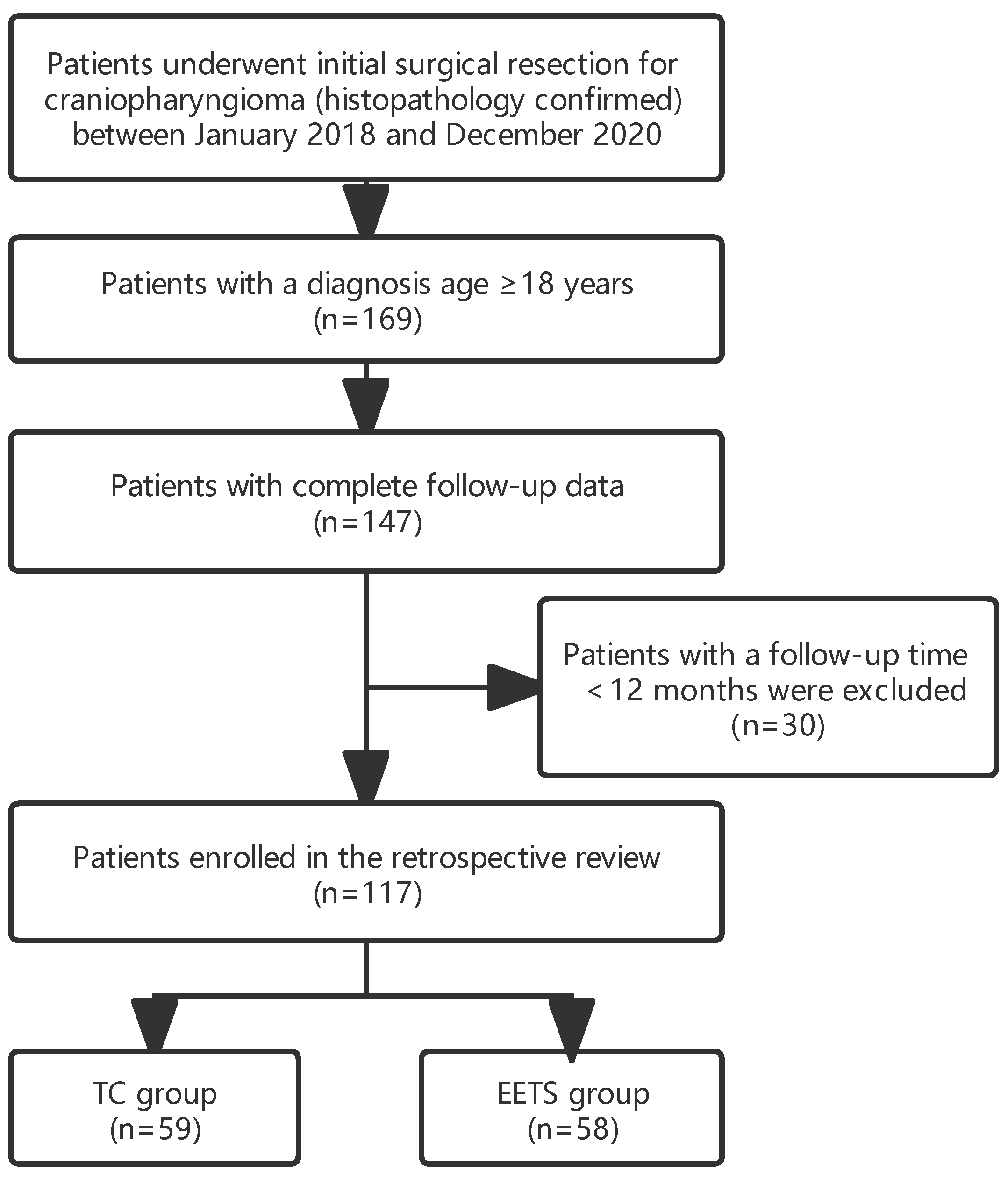
2.1. Patient Selection
2.2. Data Collection and Definition
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Extent of Surgical Resection and Hypothalamus Involvement
3.3. Hormonal Outcomes
3.4. Postsurgical Weight Change and Obesity
4. Discussion
4.1. Surgical Outcomes
4.2. Endocrinological Outcomes
4.3. Weight Control and Obesity
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Preoperative BMI Category | ALL | p (Preoperative vs. Postoperative) | Normal Weight | p (Preoperative vs. Postoperative) | Overweight | p (Preoperative vs. Postoperative) | Obesity | p (Preoperative vs. Postoperative) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | |||||
| Whole cohort | 25.50 ± 3.59 | 27.27 ± 4.24 | <0.001 | 22.60 ± 2.06 | 24.83 ± 3.38 | <0.001 | 27.03 ± 1.56 | 28.83 ± 3.98 | 0.003 | 31.62 ± 1.06 | 31.29 ± 1.96 | 0.465 |
| TC group | 25.46 ± 3.96 | 27.75 ± 3.72 | <0.001 | 22.82 ± 1.77 | 25.90 ± 3.83 | <0.001 | 26.73 ± 1.55 | 28.75 ± 3.06 | 0.003 | 31.23 ± 0.79 | 30.86 ± 1.71 | 0.596 |
| EETS group | 25.54 ± 3.21 | 26.60 ± 4.68 | 0.004 | 22.42 ± 2.29 | 23.90 ± 2.65 | <0.001 | 27.42 ± 1.52 | 28.93 ± 5.02 | 0.174 | 31.97 ± 1.19 | 31.66 ± 2.19 | 0.652 |
References
- Müller, H.L.; Merchant, T.E.; Warmuth-Metz, M.; Martinez-Barbera, J.P.; Puget, S. Craniopharyngioma. Nat. Rev. Dis. Prim. 2019, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Tatagiba, M. Surgical management of craniopharyngiomas: A review of 74 cases. Neurol. Med. Chir. 1986, 65, 22–27. [Google Scholar] [CrossRef]
- Ravindra, V.M.; Okcu, M.F.; Ruggieri, L.; Frank, T.S.; Paulino, A.C.; Mcgovern, S.L.; Horne, V.E.; Dauser, R.C.; Whitehead, W.E.; Aldave, G. Comparison of multimodal surgical and radiation treatment methods for pediatric craniopharyngioma: Long-term analysis of progression-free survival and morbidity. J. Neurosurg. Pediatr. 2021, 28, 152–159. [Google Scholar] [CrossRef]
- Clark, A.J.; Cage, T.A.; Aranda, D.; Parsa, A.T.; Sun, P.P.; Auguste, K.I.; Gupta, N. A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Child’s Nerv. Syst. 2013, 29, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.E.; Hsieh, K.; Hochm, T.; Belitskaya-Levy, I.; Wisoff, J.; Wisoff, J.H. Efficacy and safety of radical resection of primary and recurrent craniopharyngiomas in 86 children. J. Neurosurg. Pediatr. 2010, 5, 30–48. [Google Scholar] [CrossRef]
- Yang, I.; Sughrue, M.E.; Rutkowski, M.J.; Kaur, R.; Ivan, M.E.; Aranda, D.; Barani, I.J.; Parsa, A.T. Craniopharyngioma: A comparison of tumor control with various treatment strategies. Neurosurg. Focus 2010, 28, E5. [Google Scholar] [CrossRef] [PubMed]
