Hepatoid Adenocarcinoma of the Lung: A Review of the Most Updated Literature and a Presentation of Three Cases
Abstract
1. Introduction
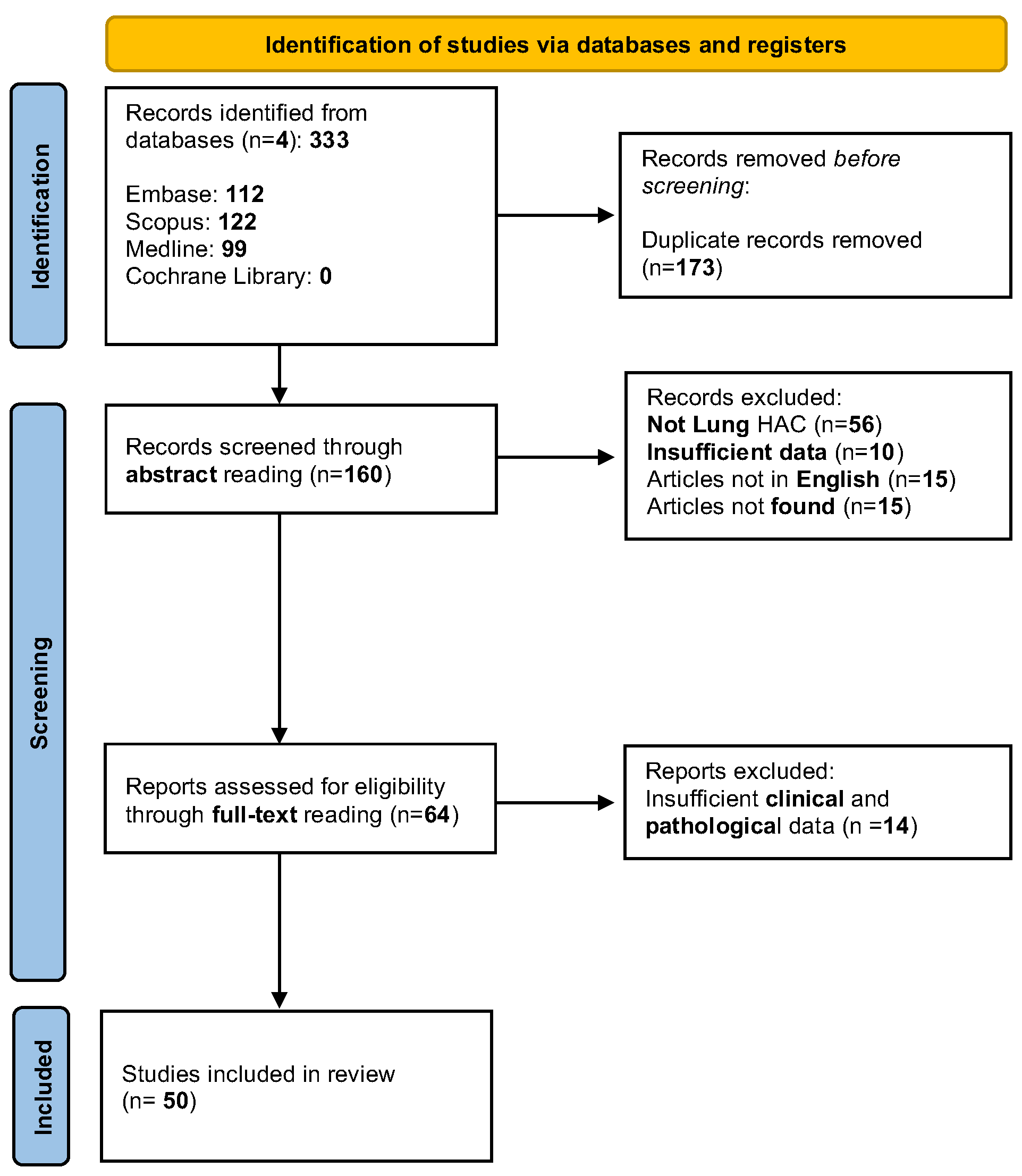
2. Materials and Methods
3. Case Presentation
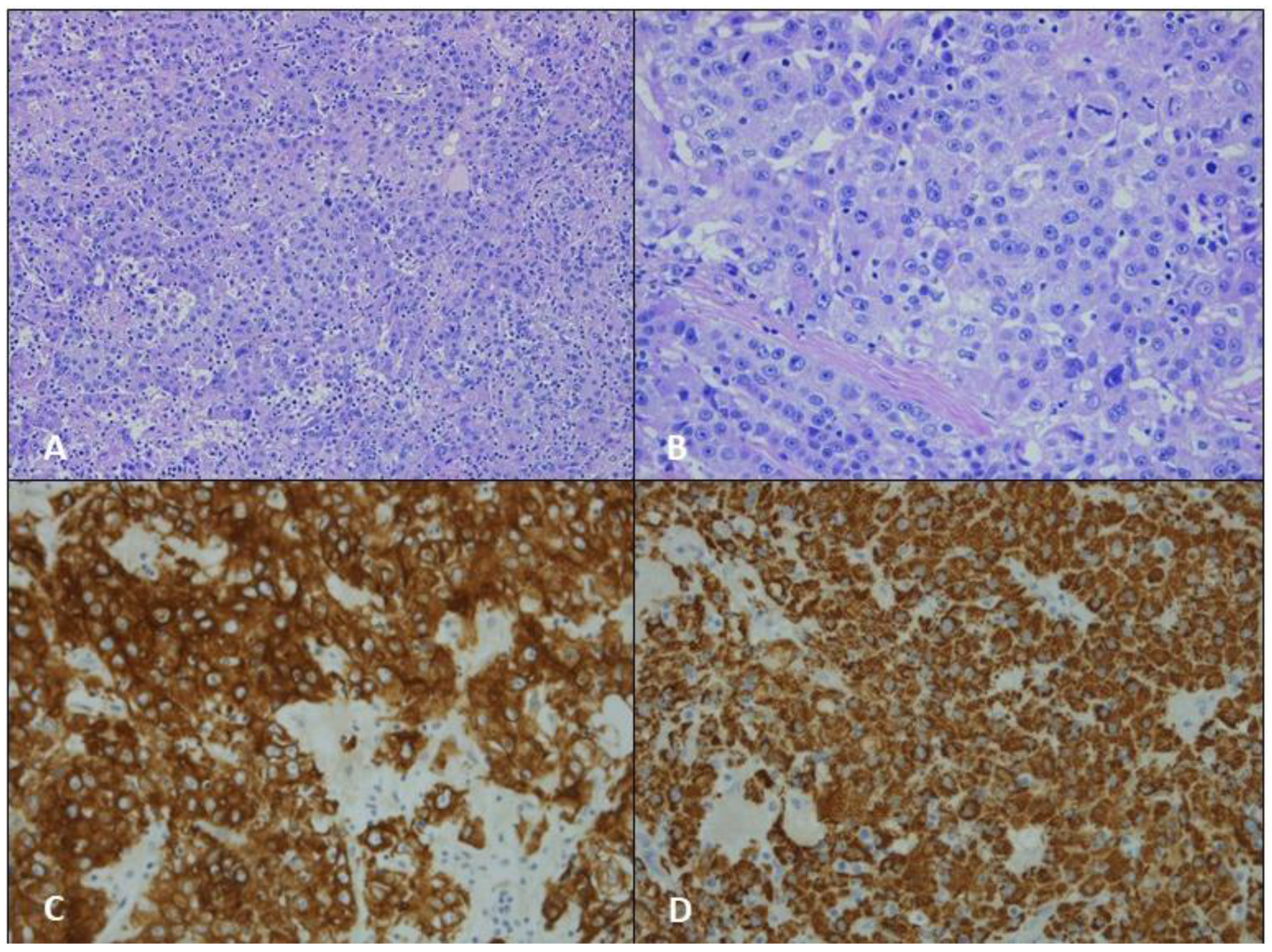
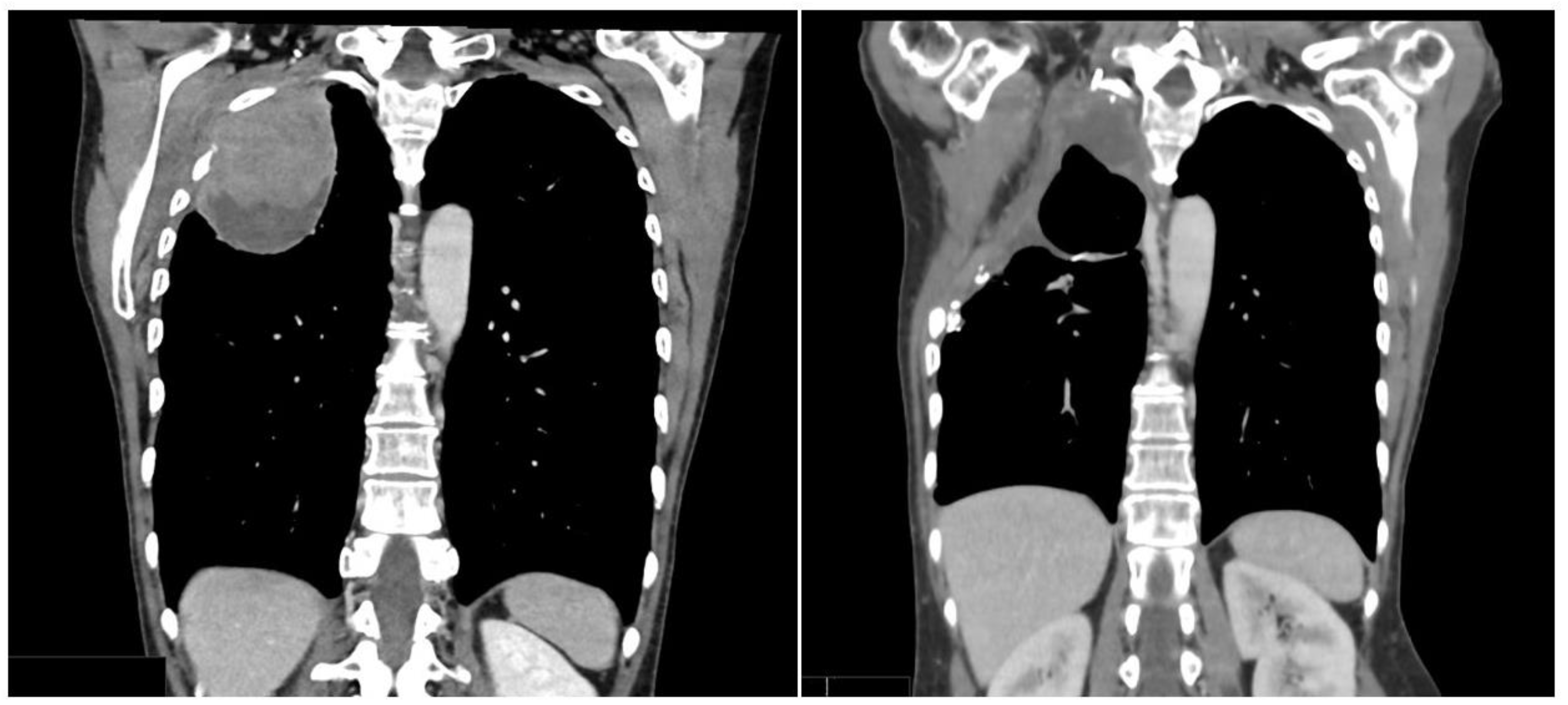
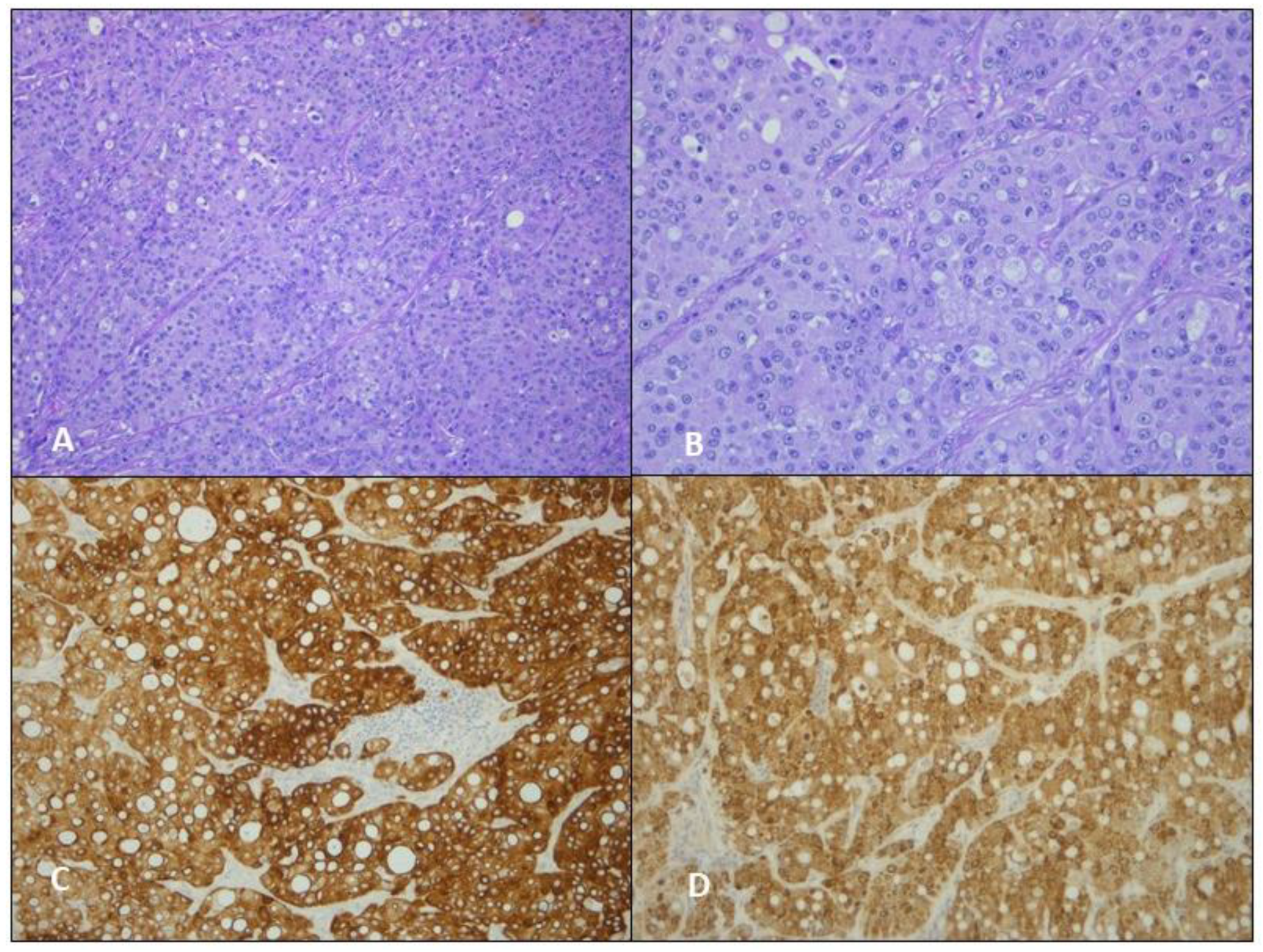
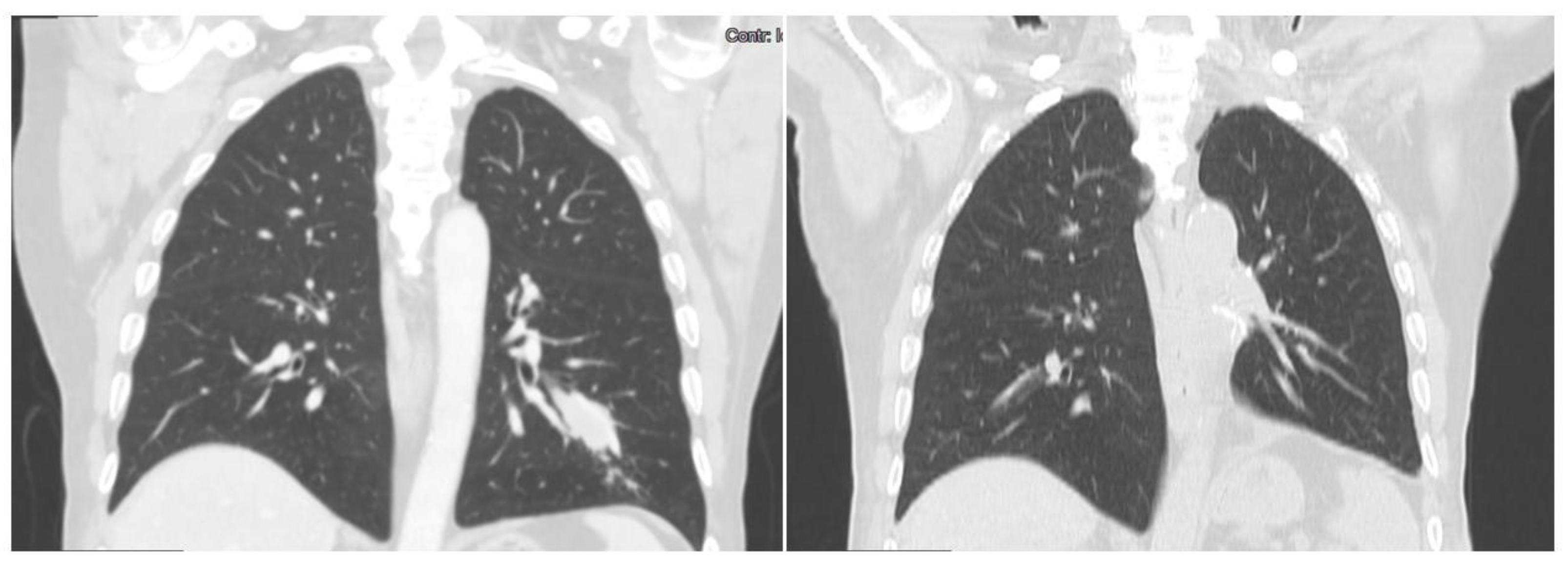
3.1. Case 1
3.2. Case 2
3.3. Case 3
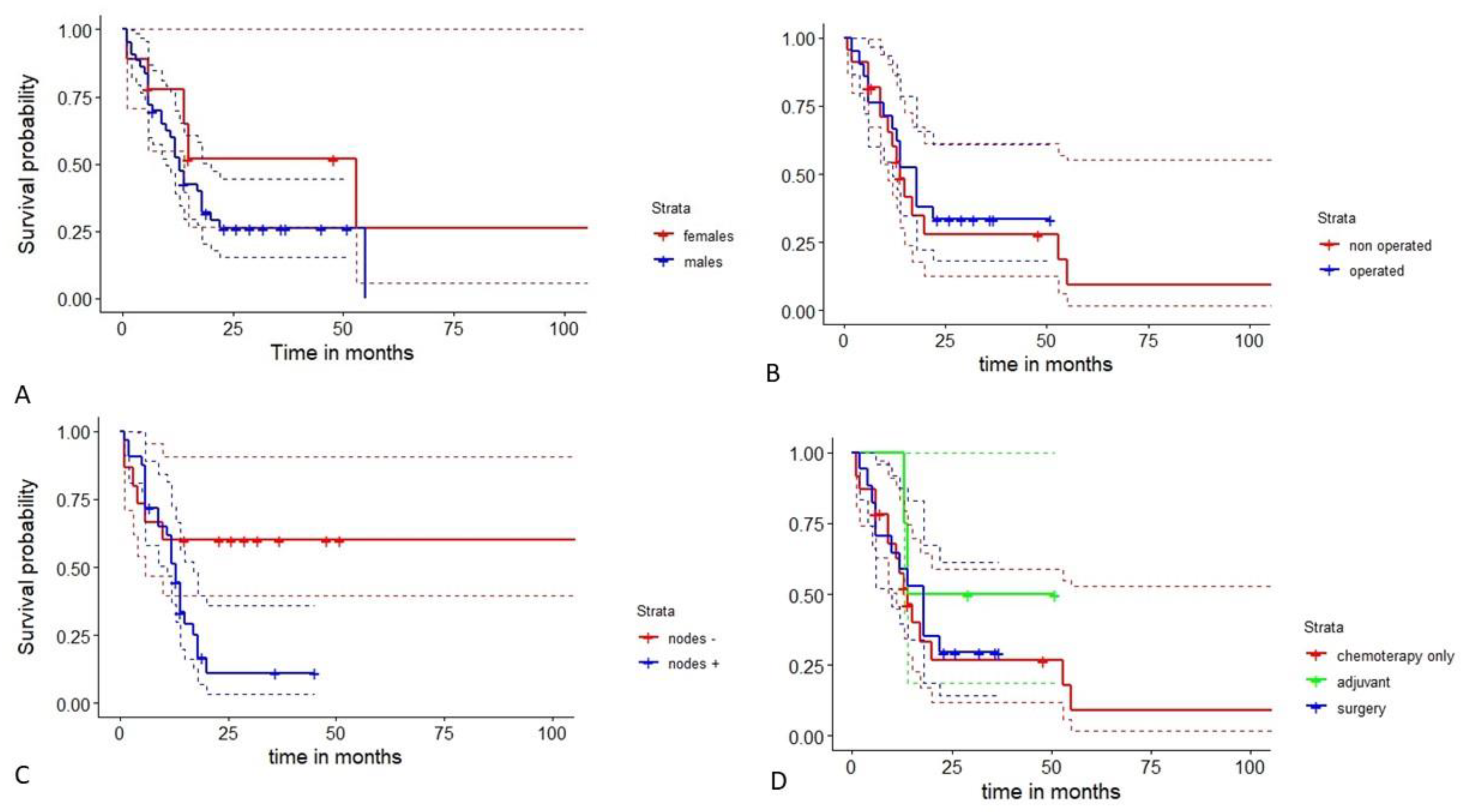
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| First Author | Second Author | Year | Case | Gender | Age |
