The Increase of Theta Power and Decrease of Alpha/Theta Ratio as a Manifestation of Cognitive Impairment in Parkinson’s Disease
Abstract
1. Introduction
- The extraction of QEEG coefficients using modern equipment and tools was conducted.
- The results of a relatively large group of patients with Parkinson’s disease who underwent comprehensive neuropsychological assessments were included.
- Data has been collected during routine diagnostics. Thus, the results were collected in real conditions, not only to assemble a group for research.
- Statistical analysis was conducted to identify biomarkers of MCI and dementia.
2. Materials and Methods
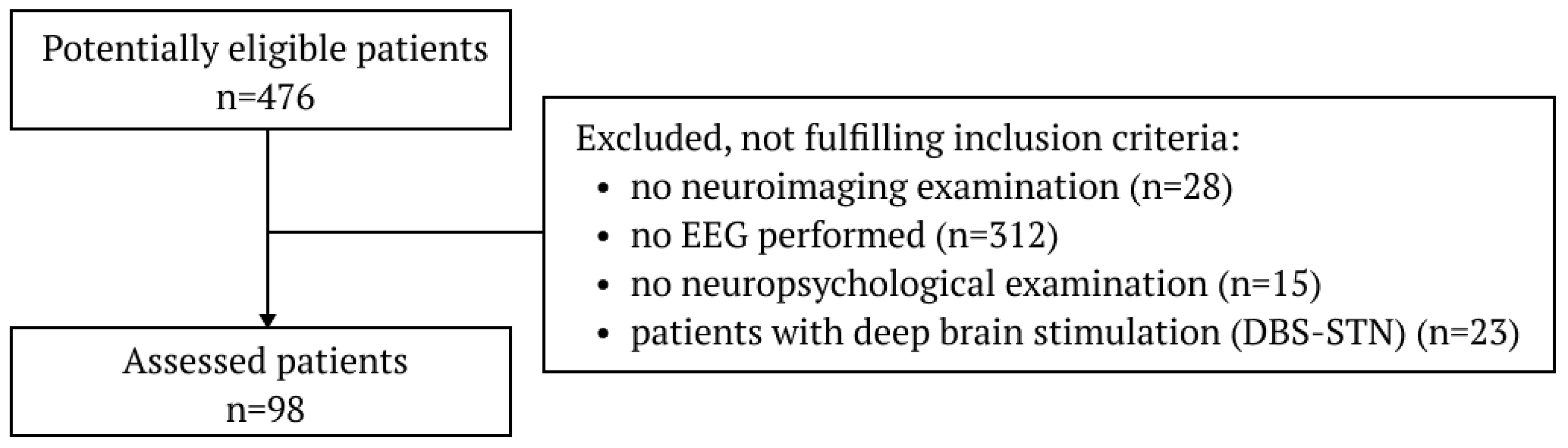
2.1. Subjects
2.2. Clinical and Neuropsychological Assessment
2.3. Eeg Recording
2.4. Processing of Eeg Data
2.5. Statistics
3. Results
3.1. Cohort Clinical Characteristics
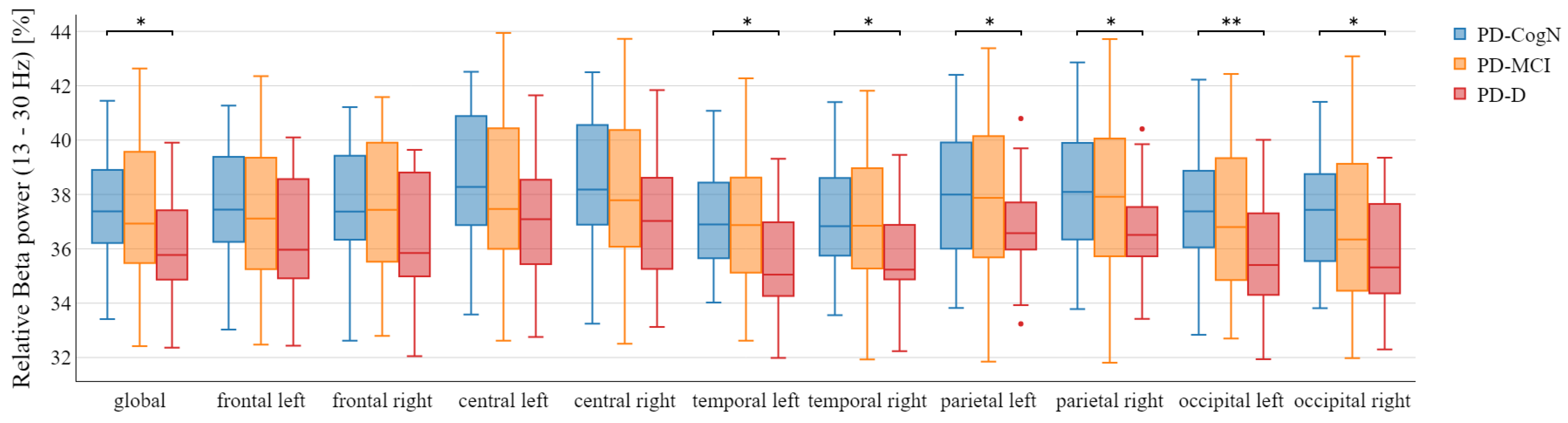
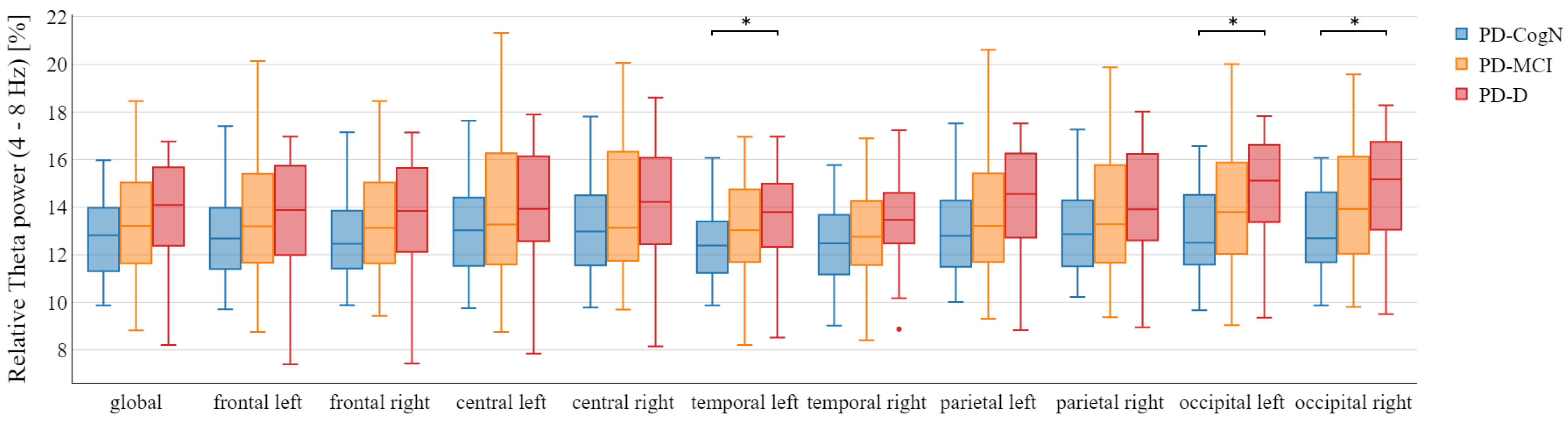
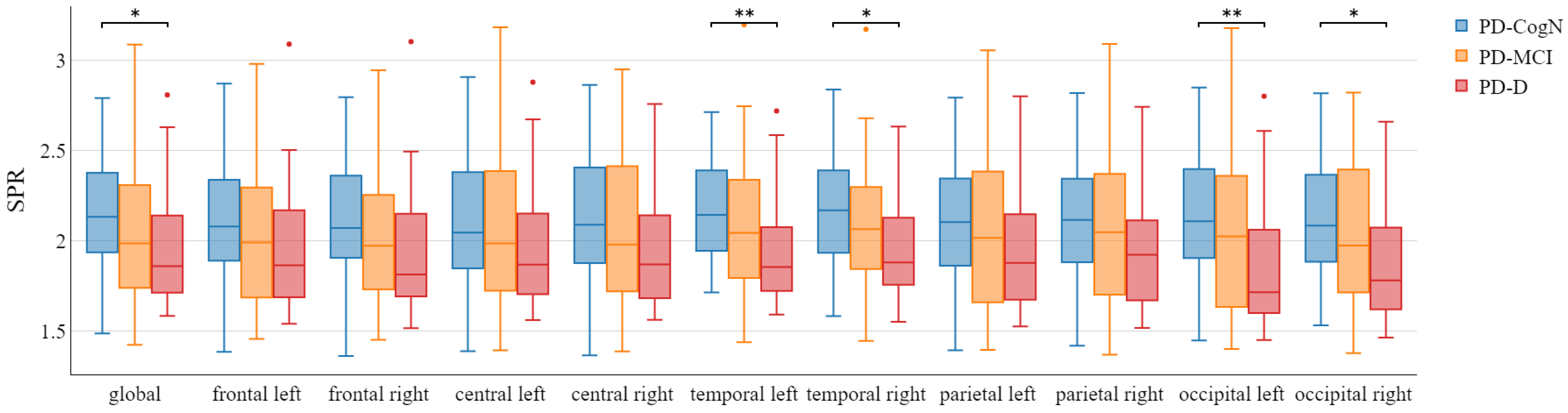
3.2. Qeeg Spectral Power Frequency Analysis
4. Discussion
5. Conclusions
- 1.
- Power spectral analysis of the PD-N, PD-MCI, and PD-D EEG recordings displays significant differences between the three groups.
- 2.