- Yaşargil, M.G.; Curcic, M.; Kis, M.; Siegenthaler, G.; Teddy, P.J.; Roth, P. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J. Neurosurg. 1990, 73, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Chuzhong, L.; Chunhui, L.; Peng, Z.; Jiwei, B.; Xinsheng, W.; Yazhuo, Z.; Songbai, G. Approach selection and outcomes of craniopharyngioma resection: A single-institute study. Neurosurg. Rev. 2021, 44, 1737–1746. [Google Scholar] [CrossRef]
- Mamelak, A.N.; Carmichael, J.; Bonert, V.H.; Cooper, O.; Melmed, S. Single-surgeon fully endoscopic endonasal transsphenoidal surgery: Outcomes in three-hundred consecutive cases. Pituitary 2013, 16, 393–401. [Google Scholar] [CrossRef]
- Feng, Z.; Li, C.; Cao, L.; Qiao, N.; Wu, W.; Bai, J.; Zhao, P.; Gui, S. Endoscopic Endonasal Transsphenoidal Surgery for Recurrent Craniopharyngiomas. Front. Neurol. 2022, 13, 847418. [Google Scholar] [CrossRef]
- Komotar, R.J.; Starke, R.M.; Raper, D.M.S.; Anand, V.K.; Schwartz, T.H. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg. 2012, 77, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Komotar, R.J.; Starke, R.M.; Raper, D.M.S.; Anand, V.K.; Schwartz, T.H. Endoscopic skull base surgery: A comprehensive comparison with open transcranial approaches. Br. J. Neurosurg. 2012, 26, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Jeswani, S.; Nuño, M.; Wu, A.; Bonert, V.; Carmichael, J.D.; Black, K.L.; Chu, R.; King, W.; Mamelak, A.N. Comparative analysis of outcomes following craniotomy and expanded endoscopic endonasal transsphenoidal resection of craniopharyngioma and related tumors: A single-institution study. J. Neurosurg. 2016, 124, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Wannemuehler, T.J.; Rubel, K.E.; Hendricks, B.K.; Ting, J.Y.; Payner, T.D.; Shah, M.V.; Cohen-Gadol, A.A. Outcomes in transcranial microsurgery versus extended endoscopic endonasal approach for primary resection of adult craniopharyngiomas. Neurosurg. Focus 2016, 41, E6. [Google Scholar] [CrossRef]
- Qiao, N.; Yang, X.; Li, C.; Ma, G.; Kang, J.; Liu, C.; Cao, L.; Zhang, Y.; Gui, S. The predictive value of intraoperative visual evoked potential for visual outcome after extended endoscopic endonasal surgery for adult craniopharyngioma. J. Neurosurg. 2021, 135, 1714–1724. [Google Scholar] [CrossRef]
- Amoo, M.; Crimmins, D.; Caird, J.; Daly, P.; Pears, J.; Owens, C.; Capra, M.; Cody, D.; Javadpour, M. Endoscopic Extended Transsphenoidal Surgery for Newly Diagnosed Paediatric Craniopharyngiomas. Pediatr. Blood Cancer 2021, 68, e29349. [Google Scholar] [CrossRef]