|---|---|---|---|---|---|
| Al-Najjar H. | Evison M. | 2015 | 1 | 1 | 71 |
| Anusha G. | 2021 | 2 | 1 | 58 | |
| Ayub A | Nunez Lopez O | 2019 | 3 | 1 | 61 |
| Basse V | Schick U | 2018 | 4 | 1 | 43 |
| Cavalcante LB | Felipe-Silva A | 2013 | 5 | 1 | 66 |
| Chandan VS | Shah SS | 2016 | 6 | 1 | 53 |
| 7 | 1 | 71 | |||
| Che YQ | Wang S | 2014 | 8 | 1 | 48 |
| Chen HF | Wang WX | 2019 | 9 | 1 | 53 |
| Chen L | Han X | 2020 | 10 | 0 | 65 |
| Chen Y. | 11 | 1 | 47 | ||
| El Khoury A. | El Khoury M. | 2019 | 12 | 1 | 59 |
| Esa N.Y.M. | Zamrud R. | 2018 | 13 | 1 | 50 |
| Fornasa F. | 2010 | 14 | 0 | 68 | |
| Gavrancic T | Park YH. | 2015 | 15 | 1 | 64 |
| Grossman K | Beasley MB | 2016 | 16 | 1 | 54 |
| Haninger DM | Kloecker GH | 2014 | 17 | 1 | 51 |
| 18 | 1 | 52 | |||
| 19 | 1 | 64 | |||
| 20 | 0 | 54 | |||
| 21 | 1 | 60 | |||
| Hayashi Y | Takanashi Y | 2002 | 22 | 1 | 55 |
| Hiroshima K | Iyoda A | 2002 | 23 | 1 | 71 |
| 24 | 1 | 70 | |||
| Hou Z | Xie J | 2021 | 25 | 1 | 66 |
| Khozin S | Roth MJ | 2012 | 26 | 0 | 56 |
| Kuan K | Khader SN | 2019 | 27 | 1 | 47 |