- The cognitive impairments in PD patients can be detected in the QEEG recordings through the following characteristics:
- (a)
- The slowing of background activity
- (b)
- The increase in relative theta power
- (c)
- The decrease in relative beta power
- (d)
- The decrease in the global alpha/theta ratio and global power spectral ratio
- (e)
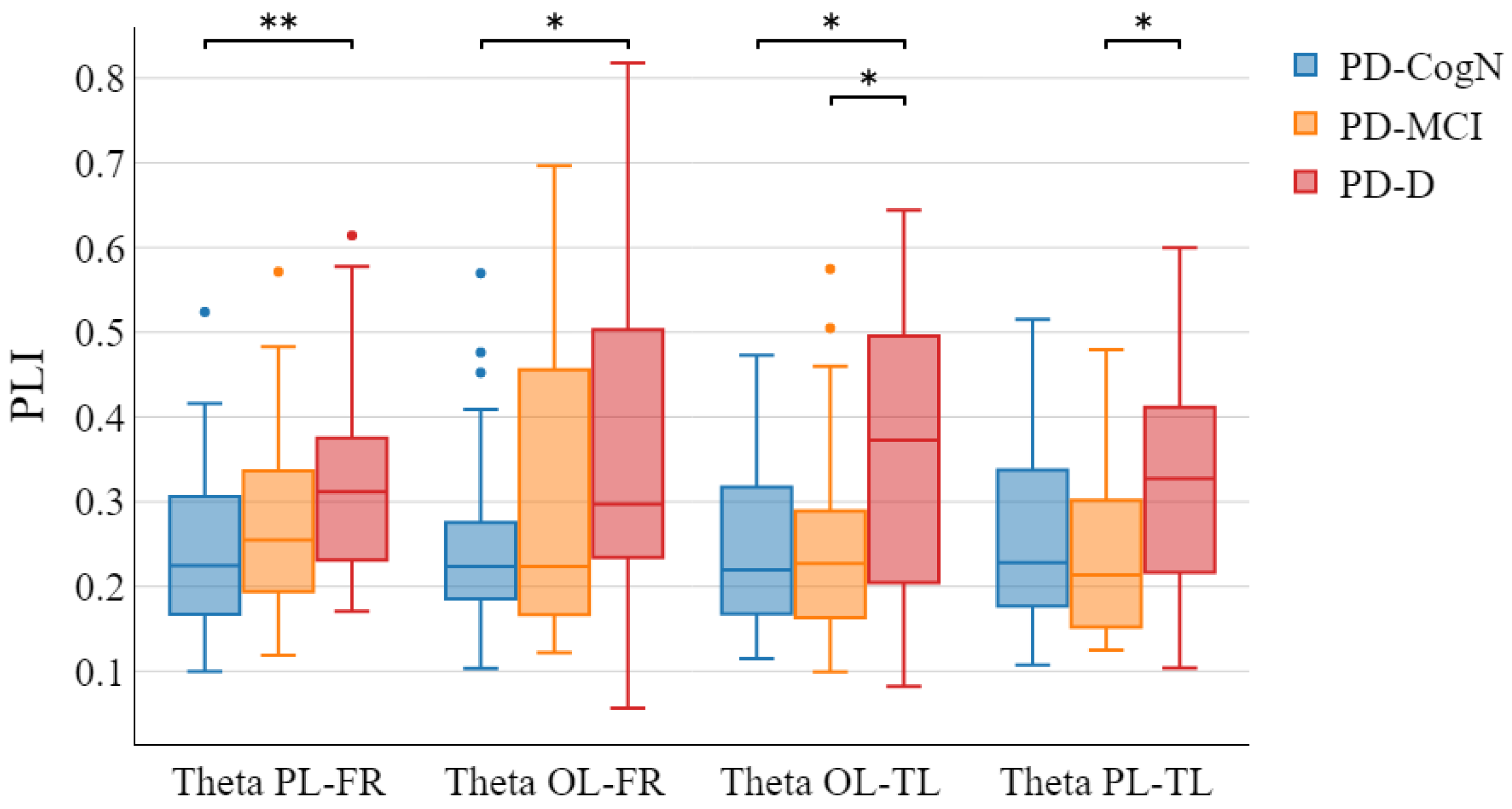
- The increase in theta PLI in specific connections between the occipital left, temporal left, frontal right, and parietal left regions
- 3.
- QEEG analysis could become a useful and complementary diagnostic tool for the neuropsychological diagnosis of cognitive impairment in patients with Parkinson’s disease, but this still requires further research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| MDS | International Parkinson and Movement Disorder Society |
| PD-CogN | Normal cognition |
| PD-MCI | Mild cognitive impairment |
| PD-D | Dementia |
| EEG | Electroencephalogram |
| QEEG | Quantitative EEG |
| H&Y | Hoehn and Yahr Scale |
| UPDRS | Unified Parkinson Disease Rating Scale |
| LED | Levodopa Equivalent Dose |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| CDT | Clock drawing test |
| ACE III | Addenbrooke’s Cognitive Examination III |
| BVRT | Benton Visual Retention Test |
| BDI | Beck Depression Inventory |
| PSD | Power spectral density |
| SPR | Spectral Power Ratio |
References
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim. 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15, 14–20. [Google Scholar] [CrossRef]
- Winter, Y.; von Campenhausen, S.; Arend, M.; Longo, K.; Boetzel, K.; Eggert, K.; Oertel, W.H.; Dodel, R.; Barone, P. Health-related quality of life and its determinants in Parkinson’s disease: Results of an Italian cohort study. Park. Relat. Disord. 2011, 17, 265–269. [Google Scholar] [CrossRef]
- Fan, Y.; Liang, X.; Han, L.; Shen, Y.; Shen, B.; Chen, C.; Sun, Y.; Wang, J.; Tang, Y. Determinants of quality of life according to cognitive status in Parkinson’s disease. Front. Aging Neurosci. 2020, 12, 269. [Google Scholar] [CrossRef]
- Lawson, R.; Yarnall, A.; Johnston, F.; Duncan, G.; Khoo, T.; Collerton, D.; Taylor, J.; Burn, D.; ICICLE-PD Study Group. Cognitive impairment in Parkinson’s disease: Impact on quality of life of carers. Int. J. Geriatr. Psychiatry 2017, 32, 1362–1370. [Google Scholar] [CrossRef]
- Aarsland, D.; Larsen, J.P.; Tandberg, E.; Laake, K. Predictors of nursing home placement in Parkinson’s disease: A population-based, prospective study. J. Am. Geriatr. Soc. 2000, 48, 938–942. [Google Scholar] [CrossRef]
- Fletcher, P.; Leake, A.; Marion, M.H. Patients with Parkinson’s disease dementia stay in the hospital twice as long as those without dementia. Mov. Disord. 2011, 26, 919. [Google Scholar] [CrossRef]
