Disparate Clinical Characteristics and Prognosis of HFpEF versus HFrEF Phenotype of Diabetic Cardiomyopathy
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Selection
2.2. Outcome Measures
2.3. Clinical and Laboratory Variables
2.4. Cardiac Structure and Function Assessment by Echocardiography
2.5. Statistical Analyses
2.6. Trial Visits
2.7. Patient and Public Involvement Statement
3. Results
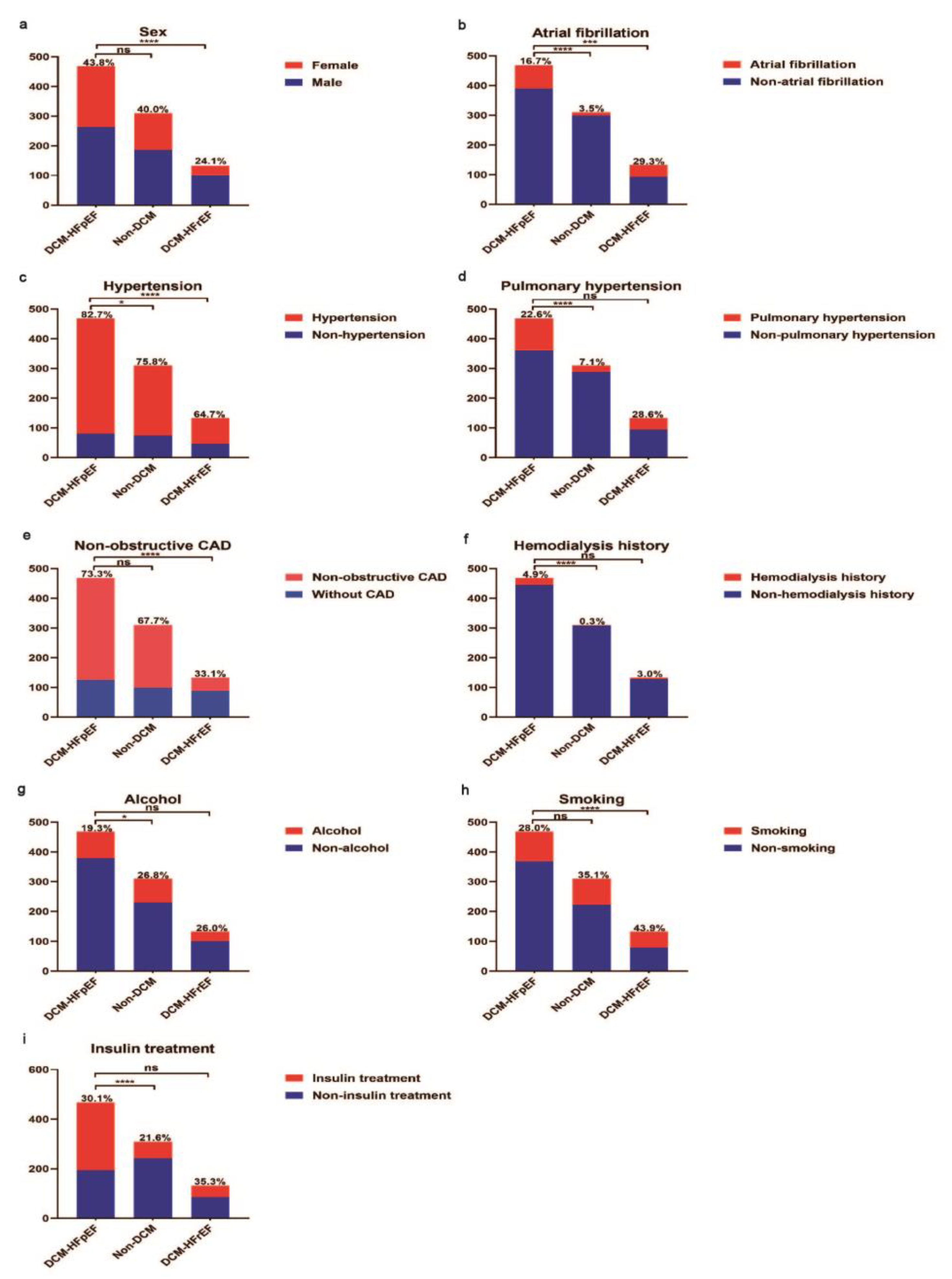
3.1. Comparison of Baseline Characteristics between Non-DCM versus DCM-HFpEF Patients
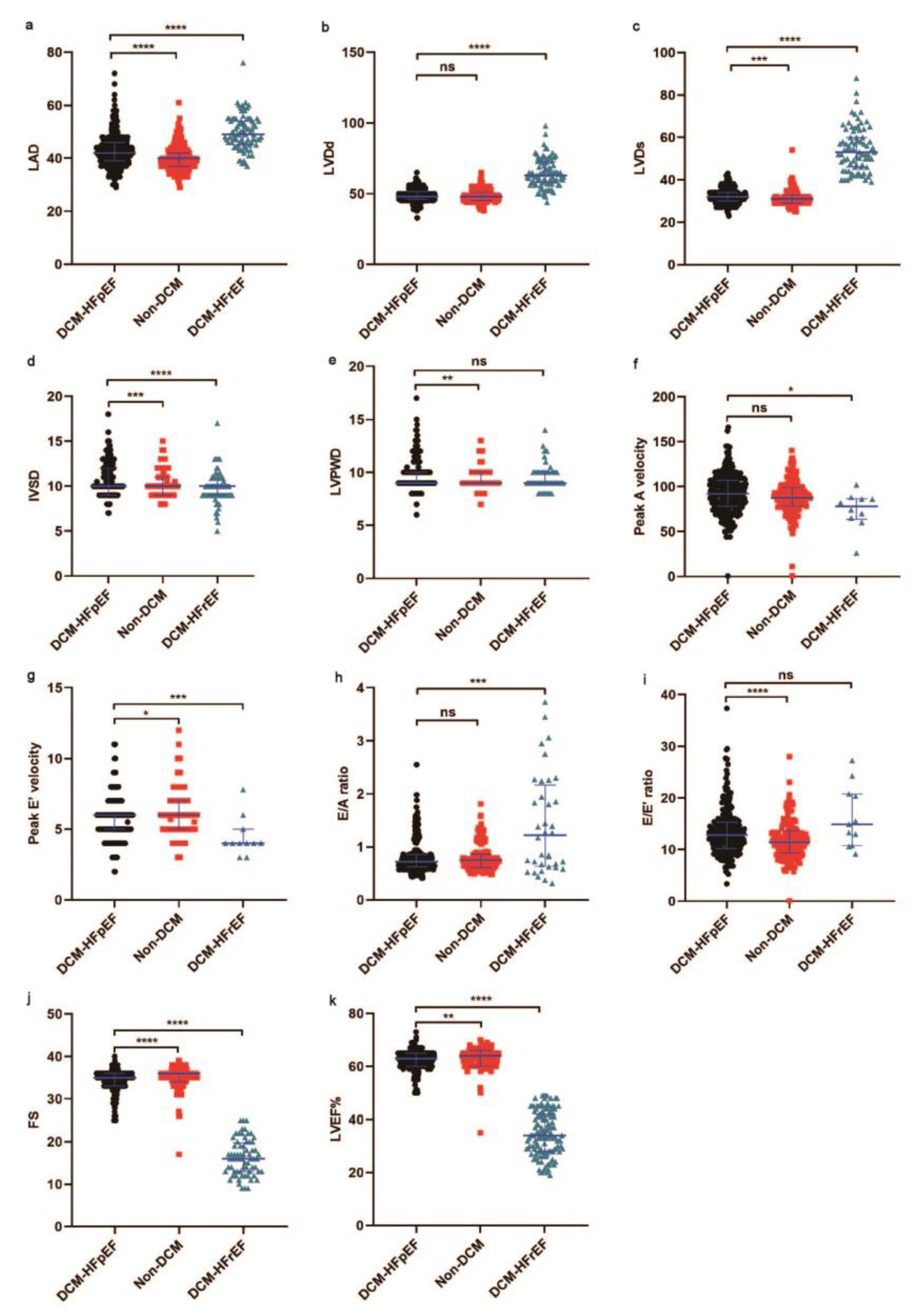
3.2. Comparison of Basic Characteristics between DCM-HFpEF and DCM-HFrEF Patients
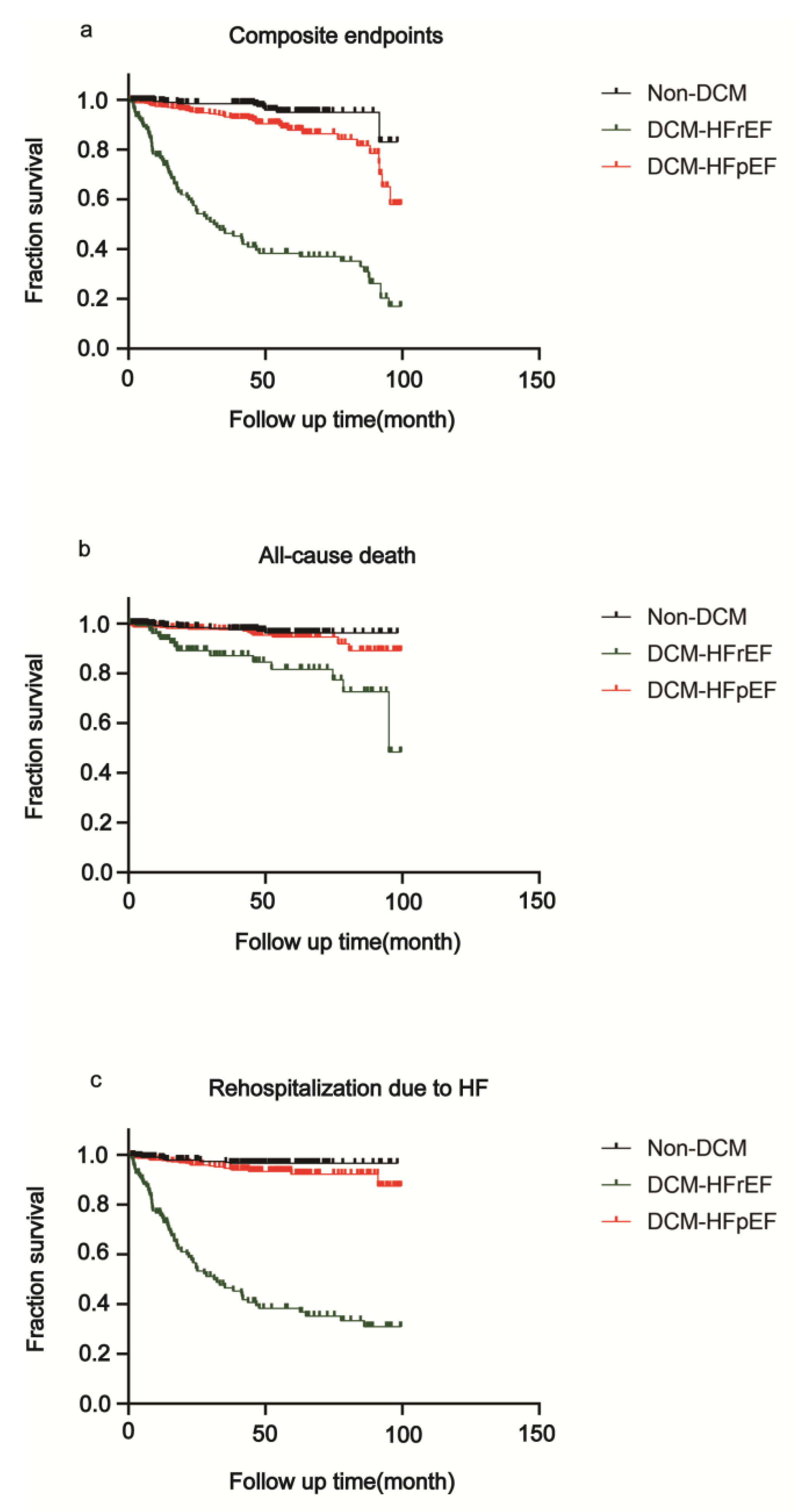
3.3. Survival Analysis of DCM-HFrEF, DCM-HFpEF and Non-DCM Patients
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Klajda, M.D.; Scott, C.G.; Rodeheffer, R.J.; Chen, H.H. Diabetes Mellitus Is an Independent Predictor for the Development of Heart Failure: A Population Study. Mayo Clin. Proc. 2020, 95, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Dandamudi, S.; Slusser, J.; Mahoney, D.W.; Redfield, M.M.; Rodeheffer, R.J.; Chen, H.H. The Prevalence of Diabetic Cardiomyopathy: A Population-Based Study in Olmsted County, Minnesota. J. Card. Fail. 2014, 20, 304–309. [Google Scholar] [CrossRef] [PubMed]
- El Hayek, M.S.; Ernande, L.; Benitah, J.-P.; Gomez, A.-M.; Pereira, L. The role of hyperglycaemia in the development of diabetic cardiomyopathy. Arch. Cardiovasc. Dis. 2021, 114, 748–760. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Reynolds, K.; Kimes, T.M.; Rosales, A.G.; Chan, W.W. Comparison of Risk of Re-hospitalization, All-Cause Mortality, and Medical Care Resource Utilization in Patients With Heart Failure and Preserved Versus Reduced Ejection Fraction. Am. J. Cardiol. 2015, 116, 1088–1092. [Google Scholar] [CrossRef]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure with Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef]