| Lagos G.G. | Feldman J.L. | 2021 | 28 | 1 | 54 |
| Li J | Qi H | 2019 | 29 | 1 | 71 |
| Miyama Y. | Fujii T. | 2020 | 30 | 1 | 58 |
| Mokrim M | Belbaraka R | 2012 | 31 | 1 | 52 |
| Motooka Y | Yoshimoto K | 2016 | 32 | 1 | 69 |
| Muroyama Y | Tamiya H | 2020 | 33 | 1 | 66 |
| Nargund A. | Agrawal M. | 2020 | 34 | 1 | 66 |
| 35 | 1 | 65 | |||
| Papatsimpas G. | Kamposioras K. | 2012 | 36 | 1 | 48 |
| Qian GQ | Yin FY | 2016 | 37 | 1 | 79 |
| Ruiz Cerrato D. | 2018 | 38 | 0 | 69 | |
| Seddon J.E. | Jayarao M. | 2021 | 39 | 1 | 33 |
| Shaib W | Sharma R | 2014 | 40 | 0 | 53 |
| Shi YF | Lu JG | 2019 | 41 | 1 | 60 |
| Sun H | Li X | 2022 | 42 | 1 | 66 |
| 43 | 1 | 64 | |||
| 44 | 1 | 64 | |||
| 45 | 1 | 63 | |||
| Sun J.N. | Zhang B.L. | 2016 | 46 | 1 | 59 |
| Tan Y. | Min J. | 2019 | 47 | 1 | 60 |
| Terracciano LM | Glatz K | 2003 | 48 | 1 | 49 |
| Tonyali O | Gonullu O | 2020 | 49 | 0 | 65 |
| Valle L | Thomas J | 2017 | 50 | 1 | 61 |
| Wang C. | Xu G. | 2019 | 51 | 1 | 70 |
| Wang S | Li M | 2016 | 52 | 1 | 56 |
| Wang W | Li G. | 2020 | 53 | 1 | 41 |
| Wu Z | Upadhyaya M | 2007 | 54 | 1 | 50 |
| Yang K | Jiang H | 2019 | 55 | 1 | 70 |
| Zhuansun Y | Bian L | 2021 | 56 | 1 | 55 |
| 57 | 1 | 61 | |||
| 58 | 1 | 49 | |||
| 59 | 1 | 64 | |||
| Chen | Ding | 2022 | 60 | 1 | 67 |
| Zhen | Xiao-Chen | 2022 | 61 | 1 | 55 |
| Yao | Guan | 2022 | 62 | 1 | 63 |
| Nakwan | Chang | 2022 | 63 | 1 | 63 |
| OUR CASES | 64 | 0 | 61 | ||
| 65 | 0 | 67 | |||
| 66 | 1 | 78 |
References
- Bade, B.C.; Cruz, C.S.D. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Rare Cancers. Available online: https://epic.iarc.fr/research/cancerworkinggroups/rarecancers.php (accessed on 5 January 2023).
