Design and Protocol for Beijing Hospital Takayasu Arteritis (BeTA) Biobank
Abstract
:1. Background
2. Method
2.1. Database Plan
2.2. Ethical Review
2.3. Venue and Population
2.4. Recruitment of Groups
2.5. Recruitment Procedure
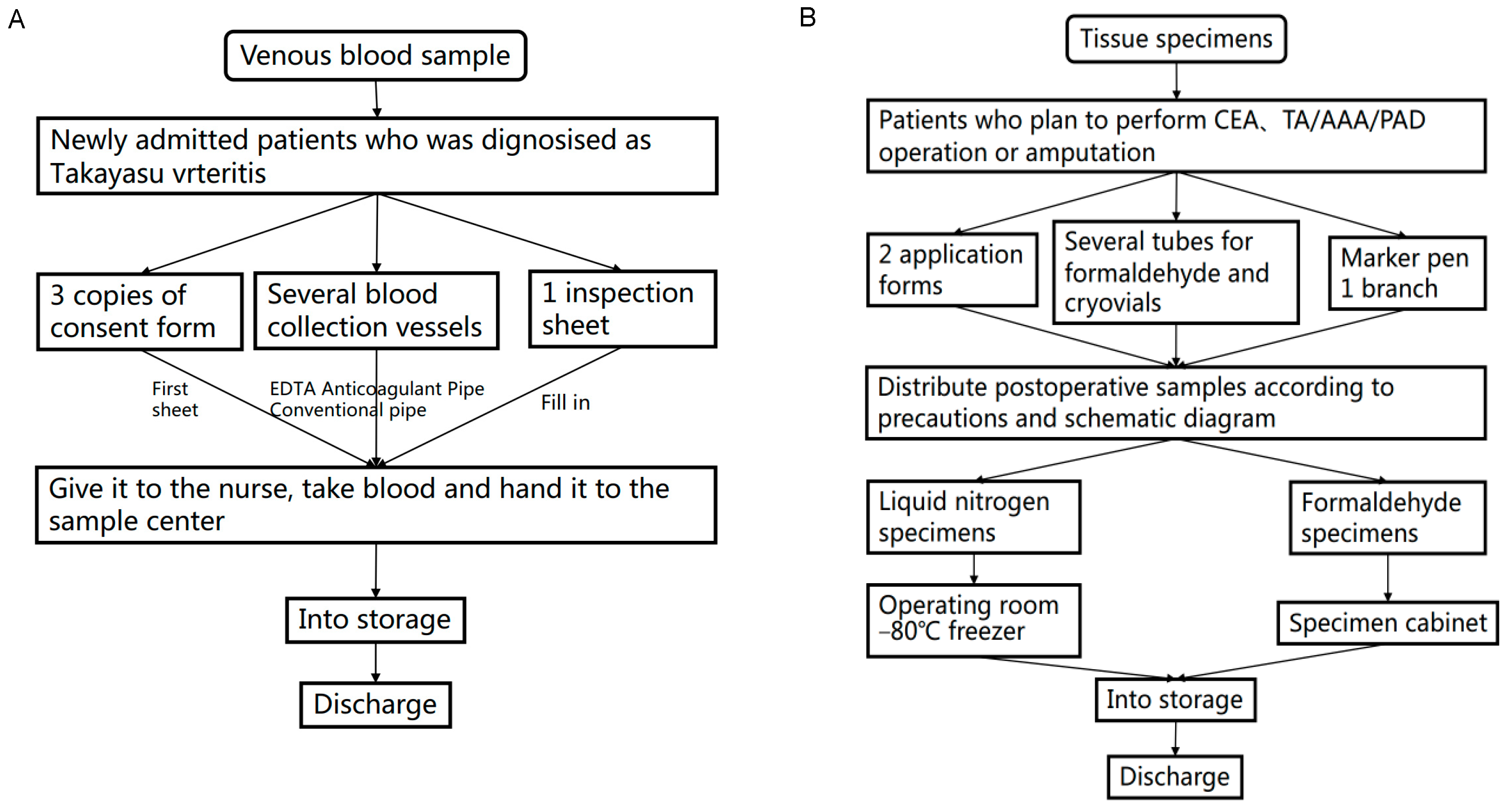
2.6. Collection and Processing of Biological Materials
2.7. Clinical Information Collection
2.8. Statistical Analysis Methods
2.9. Patient and Public Participation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, X.; Du, J.; Li, T.; Na Gao, N.; Fang, W.; Chen, S.; Qiao, Z.; Li, C.; Zhu, J.; Pan, L. Risk factors and surgical prognosis in patients with aortic valve involvement caused by Takayasu arteritis. Arthritis Res. Ther. 2022, 24, 102. [Google Scholar] [CrossRef]
- Onen, F.; Akkoç, N. Epidemiology of Takayasu arteritis. Presse Med. 2017, 46, e197–e203. [Google Scholar] [CrossRef]
- Watanabe, Y.; Miyata, T.; Tanemoto, K. Current Clinical Features of New Patients With Takayasu Arteritis Observed From Cross-Country Research in Japan: Age and Sex Specificity. Circulation 2015, 132, 1701–1709. [Google Scholar] [CrossRef]
- Mason, J.C. Surgical intervention and its role in Takayasu arteritis. Best Prac. Res. Clin. Rheumatol. 2018, 32, 112–124. [Google Scholar] [CrossRef]
- Nunes, G.; Neves, F.S.; Melo, F.M.; De Castro, G.R.W.; Zimmermann, A.F.; Pereira, I.A. Takayasu arteritis: Anti-TNF therapy in a Brazilian setting. Rev. Bras. Reumatol. 2010, 50, 291–298. [Google Scholar] [CrossRef]
- Delvino, P.; Monti, S.; Montecucco, C. Striking abdominal aortic stenosis revealing Takayasu’s arteritis. Eur. Hear. J. Cardiovasc. Imaging 2021, 22, e152. [Google Scholar] [CrossRef]
- Comarmond, C.; Biard, L.; Lambert, M.; Mekinian, A.; Ferfar, Y.; Kahn, J.E.; Benhamou, Y.; Chiche, L.; Koskas, F.; Cluzel, P.; et al. Long-Term Outcomes and Prognostic Factors of Complications in Takayasu Arteritis: A Multicenter Study of 318 Patients. Circulation 2017, 136, 1114–1122. [Google Scholar] [CrossRef]
- Chen, Z.G.; Chen, Y.X.; Diao, Y.P.; Wu, Z.Y.; Yan, S.; Ma, L.; Liu, C.W.; Li, Y.J. Simultaneous Multi-Supra-Aortic Artery Bypass Successfully Implemented in 17 Patients with Type I Ta-kayasu Arteritis. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 903–909. [Google Scholar] [CrossRef]
- Diao, Y.; Yan, S.; Premaratne, S.; Chen, Y.; Tian, X.; Chen, Z.; Wu, Z.; Miao, Y.; Zhang, W.W.; Li, Y. Surgery and Endovascular Management in Patients With Takayasu’s Arteritis: A Ten-Year Retrospective Study. Ann. Vasc. Surg. 2020, 63, 34–44. [Google Scholar] [CrossRef]