- de Simone, G.; Devereux, R.B.; Chinali, M.; Lee, E.T.; Galloway, J.M.; Barac, A.; A Panza, J.; Howard, B.V. Diabetes and incident heart failure in hypertensive and normotensive participants of the Strong Heart Study. J. Hypertens. 2010, 28, 353–360. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Xia, C.L.; Chu, P.; Liu, Y.X.; Qu, X.L.; Gao, X.F.; Wang, Z.M.; Dong, J.; Chen, S.L.; Zhang, J.X. ALDH2 rs671 polymorphism and the risk of heart failure with preserved ejection fraction (HFpEF) in patients with cardiovascular diseases. J. Hum. Hypertens. 2020, 34, 16–23. [Google Scholar] [CrossRef]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B.; Schrauben, S.J.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Yarde, M.; Wang, Z.; Bhattacharya, P.T.; Chirinos, D.A.; et al. Clinical Phenogroups in Heart Failure with Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail. 2020, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Nair, N. Epidemiology and pathogenesis of heart failure with preserved ejection fraction. Rev. Cardiovasc. Med. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Drazner, M.H. Insights from the History and Physical Examination in HFpEF or HFrEF: Similarities and Differences. JACC Heart Fail. 2021, 9, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miyoshi, T.; Yoshida, M.; Akagi, S.; Saito, Y.; Ejiri, K.; Matsuo, N.; Ichikawa, K.; Iwasaki, K.; Naito, T.; et al. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 3587. [Google Scholar] [CrossRef]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