- Puget, S.; Garnett, M.; Wray, A.; Grill, J.; Habrand, J.-L.; Bodaert, N.; Zerah, M.; Bezerra, M.; Renier, D.; Pierre-Kahn, A.; et al. Pediatric craniopharyngiomas: Classification and treatment according to the degree of hypothalamic involvement. J. Neurosurg. 2007, 106, 3–12. [Google Scholar] [CrossRef]
- Duan, D.; Wehbeh, L.; Mukherjee, D.; Hamrahian, A.H.; Rodriguez, F.J.; Gujar, S.; Khalafallah, A.M.; Hage, C.; Caturegli, P.; Gallia, G.L.; et al. Preoperative BMI Predicts Postoperative Weight Gain in Adult-onset Craniopharyngioma. J. Clin. Endocrinol. Metab. 2021, 106, e1603–e1617. [Google Scholar] [CrossRef]
- Karavitaki, N.; Brufani, C.; Warner, J.T.; Adams, C.B.T.; Richards, P.; Ansorge, O.; Shine, B.; Turner, H.E.; Wass, J.A.H. Craniopharyngiomas in children and adults: Systematic analysis of 121 cases with long-term follow-up. Clin. Endocrinol. 2005, 62, 397–409. [Google Scholar] [CrossRef]
- Wu, W.; Sun, Q.; Zhu, X.; Xiang, B.; Zhang, Q.; Miao, Q.; Wang, Y.; Li, Y.; Ye, H. Risk Factors for Hypothalamic Obesity in Patients With Adult-Onset Craniopharyngioma: A Consecutive Series of 120 Cases. Front. Endocrinol. 2021, 12, 694213. [Google Scholar] [CrossRef]
- Sterkenburg, A.S.; Hoffmann, A.; Gebhardt, U.; Warmuth-Metz, M.; Daubenbüchel, A.M.M.; Müller, H.L. Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: Newly reported long-term outcomes. Neuro Oncol. 2015, 17, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Özyurt, J.; Müller, H.L.; Warmuth-Metz, M.; Thiel, C.M. Hypothalamic tumors impact gray and white matter volumes in fronto-limbic brain areas. Cortex 2017, 89, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.J.; Carraretto, M. The neurological and cognitive consequences of hyperthermia. Crit. Care 2016, 20, 199. [Google Scholar] [CrossRef]
- Özyurt, J.; Thiel, C.M.; Lorenzen, A.; Gebhardt, U.; Calaminus, G.; Warmuth-Metz, M.; Müller, H.L. Neuropsychological outcome in patients with childhood craniopharyngioma and hypothalamic involvement. J. Pediatr. 2014, 164, 876–881.e4. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pan, C.B.; Xie, S.H.; Tang, B.; Fu, J.; Wu, X.; Tong, Z.G.; Wu, B.W.; Yang, Y.Q.; Ding, H.; et al. A propensity-adjusted comparison of endoscopic endonasal surgery versus transcranial microsurgery for pediatric craniopharyngioma: A single-center study. J. Neurosurg. Pediatr. 2022, 29, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; De Divitiis, E. Endoscopic cranial base surgery: Classification of operative approaches. Neurosurgery 2008, 62, 1003. [Google Scholar] [CrossRef]