- Gonzalez-Latapi, P.; Bayram, E.; Litvan, I.; Marras, C. Cognitive impairment in Parkinson’s disease: Epidemiology, clinical profile, protective and risk factors. Behav. Sci. 2021, 11, 74. [Google Scholar] [CrossRef]
- Litvan, I.; Aarsland, D.; Adler, C.H.; Goldman, J.G.; Kulisevsky, J.; Mollenhauer, B.; Rodriguez-Oroz, M.C.; Tröster, A.I.; Weintraub, D. MDS Task Force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov. Disord. 2011, 26, 1814–1824. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.S.; Leverenz, J.B. Profile of cognitive impairment in Parkinson’s disease. Brain. Pathol. 2010, 20, 640–645. [Google Scholar] [CrossRef]
- Hoogland, J.; Boel, J.A.; de Bie, R.M.; Geskus, R.B.; Schmand, B.A.; Dalrymple-Alford, J.C.; Marras, C.; Adler, C.H.; Goldman, J.G.; Tröster, A.I.; et al. Mild cognitive impairment as a risk factor for Parkinson’s disease dementia. Mov. Disord. 2017, 32, 1056–1065. [Google Scholar] [CrossRef]
- Weintraub, D.; Tröster, A.I.; Marras, C.; Stebbins, G. Initial cognitive changes in Parkinson’s disease. Mov. Disord. 2018, 33, 511–519. [Google Scholar] [CrossRef]
- Lin, C.H.; Wu, R.M. Biomarkers of cognitive decline in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Westman, E.; Ballard, C.; Aarsland, D. Cognitive impairment in patients with Parkinson’s disease: Diagnosis, biomarkers, and treatment. Lancet Neurol. 2012, 11, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Alvarado, M.; Gago, B.; Navalpotro-Gomez, I.; Jiménez-Urbieta, H.; Rodriguez-Oroz, M.C. Biomarkers for dementia and mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2016, 31, 861–881. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol 2017, 13, 217–231. [Google Scholar] [CrossRef]
- Nuwer, M.R. Quantitative EEG analysis in clinical settings. Brain Topogr. 1996, 8, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.L.; Dragos, H.; Pantelemon, C.; Rosu, O.V.; Strilciuc, S. The role of quantitative EEG in the diagnosis of neuropsychiatric disorders. J. Med. Life 2020, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.Q.; Gupta, N.K. Automated diagnosis of coronary heart disease using neuro-fuzzy integrated system. In Proceedings of the 2011 World Congress on Information and Communication Technologies, IEEE, Mumbai, India, 11–14 December 2011; pp. 1379–1384. [Google Scholar] [CrossRef]
- Ahsan, M.M.; Mahmud, M.P.; Saha, P.K.; Gupta, K.D.; Siddique, Z. Effect of data scaling methods on machine learning algorithms and model performance. Technologies 2021, 9, 52. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. HealthSOS: Real-time health monitoring system for stroke prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