- House, A.A.; Wanner, C.; Sarnak, M.J.; Piña, I.L.; McIntyre, C.W.; Komenda, P.; Kasiske, B.L.; Deswal, A.; Defilippi, C.R.; Cleland, J.G.F.; et al. Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 1304–1317. [Google Scholar] [CrossRef]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Li, H.; Xia, Y.-Y.; Xia, C.-L.; Qu, X.-L.; Chu, P.; Zhou, W.-Y.; Zhu, L.-L.; Li, L.; Chen, S.-L.; et al. Left atrial diameter and atrial fibrillation, but not elevated NT-proBNP, predict the development of pulmonary hypertension in patients with HFpEF. J. Geriatr. Cardiol. 2020, 17, 400–409. [Google Scholar]
- Dunlay, S.M.; Redfield, M.M.; Weston, S.A.; Therneau, T.M.; Long, K.H.; Shah, N.D.; Roger, V.L. Hospitalizations After Heart Failure Diagnosis: A Community Perspective. J. Am. Coll. Cardiol. 2009, 54, 1695–1702. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; McClelland, R.L.; Marshall, R.; Shemanski, L.; Furberg, C.D.; Kitzman, D.W.; Cushman, M.; Polak, J.; Gardin, J.M.; Gersh, B.J.; et al. Outcome of Congestive Heart Failure in Elderly Persons: Influence of Left Ventricular Systolic Function: The Cardiovascular Health Study. Ann. Intern. Med. 2002, 137, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; A Masoudi, F.; Vaccarino, V.; Radford, M.J.; Krumholz, H.M. Outcomes in heart failure patients with preserved ejection fraction: Mortality, readmission, and functional decline. J. Am. Coll. Cardiol. 2003, 41, 1510–1518. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. New Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Peterson, L.; Gropler, R.J. Metabolic and Molecular Imaging of the Diabetic Cardiomyopathy. Circ. Res. 2020, 126, 1628–1645. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; Bernardo, B.C.; McMullen, J.R.; Ritchie, R.H. Diabetic cardiomyopathy: Mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol. Ther. 2014, 142, 375–415. [Google Scholar] [CrossRef]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef]
- Xia, Y.-Y.; Shi, Y.; Li, Z.; Li, H.; Wu, L.-D.; Zhou, W.-Y.; Gu, Y.; Ling, Z.-Y.; Zhang, J.-X.; Chen, S.-L. Involvement of pyroptosis pathway in epicardial adipose tissue—Myocardium axis in experimental heart failure with preserved ejection fraction. Biochem. Biophys. Res. Commun. 2022, 636, 62–70. [Google Scholar] [CrossRef]
- Nichols, K.; Yiannikouris, F.J.C. The Role of (Pro)Renin Receptor in The Metabolic Syndrome. Curr. Hypertens. Rev. 2022, 18, 117–124. [Google Scholar]
- Fantin, F.; Giani, A.; Zoico, E.; Rossi, A.P.; Mazzali, G.; Zamboni, M. Weight Loss and Hypertension in Obese Subjects. Nutrients 2019, 11, 1667. [Google Scholar] [CrossRef]
- Osborn, J.L.; Plato, C.F.; Gordin, E.; He, X.R. Long-Term Increases in Renal Sympathetic Nerve Activity and Hypertension. Clin. Exp. Pharmacol. Physiol. 1997, 24, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Sowers, J.R. Hypertension in Diabetes: An Update of Basic Mechanisms and Clinical Disease. Hypertension 2021, 78, 1197–1205. [Google Scholar] [CrossRef]
- Winternitz, S.R.; Oparil, S. Importance of the renal nerves in the pathogenesis of experimental hypertension. Hypertension 1982, 4, III108–14. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Townsend, R.R.; Kandzari, D.E.; Kario, K.; Schmieder, R.E.; Tsioufis, K.; Pocock, S.; David, S.; Patel, K.; Rao, A.; et al. Changes in Plasma Renin Activity After Renal Artery Sympathetic Denervation. J. Am. Coll. Cardiol. 