- Müller, H.L.; Gebhardt, U.; Faldum, A.; Emser, A.; Etavard-Gorris, N.; Kolb, R.; Sörensen, N. Functional capacity and body mass index in patients with sellar masses—Cross-sectional study on 403 patients diagnosed during childhood and adolescence. Child’s Nerv. Syst. 2005, 21, 539–545. [Google Scholar] [CrossRef]
- Flynn, F.G.; Cummings, J.L.; Tomiyasu, U. Altered behavior associated with damage to the ventromedial hypothalamus: A distinctive syndrome. Behav. Neurol. 1988, 1, 49–58. [Google Scholar] [CrossRef]
- Patel, T.D.; Rullan-Oliver, B.; Ungerer, H.; Storm, P.B.; Kohanski, M.A.; Adappa, N.D.; Palmer, J.N. Outcomes of endoscopic endonasal resection of pediatric craniopharyngiomas. Int. Forum Allergy Rhinol. 2022, 12, 1517–1526. [Google Scholar] [CrossRef]
- Cohen, M.; Guger, S.; Hamilton, J. Long term sequelae of pediatric craniopharyngioma-literature review and 20 years of experience. Front. Endocrinol. 2011, 2, 81. [Google Scholar] [CrossRef]
- Yamada, S.; Fukuhara, N.; Yamaguchi-Okada, M.; Nishioka, H.; Takeshita, A.; Takeuchi, Y.; Inoshita, N.; Ito, J. Therapeutic outcomes of transsphenoidal surgery in pediatric patients with craniopharyngiomas: A single-center study. J. Neurosurg. Pediatr. 2018, 21, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Joly-Amado, A.; Cansell, C.; Denis, R.G.P.; Delbes, A.S.; Castel, J.; Martinez, S.; Luquet, S. The hypothalamic arcuate nucleus and the control of peripheral substrates. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 725–737. [Google Scholar] [CrossRef] [PubMed]
- van Iersel, L.; Brokke, K.E.; Adan, R.A.H.; Bulthuis, L.C.M.; van den Akker, E.L.T.; van Santen, H.M. Pathophysiology and Individualized Treatment of Hypothalamic Obesity Following Craniopharyngioma and Other Suprasellar Tumors: A Systematic Review. Endocr. Rev. 2019, 40, 193–235. [Google Scholar] [CrossRef] [PubMed]
- Ainiwan, Y.; Chen, Y.; Mao, C.; Peng, J.; Chen, S.; Wei, S.; Qi, S.; Pan, J. Adamantinomatous craniopharyngioma cyst fluid can trigger inflammatory activation of microglia to damage the hypothalamic neurons by inducing the production of β-amyloid. J. Neuroinflammat. 2022, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Daniel, S.; Fujioka, K.; Umashanker, D. Obesity among Asian American people in the United States: A review. Obesity 2023, 31, 316–328. [Google Scholar] [CrossRef]
- Na, Y.M.; Park, H.A.; Kang, J.H.; Cho, Y.G.; Kim, K.W.; Hur, Y.I.; Kim, Y.N.; Lee, S.H. Obesity, obesity related disease, and disability. Korean J. Fam. Med. 2011, 32, 412–422. [Google Scholar] [CrossRef]