- Beniczky, S.; Karoly, P.; Nurse, E.; Ryvlin, P.; Cook, M. Machine learning and wearable devices of the future. Epilepsia 2021, 62, S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Park, S.J. Quantitative evaluation of task-induced neurological outcome after stroke. Brain Sci. 2021, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Young, S.; Park, S.J. Driving-induced neurological biomarkers in an advanced driver-assistance system. Sensors 2021, 21, 6985. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Hossain, M.A.; Jany, R.; Bari, M.A.; Uddin, M.; Kamal, A.R.M.; Ku, Y.; Kim, J.S. Quantitative evaluation of EEG-biomarkers for prediction of sleep stages. Sensors 2022, 22, 3079. [Google Scholar] [CrossRef]
- Caviness, J.; Hentz, J.; Evidente, V.; Driver-Dunckley, E.; Samanta, J.; Mahant, P.; Connor, D.; Sabbagh, M.; Shill, H.; Adler, C. Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson’s disease. Park. Relat. Disord. 2007, 13, 348–354. [Google Scholar] [CrossRef]
- Fonseca, L.C.; Tedrus, G.M.; Carvas, P.N.; Machado, E.C. Comparison of quantitative EEG between patients with Alzheimer’s disease and those with Parkinson’s disease dementia. Clin. Neurophysiol. 2013, 124, 1970–1974. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, J.; Lu, Y.; Pan, S. Integrative frequency power of EEG correlates with progression of mild cognitive impairment to dementia in Parkinson’s disease. Clin. EEG Neurosci. 2016, 47, 113–117. [Google Scholar] [CrossRef]
- Bousleiman, H.; Zimmermann, R.; Ahmed, S.; Hardmeier, M.; Hatz, F.; Schindler, C.; Roth, V.; Gschwandtner, U.; Fuhr, P. Power spectra for screening parkinsonian patients for mild cognitive impairment. Ann. Clin. Transl. Neurol 2014, 1, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef]
- Jas, M.; Engemann, D.A.; Bekhti, Y.; Raimondo, F.; Gramfort, A. Autoreject: Automated artifact rejection for MEG and EEG data. Neuroimage 2017, 159, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Percival, D.B.; Walden, A.T. Spectral Analysis for Physical Applications; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Eichelberger, D.; Calabrese, P.; Meyer, A.; Chaturvedi, M.; Hatz, F.; Fuhr, P.; Gschwandtner, U. Correlation of visuospatial ability and EEG slowing in patients with Parkinson’s disease. Park. Dis. 2017, 2017, 3659784. [Google Scholar] [CrossRef] [PubMed]
- Guner, D.; Tiftikcioglu, B.I.; Tuncay, N.; Zorlu, Y. Contribution of quantitative EEG to the diagnosis of early cognitive impairment in patients with idiopathic Parkinson’s disease. Clin. EEG Neurosci. 2017, 48, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Caviness, J.N.; Hentz, J.G.; Belden, C.M.; Shill, H.A.; Driver-Dunckley, E.D.; Sabbagh, M.N.; Powell, J.J.; Adler, C.H. Longitudinal EEG changes correlate with cognitive measure deterioration in Parkinson’s disease. J. Park. Dis. 2015, 5, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Caviness, J.; Utianski, R.; Hentz, J.; Beach, T.; Dugger, B.; Shill, H.; Driver-Dunckley, E.; Sabbagh, M.; Mehta, S.; Adler, C. Differential spectral quantitative electroencephalography patterns between control and Parkinson’s disease cohorts. Eur. J. Neurol. 