- Grossman, K.; Beasley, M.B.; Braman, S.S. Hepatoid adenocarcinoma of the lung: Review of a rare form of lung cancer. Respir. Med. 2016, 119, 175–179. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Li, Z.; Gao, J.; Ge, S.; Zhang, C.; Yuan, J.; Wang, X.; Li, J.; Lu, Z.; et al. Hepatoid adenocarcinoma of the stomach: A unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer 2019, 22, 1183–1192. [Google Scholar] [CrossRef]
- Tonyali, O.; Gonullu, O.; Ozturk, M.A.; Kosif, A.; Civi, O.G. Hepatoid adenocarcinoma of the lung and the review of the literature. J. Oncol. Pharm. Pract. 2020, 26, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Fois, S.S.; Paliogiannis, P.; Zinellu, A.; Fois, A.G.; Cossu, A.; Palmieri, G. Molecular Epidemiology of the Main Druggable Genetic Alterations in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 612. [Google Scholar] [CrossRef]
- Li, J.; Qi, H.; Xu, B.; Zhao, J.; Gao, H.; Ma, X.; Liu, X. Genomic Profiles of a Patient of Pulmonary Hepatoid Adenocarcinoma With High AFP Level: A Case Report. Front. Oncol. 2019, 9, 1360. [Google Scholar] [CrossRef]
- Fang, W.; Ma, Y.; Yin, J.C.; Hong, S.; Zhou, H.; Wang, A.; Wang, F.; Bao, H.; Wu, X.; Yang, Y.; et al. Comprehensive Genomic Profiling Identifies Novel Genetic Predictors of Response to Anti–PD-(L)1 Therapies in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 5015–5026. [Google Scholar] [CrossRef]
- Katoh, M. Function and cancer genomics of FAT family genes. Int. J. Oncol. 2012, 41, 1913–1918. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, C.; Zhang, T.; He, Y.; Jiang, G. Primary Hepatoid Adenocarcinoma of the Lung: A Systematic Literature Review. OncoTargets Ther. 2022, 15, 609–627. [Google Scholar] [CrossRef]
- Yasunami, R.; Hashimoto, Z.; Ogura, T.; Hirao, F.; Yamamura, Y. Primary Lung Cancer Producing Al-pha-Fetoprotein: A Case Report. Cancer 1981, 47, 926–929. [Google Scholar] [CrossRef]
- Ishikura, H.; Kanda, M.; Ito, M.; Nosaka, K.; Mizuno, K. Hepatoid adenocarcinoma: A distinctive histological subtype of alpha-fetoprotein-producing lung carcinoma. Virchows Arch. 1990, 417, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Al-Najjar, H.; Evison, M.; Doran, H.; Booton, R.; Taylor, P. Primary pulmonary hepatoid adenocarcinoma: A case report. Cancer Treat. Commun. 2015, 4, 172–173. [Google Scholar] [CrossRef]
- Esa, N.Y.M.; Zamrud, R.; Bakar, N.S.; Eezamuddeen, M.N.; Hamdan, M.F. Is it liver or lung cancer? An intriguing case of lung adenocarcinoma with hepatoid differentiation. Proc. Singap. Health 2017, 27, 55–58. [Google Scholar] [CrossRef]
- Hou, Z.; Xie, J.; Zhang, L.; Dai, G.; Chen, Y.; He, L. Hepatoid Adenocarcinoma of the Lung: A Systematic Review of the Literature from 1981 to 2020. Front Oncol. 2021, 11, 702216. [Google Scholar] [CrossRef] [PubMed]