- Lee, S.G.; Ryu, J.-S.; Kim, H.O.; Oh, J.S.; Gil Kim, Y.; Lee, C.-K.; Yoo, B. Evaluation of Disease Activity Using F-18 FDG PET-CT in Patients With Takayasu Arteritis. Clin. Nucl. Med. 2009, 34, 749–752. [Google Scholar] [CrossRef]
- Tombetti, E.; Hysa, E.; Mason, J.C.; Cimmino, M.A.; Camellino, D. Blood Biomarkers for Monitoring and Prognosis of Large Vessel Vasculitides. Curr. Rheumatol. Rep. 2021, 23, 17. [Google Scholar] [CrossRef]
- Alibaz-Oner, F.A.T.M.A.; Yentür, S.P.; Saruhan-Direskeneli, G.; Direskeneli, H. Serum cytokine profiles in Takayasu’s arteritis: Search for biomarkers. Clin. Exp. Rheumatol. 2015, 33 (Suppl. S89), S-32–S-35. [Google Scholar]
- Cui, X.; Qin, F.; Song, L.; Wang, T.; Geng, B.; Zhang, W.; Jin, L.; Wang, W.; Li, S.; Tian, X.; et al. Novel Biomarkers for the Precisive Diagnosis and Activity Classification of Takayasu Arteritis. Circ. Genom. Precis. Med. 2019, 12, e002080. [Google Scholar] [CrossRef]
- Keser, G.; Aksu, K.; Direskeneli, H. Discrepancies between vascular and systemic inflammation in large vessel vasculitis: An important problem revisited. Rheumatology 2017, 57, 784–790. [Google Scholar] [CrossRef]
- Singh, K.; Rathore, U.; Rai, M.K.; Behera, M.R.; Jain, N.; Ora, M.; Bhadauria, D.; Sharma, S.; Pande, G.; Gambhir, S.; et al. Novel Th17 Lymphocyte Populations, Th17.1 and PD1+Th17, are Increased in Takayasu Arteritis, and Both Th17 and Th17.1 Sub-Populations Associate with Active Disease. J. Inflamm. Res. 2022, 15, 1521–1541. [Google Scholar] [CrossRef]
- Wu, G.; Mahajan, N.; Dhawan, V. Acknowledged Signatures of Matrix Metalloproteinases in Takayasu’s Arteritis. BioMed Res. Int. 2014, 2014, 827105. [Google Scholar] [CrossRef]
- Soto, M.E.; Espinola-Zavaleta, N.; Ramirez-Quito, O.; Reyes, P.A. Echocardiographic follow-up of patients with Takayasu’s arteritis: Five-year survival. Echocardiography 2006, 23, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Hotchi, M. Pathological studies on Takayasu arteritis. Hear. Vessel. 1992, 7, 11–17. [Google Scholar] [CrossRef]
- Mirault, T.; Guillet, H.; Messas, E. Immune response in Takayasu arteritis. Presse Med. 2017, 46, e189–e196. [Google Scholar] [CrossRef]
- Kong, X.; Sun, Y.; Ma, L.; Chen, H.; Wei, L.; Wu, W.; Ji, Z.; Ma, L.; Zhang, Z.; Zhang, Z.; et al. The critical role of IL-6 in the pathogenesis of Takayasu arteritis. Clin. Exp. Rheumatol. 2015, 34, S21–S27. [Google Scholar]