- Van Gompel, J.J.; Nippoldt, T.B.; Higgins, D.M.; Meyer, F.B. Magnetic resonance imaging-graded hypothalamic compression in surgically treated adult craniopharyngiomas determining postoperative obesity. Neurosurg. Focus 2010, 28, E3. [Google Scholar] [CrossRef]
- Daousi, C.; Dunn, A.J.; Foy, P.M.; MacFarlane, I.A.; Pinkney, J.H. Endocrine and neuroanatomic features associated with weight gain and obesity in adult patients with hypothalamic damage. Am. J. Med. 2005, 118, 45–50. [Google Scholar] [CrossRef]
- Steele, C.A.; Cuthbertson, D.J.; MacFarlane, I.A.; Javadpour, M.; Das, K.S.V.; Gilkes, C.; Wilding, J.P.; Daousi, C. Hypothalamic obesity: Prevalence, associations and longitudinal trends in weight in a specialist adult neuroendocrine clinic. Eur. J. Endocrinol. 2013, 168, 501–507. [Google Scholar] [CrossRef]
- Park, S.W.; Jung, H.W.; Lee, Y.A.; Shin, C.H.; Yang, S.W.; Cheon, J.-E.; Kim, I.-O.; Phi, J.H.; Kim, S.-K.; Wang, K.-C. Tumor origin and growth pattern at diagnosis and surgical hypothalamic damage predict obesity in pediatric craniopharyngioma. J. Neurooncol. 2013, 113, 417–424. [Google Scholar] [CrossRef]
- Müller, H.L.; Bueb, K.; Bartels, U.; Roth, C.; Harz, K.; Graf, N.; Korinthenberg, R.; Bettendorf, M.; Kühl, J.; Gutjahr, P.; et al. Obesity after childhood craniopharyngioma--German multicenter study on pre-operative risk factors and quality of life. Klin. Pädiatrie 2001, 213, 244–249. [Google Scholar] [CrossRef]
- De Vile, C.J.; Grant, D.B.; Kendall, B.E.; Neville, B.G.; Stanhope, R.; Watkins, K.E.; Hayward, R.D. Management of childhood craniopharyngioma: Can the morbidity of radical surgery be predicted? J. Neurosurg. 1996, 85, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Andereggen, L.; Hess, B.; Andres, R.; El-Koussy, M.; Mariani, L.; Raabe, A.; Seiler, R.W.; Christ, E. A ten-year follow-up study of treatment outcome of craniopharyngiomas. Swiss Med. Wkly. 2018, 148, w14521. [Google Scholar] [CrossRef] [PubMed]
- Bozbulut, R.; Ertaş-Öztürk, Y.; Döğer, E.; Bideci, A.; Köksal, E. Increased Obesity Awareness and Adherence to Healthy Lifestyle-Diet Reduce Metabolic Syndrome Risk in Overweight Children. J. Am. Coll. Nutr. 2020, 39, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Post, S.R.; Srivannaboon, K.; Rose, S.R.; Danish, R.K.; Burghen, G.A.; Xiong, X.; Wu, S.; Merchant, T.E. Risk factors for the development of obesity in children surviving brain tumors. J. Clin. Endocrinol. Metab. 2003, 88, 611–616. [Google Scholar] [CrossRef]
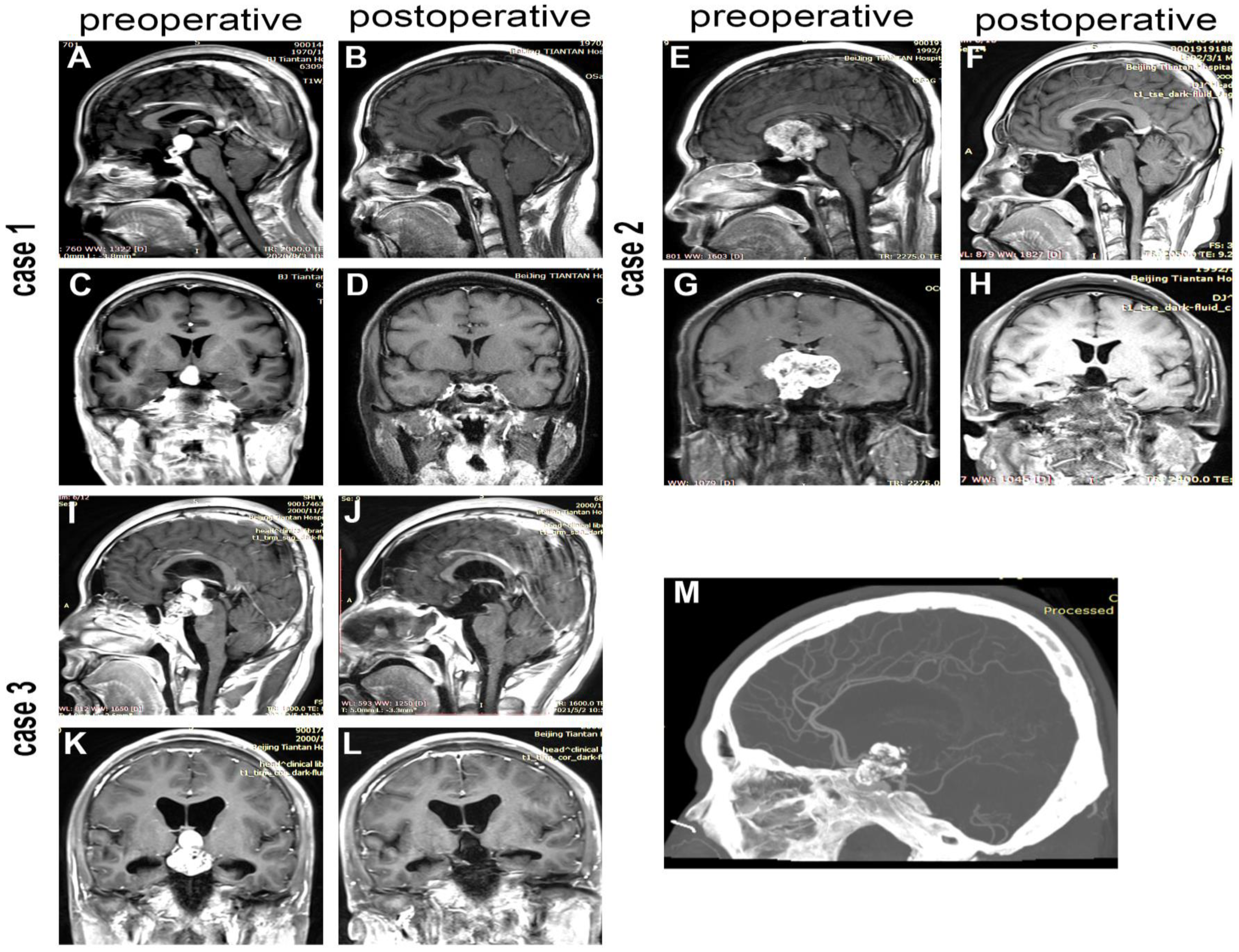