2021, 77, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, B.; Li, M.; Yu, Y.; Wang, Z.; Chen, S. Improvement of cardiac dysfunction by bilateral surgical renal denervation in animals with diabetes induced by high fructose and high fat diet. Diabetes Res. Clin. Pract. 2016, 115, 140–149. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- I McFarlane, S.; Kumar, A.; Sowers, J.R. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am. J. Cardiol. 2003, 91, 30–37. [Google Scholar] [CrossRef]
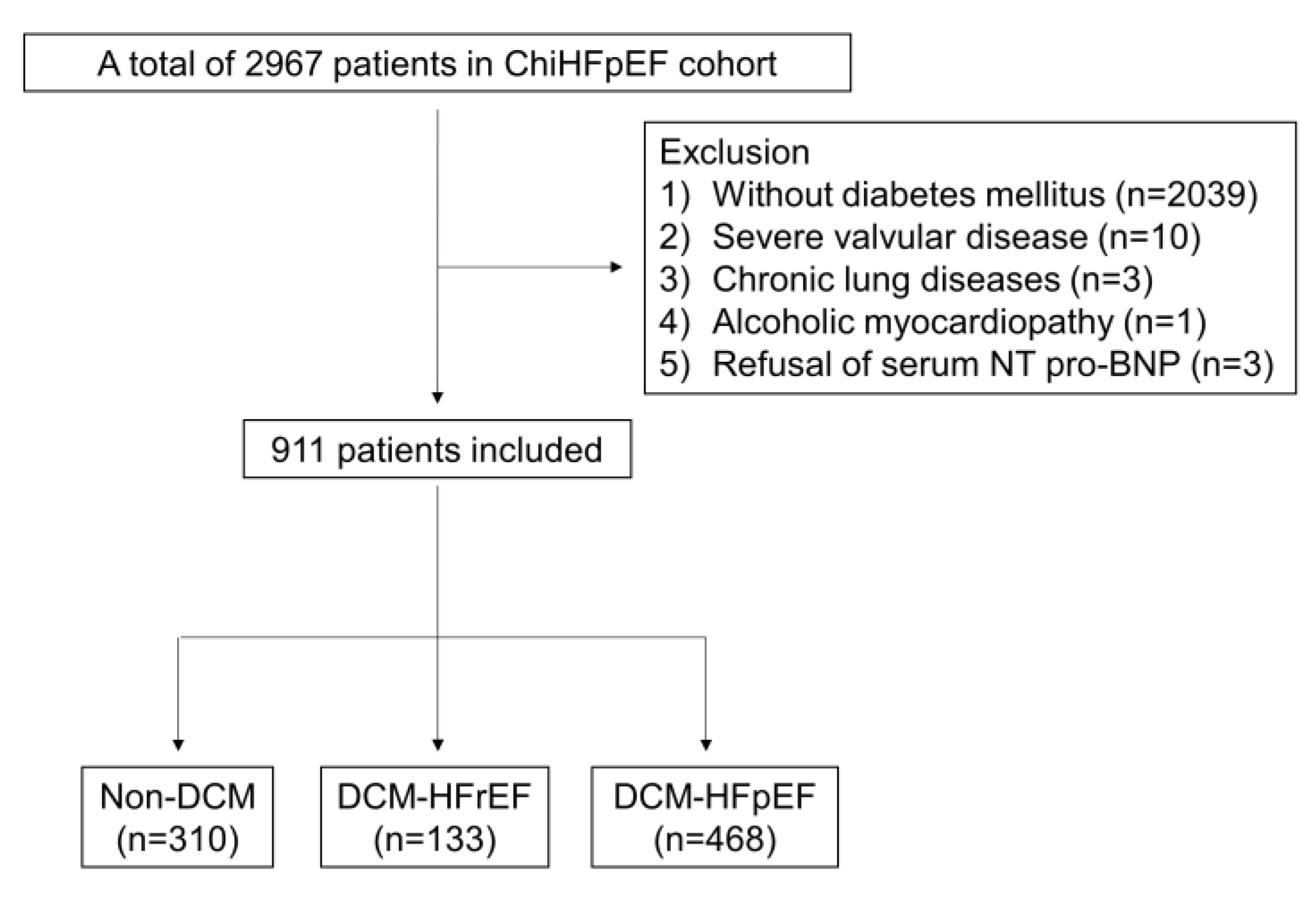


| Variables | DCM-HFpEF (n = 468) | Non-HF (n = 310) | DCM-HFrEF (n = 133) | p Value * | p Value † |
|---|---|---|---|---|---|
| Age, y | 70.2 ± 10.0 | 65.5 ± 10.2 | 65.1 ± 11.8 | <0.001 | <0.001 |
| Female, n(%) | 205 (43.8) | 124 (40.0) | 32 (24.1) | 0.293 | <0.001 |
| Hypertension, n(%) | 387 (82.7) | 235 (75.8) | 86 (64.7) | 0.019 | <0.001 |
| Non-obstructive coronary heart disease, n(%) | 343 (73.3) | 210 (67.7) | 44 (33.1) | 0.095 | <0.001 |
| Atrial fibrillation, n(%) | 78 (16.7) | 11 (3.5) | 39 (29.3) | <0.001 | 0.001 |
| Pulmonary hypertension, n(%) | 106 (22.6) | 22 (7.1) | 38 (28.6) | <0.001 | 0.158 |
| Use of insulin, n(%) | 274 (30.1) | 67 (21.6) | 47 (35.3) | <0.001 | 0.805 |
| Duration of diabetes, week | 480 (192–696) | 240 (96–480) | 240 (48–480) | <0.001 | <0.001 |
| Hemodialysis history, n(%) | 23 (4.9) | 1 (0.3) | 4 (3.0) | <0.001 | 0.349 |
| Alcohol habit, n(%) | 88 (19.3) | 80 (26.8) | 32 (26.0) | 0.016 | 0.105 |
| Smoking habit, n(%) | 99 (28.0) | 87 (35.1) | 54 (43.9) | 0.063 | 0.001 |
| HR at admission, bpm | 72 (67–79) | 72 (67–78) | 82 (72–95) | 0.920 | <0.001 |