- Saadoun, D.; Garrido, M.; Comarmond, C.; Desbois, A.C.; Domont, F.; Savey, L.; Terrier, B.; Geri, G.; Rosenzwajg, M.; Klatzmann, D.; et al. Th1 and Th17 Cytokines Drive Inflammation in Takayasu Arteritis. Arthritis Rheumatol. 2015, 67, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.D.; Coit, P.; Saruhan-Direskeneli, G.; Hernández-Rodríguez, J.; Cid, M.C.; Solans, R.; Castañeda, S.; Vaglio, A.; Direskeneli, H.; Merkel, P.A.; et al. Analysis of the common genetic component of large-vessel vasculitides through a meta-Immunochip strategy. Sci. Rep. 2017, 7, 43953. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Fernández, L.; Carmona, F.D.; López-Mejías, R.; González-Escribano, M.F.; Lyons, P.A.; Morgan, A.W.; Sawalha, A.H.; Merkel, P.A.; Smith, K.G.; González-Gay, M.A.; et al. Cross-phenotype analysis of Immunochip data identifies KDM4C as a relevant locus for the de-velopment of systemic vasculitis. Ann. Rheum. Dis. 2018, 77, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Saruhan-Direskeneli, G.; Hughes, T.; Aksu, K.; Keser, G.; Coit, P.; Aydin, S.Z.; Alibaz-Oner, F.; Kamalı, S.; Inanc, M.; Carette, S.; et al. Identification of Multiple Genetic Susceptibility Loci in Takayasu Arteritis. Am. J. Hum. Genet. 2013, 93, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Yuqing, M.; Shang, G.; Qing, G.; Jiyang, W.; Ruihao, L.; Zuoguan, C.; Yongpeng, D.; Zhiyuan, W.; Yongjun, L. Transcriptome profiling of abdominal aortic tissues reveals alterations in mRNAs of Takayasu arteritis. Front. Genet. 2022, 13, 1036233. [Google Scholar] [CrossRef]
- Qing, G.; Wu, Z.Y.; Yu, J.G.; Miao, Y.Q.; Chen, Z.G.; Diao, Y.P.; Yin, J.F.; Jia, J.N.; Guo, Y.J.; Li, W.M.; et al. Single-Cell RNA Sequencing Revealed CD14+ Monocytes Increased in Patients With Takayasu’s Arteritis Re-quiring Surgical Management. Front. Cell Dev. Biol. 2021, 9, 761300. [Google Scholar] [CrossRef]
| Enrollment Criteria | Exclusion Criteria |
|---|---|
| The diagnosis of Takayasu arteritis conforms to the 2022 ACR/EULAR TAK classification criteria | Patients with other systemic autoimmune diseases |
| Persistent active disease remains under immunosuppressive therapy | Patients with severe hematological and endocrine system lesions or tumors |
| Conformance indications for surgical intervention: 1. Imaging examination shows severe stenosis (> 70%) or occlusion of the aorta and its branches 2. Intractable renovascular or aortic constricting hypertension 3. Ischemia of heart, brain, limbs and other organs 4. Aortic aneurysm-like lesions | Patients with severe heart, lung, liver, kidney and other important organ lesions, unable to tolerate anesthesia and surgery |
| Patients with acute or chronic infectious diseases (except tuberculosis) | |