| Preoperative Characterstics | Total Group (n = 117) | TC Group (n = 59) | EETS Group (n = 58) | p |
|---|---|---|---|---|
| Age at diagnosis (mean ± SD, year) | 41.92 ± 15.51 | 41.02 ± 10.20 | 42.84 ± 12.74 | 0.393 |
| Tumor size (mean ± SD, cm) | 2.99 ± 0.95 | 2.89 ± 0.87 | 3.10 ± 1.03 | 0.245 |
| Follow–up time (median, IQR, month) | 26 (22–30) | 26 (20–32) | 25.50 (23–29) | 0.724 |
| Male sex | 43 (36.7%) | 22 (37.3%) | 21 (36.2%) | 0.904 |
| Histopathology subtype (ACP) | 72 (61.5%) | 33 (55.9%) | 39 (67.2%) | 0.209 |
| Calcification | 77 (65.8%) | 39 (66.1%) | 38 (65.5%) | 0.947 |
| Preoperative BMI (mean ± SD, kg/m2) | 25.50 ± 3.59 | 25.46 ± 3.96 | 25.54 ± 3.21 | 0.111 |
| Preoperative BMI (category) | 0.569 | |||
| Normal weight | 56 (47.9%) | 26 (44.1%) | 30 (51.7%) | |
| Overweight | 46 (39.3%) | 26 (44.1) | 20 (34.5%) | |
| Obesity | 15 (12.8%) | 7 (11.8) | 8 (11.8%) | |
| Preoperative HI | 0.629 | |||
| Grade 0 | 3 (2.6%) | 2 (3.4%) | 1 (1.7%) | |
| Grade 1 | 42 (35.9%) | 19 (32.2%) | 23 (39.7%) | |
| Grade 2 | 72 (61.5%) | 38 (64.4%) | 34 (58.6%) | |
| Surgical outcomes | ||||
| Extent of surgical resection | 0.027 | |||
| GTR | 99 (84.6%) | 46 (78.0%) | 53 (91.4%) | |
| STR | 18 (15.4%) | 13 (22.0%) | 5 (8.6%) | |
| Postoperative HI | <0.001 | |||
| Grade 0 | 38 (32.5%) | 18 (13.6%) | 20 (34.5%) | |
| Grade 1 | 50 (42.7%) | 17 (28.8%) | 33 (56.9%) | |
| Grade 2 | 29 (24.8%) | 24 (40.7%) | 5 (8.6%) | |
| HI change | 0.053 | |||
| improved | 77 (65.8%) | 35 (59.3%) | 42 (72.4%) | |
| no change | 35 (29.9%) | 19 (32.2%) | 16 (27.6%) | |
| worse | 5 (4.3%) | 5 (8.5%) | 0 (0%) |
| Characteristics | GTR | HI Improvement | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95%CI) | p | Adjusted OR (95%CI) | p | Unadjusted OR (95%CI) | p | Adjusted OR (95%CI) | p | |
| Age | 1.01 (0.97–1.06) | 0.686 | 1.03 (0.97–1.09) | 0.403 | 0.99 (0.96–1.02) | 0.59 | 0.99 (0.951–1.03) | 0.584 |
| Tumor size | 1.03 (0.60–1.77) | 0.928 | 1.02 (0.53–1.97) | 0.946 | 1.45 (0.95–2.24) | 0.09 | 1.12 (0.66–1.91) | 0.672 |
| Sex (female vs. male) | 0.48 (0.15–1.58) | 0.229 | 0.43 (0.11–1.62) | 0.209 | 0.84 (0.38–1.86) | 0.67 | 1.08 (0.42–2.73) | 0.878 |
| Histopathology subtype (ACP vs. PCP) | 2.65 (0.93–7.58) | 0.068 | 2.93 (0.85–10.07) | 0.088 | 1.66 (0.77–3.61) | 0.2 | 1.39 (0.55–3.52) | 0.488 |
| Calcification (yes vs. no) | 1.06 (0.36–3.11) | 0.917 | 1.05 (0.29–3.77) | 0.947 | 1.00 (0.45–2.22) | 0.99 | 0.78 (0.28–2.17) | 0.64 |
| Operation (EEA vs. TC) | 3.82 (1.16–12.51) | 0.027 * | 4.08 (1.15–14.43) | 0.029 * | 1.93 (0.89–4.18) | 0.1 | 2.58 (1.04–6.42) | 0.041 * |