| SBP at admission, mmHg | 130 (124–145) | 130 (120–140) | 129 (120–140) | 0.182 | <0.001 |
| DBP at admission, mmHg | 79 (70–80) | 80 (70–80) | 80 (70–84) | 0.397 | 0.301 |
| NT-proBNP, pg/mL | 558 (326–1152) | 28 (18–42) | 2711 (915–9095) | <0.001 | <0.001 |
| HbA1c, % | 7.1 (6.4–7.9) | 7.0 (6.2–8.1) | 7.1 (6.3–8.2) | 0.896 | 0.568 |
| Alanine aminotransferase, U/L | 18 (13–26) | 20 (15–31) | 23 (14–36) | <0.001 | <0.001 |
| Aspartate aminotransferase, U/L | 19 (16–24) | 19 (16–24) | 22 (18–30) | 0.186 | <0.001 |
| Potassium, mmol/L | 3.9 (3.6–4.1) | 3.9 (3.6–4.1) | 4.0 (3.6–4.2) | 0.896 | 0.119 |
| Sodium, mmol/L | 141 (139–142) | 141 (139–143) | 140 (138–142) | 0.026 | 0.135 |
| Chlorine, mmol/L | 103 (101–106) | 104 (101–106) | 103 (100–105) | 0.402 | 0.080 |
| Creatinine, umol/L | 78 (64–108) | 67 (56–81) | 91 (75–125) | <0.001 | <0.001 |
| Urea, mmol/L | 6.3 (5.1–8.8) | 5.7 (4.6–6.9) | 8.0 (6.4–11.1) | <0.001 | <0.001 |
| Uric acid, umol/L | 338 (267–434) | 309 (250–365) | 432 (346–579) | 0.003 | <0.001 |
| Glucose, mmmol/L | 6.8 (5.5–8.7) | 7.0 (6.0–8.4) | 7.4 (5.8–9.1) | 0.239 | 0.095 |
| Total cholesterol, mmol/L | 3.64 (3.02–4.47) | 3.82 (3.13–4.57) | 3.65 (2.93–4.38) | 0.103 | 0.883 |
| Triglycerides, mmol/L | 1.39 (1.00–1.97) | 1.54 (1.06–2.22) | 1.15 (0.92–1.57) | 0.010 | <0.001 |
| HDL-C, mmol/L | 0.97 (0.84–1.13) | 0.97 (0.83–1.14) | 0.92 (0.75–1.08) | 0.809 | 0.115 |
| LDL-C, mmol/L | 2.03 (1.53–2.64) | 2.11 (1.61–2.70) | 2.23 (1.72–2.82) | 0.322 | 0.010 |
| Lipoprotein A1, mg/L | 1.20 (1.07–1.35) | 1.22 (1.05–1.35) | 1.12 (0.89–1.27) | 0.573 | <0.001 |
| Lipoprotein B, mg/L | 0.71 (0.56–0.90) | 0.76 (0.60–0.93) | 0.74 (0.59–0.87) | 0.078 | 0.540 |
| Lpa, mg/L | 166 (86–388) | 129 (66–315) | 162 (91–308) | 0.016 | 0.762 |