| Patients with mental disorders or who are unable or unwilling to cooperate with treatment for other reasons | |
| Patients who are pregnant before surgical intervention |
| Biomaterials | Acquisition Time | Saved State | Position |
|---|---|---|---|
| Peripheral blood: EDTA anticoagulation | The morning after admission | −80 °C RNAlater | Peripheral venous blood |
| Peripheral blood: routine biochemical serum | The morning after admission | 4 °C preservation | Peripheral venous blood |
| Samples of the vessel wall of Takayasu arteritis | Intraoperative dispensing | Formalin specimen, encapsulated at normal temperature | Diseased vascular segments |
| Samples of the vessel wall of Takayasu arteritis | Intraoperative dispensing | −80 °C RNAlater | Diseased vascular segments |
| Follow-Up Programs | Enlistment | 1 Month | 3 Months | 6 Months | 9 Months | 12 Months | |
|---|---|---|---|---|---|---|---|
| Medical history | Symptom | √ | √ | √ | √ | √ | √ |
| Sign | √ | √ | √ | √ | √ | √ | |
| Blood | ESR | √ | √ | √ | √ | ||
| C-reactive protein | √ | √ | √ | √ | |||
| Image | Ultrasound of the target vessel | √ | √ | √ | √ | ||
| Target vascular CTA | √ | √ | √ | ||||
| Medication | Immunotherapy | √ | √ | √ | √ | √ | √ |
| Antihypertensive therapy | √ | √ | √ | √ | √ | √ | |
| Antithrombotic therapy | √ | √ | √ | √ | √ | √ | |
| Quality of life | Preoperatively SF-36 | √ | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Wu, Z.-Y.; Miao, Y.-Q.; Lu, Z.-B.; Tan, S.-P.; Wang, J.-Y.; Lu, C.-R.; Xu, Z.-X.; Li, P.; Lan, Y.; et al. Design and Protocol for Beijing Hospital Takayasu Arteritis (BeTA) Biobank. J. Clin. Med. 2023, 12, 1516. https://doi.org/10.3390/jcm12041516
Gao S, Wu Z-Y, Miao Y-Q, Lu Z-B, Tan S-P, Wang J-Y, Lu C-R, Xu Z-X, Li P, Lan Y, et al. Design and Protocol for Beijing Hospital Takayasu Arteritis (BeTA) Biobank. Journal of Clinical Medicine. 2023; 12(4):1516. https://doi.org/10.3390/jcm12041516
Chicago/Turabian StyleGao, Shang, Zhi-Yuan Wu, Yu-Qing Miao, Zhen-Bo Lu, Shu-Ping Tan, Ji-Yang Wang, Cheng-Ran Lu, Zheng-Xi Xu, Peng Li, Yong Lan, and et al. 2023. "Design and Protocol for Beijing Hospital Takayasu Arteritis (BeTA) Biobank" Journal of Clinical Medicine 12, no. 4: 1516. https://doi.org/10.3390/jcm12041516
APA StyleGao, S., Wu, Z.-Y., Miao, Y.-Q., Lu, Z.-B., Tan, S.-P., Wang, J.-Y., Lu, C.-R., Xu, Z.-X., Li, P., Lan, Y., Diao, Y.-P., Chen, Z.-G., & Li, Y.-J. (2023). Design and Protocol for Beijing Hospital Takayasu Arteritis (BeTA) Biobank. Journal of Clinical Medicine, 12(4), 1516. https://doi.org/10.3390/jcm12041516