| Preoperative HI (G2 vs. G1&G0) | 2.00 (0.71–5.64) | 0.19 | 3.00 (0.82–11.05) | 0.098 | 6.21 (2.70–14.32) | <0.001 * | 8.86 (3.14–25.01) | <0.001 * |
| Preoperative BMI (normal weight, reference) | — | — | — | — | — | — | — | — |
| Overweight | 1.37 (0.415–4.50) | 0.608 | 1.18 (0.30–4.65) | 0.814 | 1.24 (0.55–2.82) | 0.61 | 0.74 (0.28–1.02) | 0.561 |
| Obesity | 0.458 (0.12–1.80) | 0.263 | 0.12 (0.02–0.81) | 0.03 * | 1.20 (0.36–3.99) | 0.77 | 0.34 (0.07–1.60) | 0.175 |
| Pre-Existing Hormonal Outcomes | Total (n = 117) | TC Group (n = 59) | EETS Group (n = 58) | p |
|---|---|---|---|---|
| Anterior pituitary dysfunction | ||||
| Adrenocorticotropic hormone deficiency | 10 (8.5%) | 7 (11.9%) | 3 (5.2%) | 0.322 |
| Thyroid-stimulating hormone deficiency | 25 (21.4%) | 12 (20.3%) | 13 (22.4%) | 0.827 |
| Growth hormone deficiency | 14 (12.0%) | 7 (11.9%) | 7 (12.1%) | 1 |
| Gonadotropic hormone deficiency | 29 (24.8%) | 11 (18.6%) | 18 (31.0%) | 0.121 |
| Posterior pituitary dysfunction | ||||
| Antidiuretic hormone deficiency | 23 (19.7%) | 12 (20.3%) | 11 (19.0%) | 0.8517 |
| ≥3 hormone deficiency | 13 (11.1%) | 6 (10.2%) | 7 (12.1%) | 0.744 |
| Hypopituitarism | 13 (11.1%) | 6 (10.2%) | 7 (12.1%) | 0.744 |
| New-onset hormonal outcomes | Total | TC group | EETS group | p |
| Anterior pituitary function worsening | 52 (44.4%) | 30 (50.8%) | 22 (37.9%) | 0.161 |
| Posterior pituitary dysfunction # | 43 (45.7%) | 27 (57.4%) | 16 (34.0%) | 0.024 * |
| ≥3 hormone deficiency $ | 47 (45.2%) | 27 (50.9%) | 20 (39.2%) | 0.231 |
| Hypopituitarism & | 48 (46.2%) | 31 (58.5%) | 18 (45.0%) | 0.019 * |
| Characteristics | Preoperative Record (Diagnosis) | Postoperative Record (Final Follow-Up) | p-Value |
|---|---|---|---|
| BMI | 25.50 ± 3.59 | 27.27 ± 4.24 | <0.001 |
| BMI category | 0.003 | ||
| normal weight | 56 | 34 | |
| overweight | 46 | 51 | |
| obesity | 15 | 32 | |
| Weight change | — | ||
| weight loss > 5% | — | 10 | |
| weight stable | — | 52 | |
| weight gain > 5% | — | 55 |
| Characteristics | Weight Loss > 5% # | Weight Gain > 5% $ | Significant Weight Change | New-Onset Obesity & | ||||
|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95%) | p | Adjusted OR (95%) | p | Adjusted OR (95%) | p | Adjusted OR (95%) | p | |
| Age | 1.034 (0.965–1.108) | 0.34 | 1.059 (1.016–1.103) | 0.007 ** | 1.055 (1.016–1.095) | 0.005 ** | 1.040 (0.990–1.091) | 0.117 |
| Tumor size | 1.085 (0.399–2.950) | 0.87 | 1.072 (0.651–1.765) | 0.785 | 1.069 (0.667–1.713) | 0.782 | 1.486 (0.765–2.887) | 0.242 |