| Haemoglobin, g/dl | 127 (115–138) | 135 (124–143) | 135 (120–147) | <0.001 | <0.001 |
| White blood cells, 109/L | 6.48 (5.24–7.67) | 6.49 (5.61–7.69) | 6.79 (5.57–8.15) | 0.434 | 0.064 |
| LVEF, % | 64 (62–65) | 65 (63–66) | 33 (28–39) | 0.008 | <0.001 |
| AOD, mm | 33 (31–36) | 33 (31–36) | 33 (31–36) | 0.650 | 0.097 |
| LAD, mm | 42 (39–46) | 40 (31–42) | 49 (45–54) | <0.001 | <0.001 |
| LVDd, mm | 48 (46–51) | 48 (45–51) | 64 (58–73) | 0.081 | <0.001 |
| LVDs, mm | 32 (30–34) | 31 (29–33) | 54 (47–63) | 0.001 | <0.001 |
| IVSD, mm | 10.0 (9.0–12.0) | 10.0 (9.0–11.0) | 9.5 (9.0–10.0) | 0.001 | <0.001 |
| LVPWD, mm | 9.0 (9.0–10.0) | 9.0 (9.0–10.0) | 9.0 (9.0–10.0) | 0.006 | 0.089 |
| FS, % | 35 (33–36) | 36 (34–36) | 16 (13–18) | <0.001 | <0.001 |
| SV, ml | 70 (60–77) | 70 (60–77) | 64 (41–71) | 0.363 | 0.549 |
| Peak E velocity, cm/s | 70 (57–85) | 65 (56–77) | 58 (51–87) | 0.101 | 0.522 |
| Peak A velocity, cm/s | 92 (78–107) | 88 (79–99) | 78 (64–87) | 0.116 | 0.018 |
| Peak E’ velocity, cm/s | 6.0 (5.0–6.0) | 6.0 (5.0–7.0) | 4.0 (3.8–4.3) | 0.011 | 0.004 |
| Peak A’ velocity, cm/s | 10.0 (8.0–11.0) | 10.0 (9.0–11.0) | 9.5 (7.4–10.8) | 0.402 | 0.637 |
| E/A ratio | 0.72 (0.63–0.86) | 0.74 (0.61–0.86) | 0.79 (0.59–1.30) | 0.566 | 0.002 |
| E’/A’ ratio | 0.60 (0.50–0.70) | 0.62 (0.50–0.71) | 0.67 (0.55–0.80) | 0.244 | 0.064 |
| E/E’ ratio | 12.80 (10.20–15.31) | 11.43 (9.29–13.67) | 14.38 (10.71–21.63) | <0.001 | 0.108 |
| Clinical Outcomes | Group | Number (%) | HR (95% CI) | p Value |
|---|---|---|---|---|
| Primary end point | ||||
| Composite endpoints | DCM-HFpEF (n = 468) | 41 (8.8) | Reference | … |
| Non-DCM (n = 310) | 10 (3.2) | 0.38 (0.22–0.66) | 0.0021 | |