2016, 23, 387–392. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, L.; Pang, J.; Zhu, X.; Ming, D. Characterization of EEG data revealing relationships with cognitive and motor symptoms in Parkinson’s disease: A systematic review. Front. Aging Neurosci. 2020, 12, 587396. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.; Chen, J.; Xie, C.; Gan, R.; Wang, L.; Wang, L. Changes in theta activities in the left posterior temporal region, left occipital region and right frontal region related to mild cognitive impairment in Parkinson’s disease patients. Int. J. Neurosci. 2017, 127, 66–72. [Google Scholar] [CrossRef]
- Klassen, B.; Hentz, J.; Shill, H.; Driver-Dunckley, E.; Evidente, V.; Sabbagh, M.; Adler, C.; Caviness, J. Quantitative EEG as a predictive biomarker for Parkinson disease dementia. Neurology 2011, 77, 118–124. [Google Scholar] [CrossRef]
- Jaramillo-Jimenez, A.; Suarez-Revelo, J.X.; Ochoa-Gomez, J.F.; Arroyave, J.A.C.; Bocanegra, Y.; Lopera, F.; Buriticá, O.; Pineda-Salazar, D.A.; Gómez, L.M.; Quintero, C.A.T.; et al. Resting-state EEG alpha/theta ratio related to neuropsychological test performance in Parkinson’s Disease. Clin. Neurophysiol. 2021, 132, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Bousleiman, H.; Chaturvedi, M.; Gschwandtner, U.; Hatz, F.; Schindler, C.; Zimmermann, R.; Fuhr, P. P122. Alpha1/theta ratio from quantitative EEG (qEEG) as a reliable marker for mild cognitive impairment (MCI) in patients with Parkinson’s disease (PD). Clin. Neurophysiol. 2015, 126, e150–e151. [Google Scholar] [CrossRef]
- Morita, A.; Kamei, S.; Mizutani, T. Relationship between slowing of the EEG and cognitive impairment in Parkinson disease. J. Clin. Neurophysiol. 2011, 28, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Dubbelink, K.T.O.; Stoffers, D.; Deijen, J.B.; Twisk, J.W.; Stam, C.J.; Berendse, H.W. Cognitive decline in Parkinson’s disease is associated with slowing of resting-state brain activity: A longitudinal study. Neurobiol. Aging 2013, 34, 408–418. [Google Scholar] [CrossRef]
- Benz, N.; Hatz, F.; Bousleiman, H.; Ehrensperger, M.M.; Gschwandtner, U.; Hardmeier, M.; Ruegg, S.; Schindler, C.; Zimmermann, R.; Monsch, A.U.; et al. Slowing of EEG background activity in Parkinson’s and Alzheimer’s disease with early cognitive dysfunction. Front. Aging Neurosci. 2014, 6, 314. [Google Scholar] [CrossRef]
- Cozac, V.V.; Gschwandtner, U.; Hatz, F.; Hardmeier, M.; Rüegg, S.; Fuhr, P. Quantitative EEG and cognitive decline in Parkinson’s disease. Park. Dis. 2016, 2016, 9060649. [Google Scholar] [CrossRef]
- Geraedts, V.J.; Boon, L.I.; Marinus, J.; Gouw, A.A.; van Hilten, J.J.; Stam, C.J.; Tannemaat, M.R.; Contarino, M.F. Clinical correlates of quantitative EEG in Parkinson disease: A systematic review. Neurology 2018, 91, 871–883. [Google Scholar] [CrossRef]