| Follow-up | 0.930 (0.794–1.088) | 0.37 | 1.017 (0.932–1.111) | 0.7 | 1.008 (0.930–1.093) | 0.84 | 1.028 (0.927–1.14) | 0.598 |
| Sex (female vs. male) | 0.394 (0.079–1.971) | 0.26 | 0.697 (0.271–1.795) | 0.454 | 0.577 (0.241–1.380) | 0.216 | 1.073 (0.334–3.443) | 0.905 |
| Histopathology subtype (ACP vs. PCP) | 0.472 (0.067–3.324) | 0.45 | 1.323 (0.506–3.455) | 0.568 | 1.142 (0.462–2.824) | 0.773 | 1.420 (0.390–5.177) | 0.595 |
| Calcification (yes vs. no) | 1.453 (0.212–9.982) | 0.7 | 0.892 (0.328–2.426) | 0.823 | 1.032 (0.410–2.593) | 0.947 | 0.408 (0.108–1.543) | 0.186 |
| Operation (EETS vs. TC) | 0.379 (0.079–1.971) | 0.25 | 0.376 (0.152–0.930) | 0.034 * | 0.379 (0.165–0.870) | 0.022 * | 0.259 (0.075–0.892) | 0.032 * |
| Preoperative HI (G2 vs. G1&G0) | 0.693 (0.094–5.094) | 0.72 | 1.395 (0.531–3.666) | 0.5 | 1.293 (0.515–3.248) | 0.585 | 2.534 (0.629–10.207) | 0.191 |
| Preoperative BMI (normal weight, reference) | — | — | — | — | — | — | — | — |
| Overweight | 1.684 (0.204–13.938) | 0.63 | 0.510 (0.195–1.334) | 0.17 | 0.559 (0.227–1.381) | 0.208 | 10.323 (3.646–29.227) | <0.001 *** |
| Obesity | 3.312 (0.260–42.278) | 0.36 | 0.029 (0.003–0.289) | 0.003 ** | 0.131 (0.031–0.554) | 0.006 ** | — & | — & |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xiao, Y.; Wu, W.; Jin, L.; Jia, Y.; Cai, K.; Qiao, N.; Cao, L.; Gui, S. Effects of Craniotomy and Endoscopic Endonasal Transsphenoidal Surgery on Bodyweight in Adult-Onset Craniopharyngioma: A Single-Center Retrospective Study. J. Clin. Med. 2023, 12, 1578. https://doi.org/10.3390/jcm12041578
Li Y, Xiao Y, Wu W, Jin L, Jia Y, Cai K, Qiao N, Cao L, Gui S. Effects of Craniotomy and Endoscopic Endonasal Transsphenoidal Surgery on Bodyweight in Adult-Onset Craniopharyngioma: A Single-Center Retrospective Study. Journal of Clinical Medicine. 2023; 12(4):1578. https://doi.org/10.3390/jcm12041578
Chicago/Turabian StyleLi, Yanbin, Youchao Xiao, Wentao Wu, Lu Jin, Yanfei Jia, Kefan Cai, Ning Qiao, Lei Cao, and Songbai Gui. 2023. "Effects of Craniotomy and Endoscopic Endonasal Transsphenoidal Surgery on Bodyweight in Adult-Onset Craniopharyngioma: A Single-Center Retrospective Study" Journal of Clinical Medicine 12, no. 4: 1578. https://doi.org/10.3390/jcm12041578
APA StyleLi, Y., Xiao, Y., Wu, W., Jin, L., Jia, Y., Cai, K., Qiao, N., Cao, L., & Gui, S. (2023). Effects of Craniotomy and Endoscopic Endonasal Transsphenoidal Surgery on Bodyweight in Adult-Onset Craniopharyngioma: A Single-Center Retrospective Study. Journal of Clinical Medicine, 12(4), 1578. https://doi.org/10.3390/jcm12041578