| DCM-HFrEF (n = 133) | 71 (53.4) | 11.76 (7.47–18.52) | <0.0001 | |
| Secondary end point | ||||
| All-cause death | DCM-HFpEF (n = 468) | 17 (3.6) | Reference | … |
| Non-DCM (n = 310) | 8 (2.6) | 0.60 (0.27–1.33) | 0.2074 | |
| DCM-HFrEF (n = 133) | 16 (12.0) | 2.99 (1.37–6.55) | 0.0061 | |
| Rehospitalization due to HF | DCM-HFpEF (n = 468) | 25 (5.3) | Reference | … |
| Non-DCM (n = 310) | 9 (2.9) | 0.52 (0.26–1.03) | 0.0615 | |
| DCM-HFrEF (n = 133) | 66 (49.6) | 30.46 (18.07–51.32) | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Shi, Y.; Xia, Y.; Wu, L.; Li, H.; Zhou, R.; Gao, X.; Zhang, H.; Jin, X.; Zhang, J. Disparate Clinical Characteristics and Prognosis of HFpEF versus HFrEF Phenotype of Diabetic Cardiomyopathy. J. Clin. Med. 2023, 12, 1565. https://doi.org/10.3390/jcm12041565
Li Z, Shi Y, Xia Y, Wu L, Li H, Zhou R, Gao X, Zhang H, Jin X, Zhang J. Disparate Clinical Characteristics and Prognosis of HFpEF versus HFrEF Phenotype of Diabetic Cardiomyopathy. Journal of Clinical Medicine. 2023; 12(4):1565. https://doi.org/10.3390/jcm12041565
Chicago/Turabian StyleLi, Zheng, Yi Shi, Yiyuan Xia, Lida Wu, Hui Li, Rong Zhou, Xiaofei Gao, Hongsong Zhang, Xiaoping Jin, and Junxia Zhang. 2023. "Disparate Clinical Characteristics and Prognosis of HFpEF versus HFrEF Phenotype of Diabetic Cardiomyopathy" Journal of Clinical Medicine 12, no. 4: 1565. https://doi.org/10.3390/jcm12041565
APA StyleLi, Z., Shi, Y., Xia, Y., Wu, L., Li, H., Zhou, R., Gao, X., Zhang, H., Jin, X., & Zhang, J. (2023). Disparate Clinical Characteristics and Prognosis of HFpEF versus HFrEF Phenotype of Diabetic Cardiomyopathy. Journal of Clinical Medicine, 12(4), 1565. https://doi.org/10.3390/jcm12041565