- Babiloni, C.; De Pandis, M.F.; Vecchio, F.; Buffo, P.; Sorpresi, F.; Frisoni, G.B.; Rossini, P.M. Cortical sources of resting state electroencephalographic rhythms in Parkinson’s disease related dementia and Alzheimer’s disease. Clin. Neurophysiol. 2011, 122, 2355–2364. [Google Scholar] [CrossRef]
- Bočková, M.; Rektor, I. Impairment of brain functions in Parkinson’s disease reflected by alterations in neural connectivity in EEG studies: A viewpoint. Clin. Neurophysiol. 2019, 130, 239–247. [Google Scholar] [CrossRef]
- Steriade, M.; Gloor, P.; Llinas, R.R.; Da Silva, F.L.; Mesulam, M.M. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 481–508. [Google Scholar] [CrossRef]
- Massa, F.; Meli, R.; Grazzini, M.; Famà, F.; De Carli, F.; Filippi, L.; Arnaldi, D.; Pardini, M.; Morbelli, S.; Nobili, F. Utility of quantitative EEG in early Lewy body disease. Park. Relat. Disord. 2020, 75, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Emre, M.; Aarsland, D.; Albanese, A.; Byrne, E.J.; Deuschl, G.; De Deyn, P.P.; Durif, F.; Kulisevsky, J.; Van Laar, T.; Lees, A.; et al. Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med. 2004, 351, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010, 9, 1200–1213. [Google Scholar] [CrossRef] [PubMed]
- Gratwicke, J.; Jahanshahi, M.; Foltynie, T. Parkinson’s disease dementia: A neural networks perspective. Brain 2015, 138, 1454–1476. [Google Scholar] [CrossRef]
- Pereira, J.B.; Junqué, C.; Martí, M.J.; Ramirez-Ruiz, B.; Bargalló, N.; Tolosa, E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson’s disease. Mov. Disord. 2009, 24, 1193–1199. [Google Scholar] [CrossRef]
- Baggio, H.C.; Segura, B.; Sala-Llonch, R.; Marti, M.J.; Valldeoriola, F.; Compta, Y.; Tolosa, E.; Junque, C. Cognitive impairment and resting-state network connectivity in Parkinson’s disease. Hum. Brain Mapp. 2015, 36, 199–212. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Salat, D.H.; Bates, J.F.; Atiya, M.; Killiany, R.J.; Greve, D.N.; Dale, A.M.; Stern, C.E.; Blacker, D.; Albert, M.S.; et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 2004, 56, 27–35. [Google Scholar] [CrossRef]
- Hammond, C.; Bergman, H.; Brown, P. Pathological synchronization in Parkinson’s disease: Networks, models and treatments. Trends Neurosci. 2007, 30, 357–364. [Google Scholar] [CrossRef]


| PD-CogN | PD-MCI | PD-D | p | |
|---|---|---|---|---|
| N | 43 | 30 | 25 | - |
| Age (years) | 61.79 (12.49) | 68.27(6.20) | 71.36 (5.34) | |
| PD duration (years) | 8.65 (6.80) | 8.40 (6.17) | 13.88 (4.55) | |
| MDS-UPDRS III OFF | 41.05 (14.82) | 44.00 (18.85) | 58.46 (15.65) | |
| Hoehn-Yahr stage | 2.72 (0.80) | 2.87 (0.86) | 3.44 (0.87) | |
| LED (mg) | 808.14(732.16) | 845.07(532.14) | 1291.88(685.37) |
| PD-CogN | PD-MCI | PD-D | p | |
|---|---|---|---|---|
| Delta (dB) | 39.78 (2.73) | 40.60 (3.52) | 42.07(4.17) | 0.0855 |
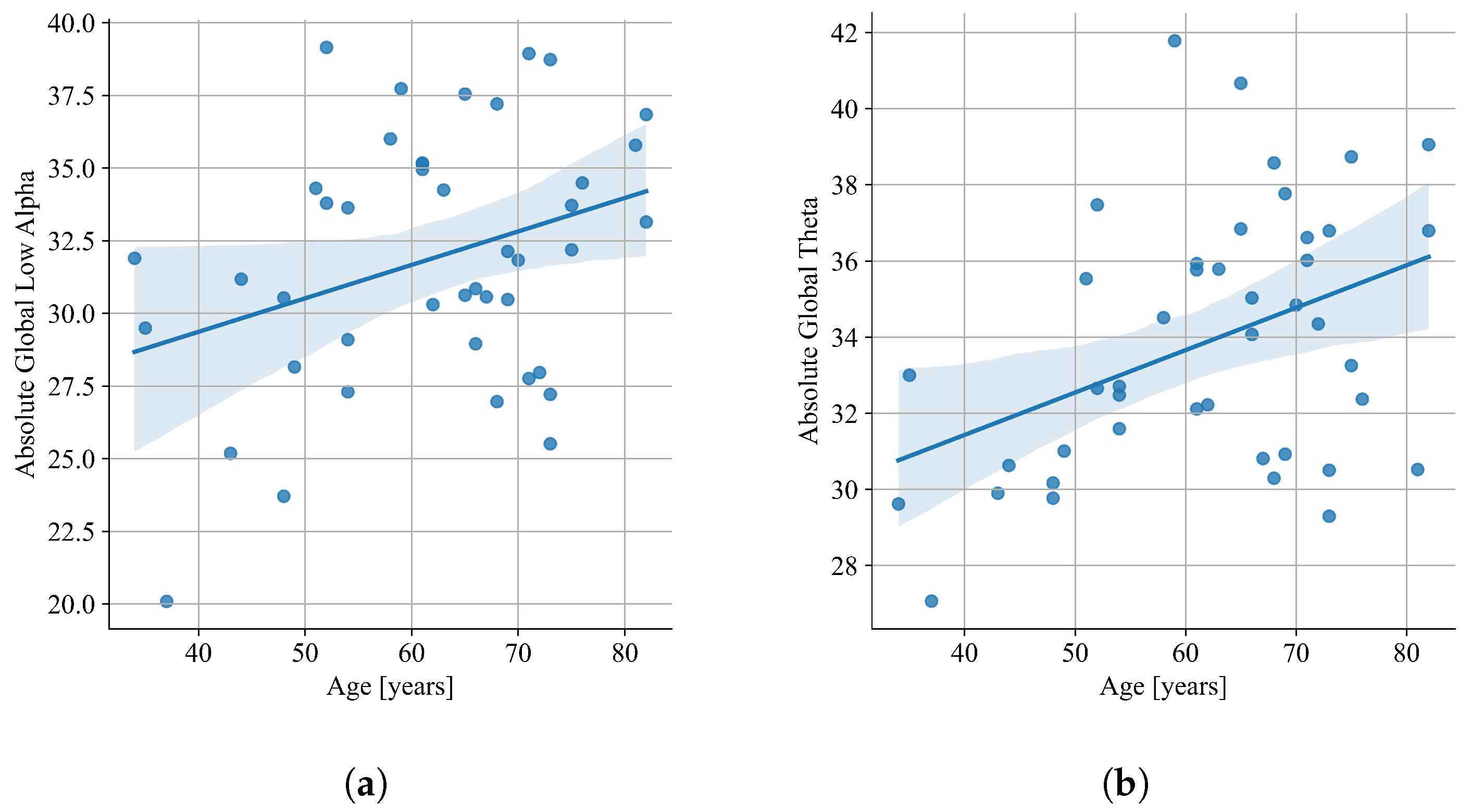
| Theta (dB) | 33.86 (3.41) | 35.62 (3.93) | 37.03 (4.03) | 0.0036 |
| Alpha (dB) | 34.51 (4.00) | 34.86 (3.30) | 34.12 (3.14) | 0.7501 |
| Low Alpha (dB) | 31.87 (4.36) | 32.30 (3.53) | 31.76 (3.43) | 0.8539 |
| High Alpha (dB) | 30.58 (3.88) | 30.77 (3.64) | 30.19 (2.99) | 0.8367 |
| Beta (dB) | 32.67 (3.08) | 32.77 (3.44) | 32.63 (3.32) | 0.8662 |
| Gamma (dB) | 25.80 (2.99) | 25.66 (3.91) | 26.86 (4.86) | 0.8574 |
| PD-CogN | PD-MCI | PD-D | p | |
|---|---|---|---|---|
| Delta (%) | 12.38 (1.64) | 12.69 (2.18) | 12.96 (1.72) | 0.4531 |
| Theta (%) | 12.62 (1.64) | 13.36 (2.32) | 13.76 (2.14) | 0.0656 |
| Alpha (%) | 15.16 (1.43) | 15.22 (1.30) | 14.80 (1.55) | 0.5107 |
| Low Alpha (%) | 6.51 (0.81) | 6.57 (0.77) | 6.39 (0.78) | 0.7040 |
| High Alpha (%) | 8.65 (0.78) | 8.65 (0.74) | 8.41 (0.80) | 0.4210 |
| Beta (%) | 37.69 (2.06) | 37.32 (2.61) | 36.27 (1.93) | 0.0413 |
| Gamma (%) | 22.15 (3.00) | 21.41 (3.65) | 22.22 (4.1) | 0.6117 |
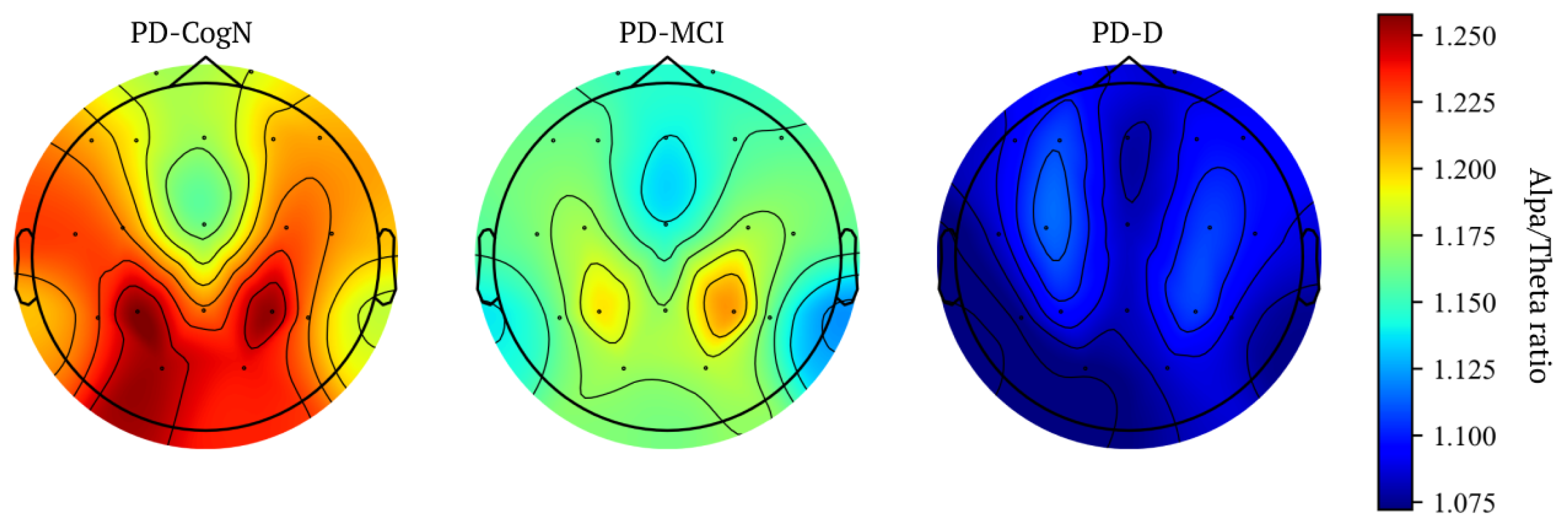
| alpha/theta ratio | 1.21 (0.13) | 1.17 (0.18) | 1.09 (0.13) | 0.0040 |
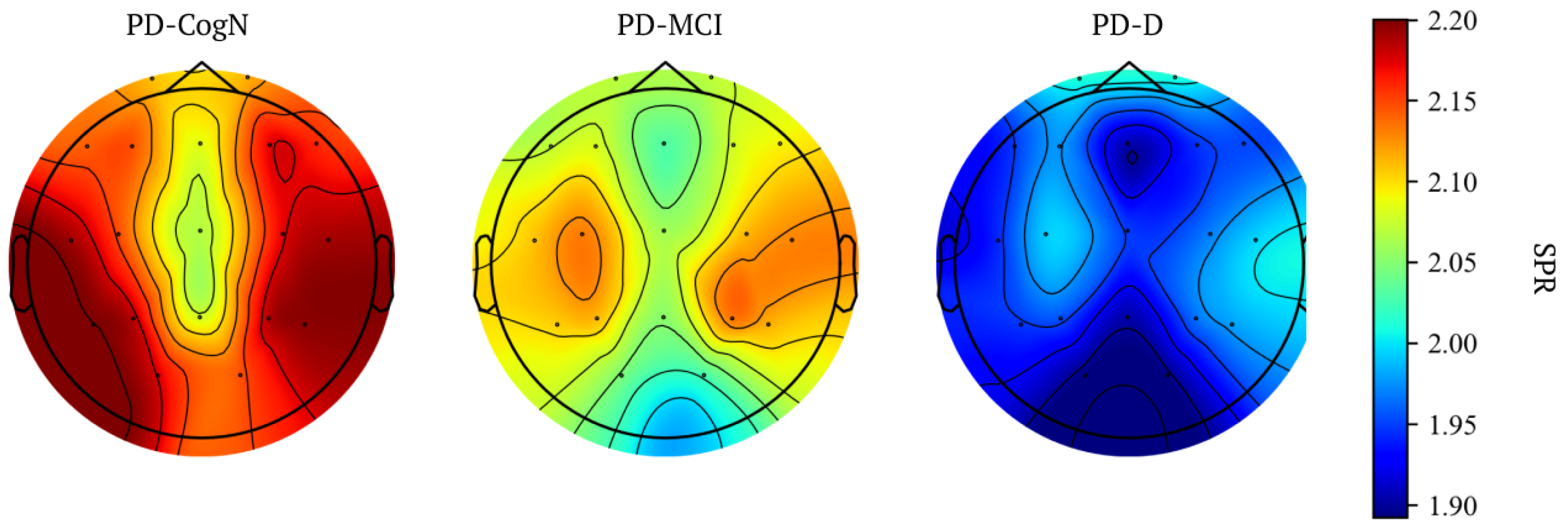
| SPR | 2.15 (0.31) | 2.09 (0.43) | 1.96 (0.34) | 0.0385 |
| Advantages | Drawbacks | |
|---|---|---|
| Proposed work |
|
|
| Cozac et al., 2016 [48] |
|
|
| Caviness et al., 2007 [29] |
|
|
| Caviness et al., 2015 [38] |
|
|
| Moritta et al., 2011 [45] |
|
|
| Bousleimann et al., 2014 [32] |
|
|
| Gu et al., 2016 [31] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawiślak-Fornagiel, K.; Ledwoń, D.; Bugdol, M.; Romaniszyn-Kania, P.; Małecki, A.; Gorzkowska, A.; Mitas, A.W. The Increase of Theta Power and Decrease of Alpha/Theta Ratio as a Manifestation of Cognitive Impairment in Parkinson’s Disease. J. Clin. Med. 2023, 12, 1569. https://doi.org/10.3390/jcm12041569
Zawiślak-Fornagiel K, Ledwoń D, Bugdol M, Romaniszyn-Kania P, Małecki A, Gorzkowska A, Mitas AW. The Increase of Theta Power and Decrease of Alpha/Theta Ratio as a Manifestation of Cognitive Impairment in Parkinson’s Disease. Journal of Clinical Medicine. 2023; 12(4):1569. https://doi.org/10.3390/jcm12041569
Chicago/Turabian StyleZawiślak-Fornagiel, Katarzyna, Daniel Ledwoń, Monika Bugdol, Patrycja Romaniszyn-Kania, Andrzej Małecki, Agnieszka Gorzkowska, and Andrzej W. Mitas. 2023. "The Increase of Theta Power and Decrease of Alpha/Theta Ratio as a Manifestation of Cognitive Impairment in Parkinson’s Disease" Journal of Clinical Medicine 12, no. 4: 1569. https://doi.org/10.3390/jcm12041569
APA StyleZawiślak-Fornagiel, K., Ledwoń, D., Bugdol, M., Romaniszyn-Kania, P., Małecki, A., Gorzkowska, A., & Mitas, A. W. (2023). The Increase of Theta Power and Decrease of Alpha/Theta Ratio as a Manifestation of Cognitive Impairment in Parkinson’s Disease. Journal of Clinical Medicine, 12(4), 1569. https://doi.org/10.3390/jcm12041569