- Papatsimpas, G.; Kamposioras, K.; Goula, K.; Papaparaskeva, K.; Loukides, S.; Kotoulas, C.; Kelekis, N.; Xiros, N.; Pectasides, D.; Koumarianou, A. Hepatoid Pancoast tumor. A case report and review of the literature. Lung Cancer 2012, 77, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.-X.; Liu, C.; Zhao, Y.-Y.; Ding, G.-S.; Ma, J.-Q.; Teng, F.; Guo, W.-Y. Merged hepatopulmonary features in hepatoid adenocarcinoma of the lung: A systematic review. Am. J. Transl. Res . 2021, 13, 898–922. [Google Scholar]
- Ayub, A.; Lopez, O.N.; Booth, A.; Okereke, I. Pulmonary hepatoid adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2019, 158, e139–e140. [Google Scholar] [CrossRef]
- Haninger, D.M.; Kloecker, G.H.; Ii, M.B.; Nowacki, M.R.; Slone, S.P. Hepatoid adenocarcinoma of the lung: Report of five cases and review of the literature. Mod. Pathol. 2014, 27, 535–542. [Google Scholar] [CrossRef]
- Kuan, K.; Khader, S.N.; El Hussein, S. Hepatoid adenocarcinoma of the lung. Diagn. Cytopathol. 2019, 47, 831–833. [Google Scholar] [CrossRef]
- Khozin, S.; Roth, M.J.; Rajan, A.; Smith, K.; Thomas, A.; Berman, A.; Giaccone, G. Hepatoid Carcinoma of the Lung with Anaplastic Lymphoma Kinase Gene Rearrangement. J. Thorac. Oncol. 2012, 7, e29–e31. [Google Scholar] [CrossRef]
- Lei, L.; Yang, L.; Xu, Y.-Y.; Chen, H.-F.; Zhan, P.; Wang, W.-X.; Xu, C.-W. Hepatoid adenocarcinoma of the lung: An analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Open Med. 2021, 16, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- The Jamovi Project. Jamovi (Version 2.3.18). 2022. Available online: https://www.jamovi.org (accessed on 3 January 2023).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 3 January 2023).
- Anusha, G.; Subramanian, B.V.; Das, P.; Kalawat, T.; Prayaga, A.K. Hepatoid Adenocarcinoma of Lung Presenting as Unknown Primary with Cervical Lymphadenopathy: A Rare Case Report. J. Clin. Diagn. Res. 2021, 15, 1–3. [Google Scholar] [CrossRef]
- Basse, V.; Schick, U.; Guéguen, P.; Le Maréchal, C.; Quintin-Roué, I.; Descourt, R.; Simon, H.; Uguen, A.; Quéré, G. A Mismatch Repair–Deficient Hepatoid Adenocarcinoma of the Lung Responding to Anti–PD-L1 Durvalumab Therapy Despite No PD-L1 Expression. J. Thorac. Oncol. 2018, 13, e120–e122. [Google Scholar] [CrossRef]
- Chandan, V.S.; Shah, S.S.; Torbenson, M.S.; Wu, T.-T. Arginase-1 is frequently positive in hepatoid adenocarcinomas. Hum. Pathol. 2016, 55, 11–16. [Google Scholar] [CrossRef]
- Che, Y.Q.; Wang, S.; Luo, Y.; Wang, J.B.; Wang, L.H. Hepatoid adenocarcinoma of the lung: Presenting me-diastinal metastasis without transfer to the liver. Oncol. Lett. 2014, 8, 105–110. [Google Scholar] [CrossRef]
- Chen, H.-F.; Wang, W.-X.; Li, X.-L.; Xu, C.-W.; Du, K.-Q.; Zhu, Y.-C.; Fang, M.-Y. Hepatoid Adenocarcinoma of the Lung with EGFR Mutation and the Response to Tyrosine Kinase Inhibitors. J. Thorac. Oncol. 2019, 14, e217–e219. [Google Scholar] [CrossRef]
- Chen, L.; Han, X.; Gao, Y.; Zhao, Q.; Wang, Y.; Jiang, Y.; Liu, S.; Wu, X.; Miao, L. Anti-PD-1 Therapy Achieved Disease Control after Multiline Chemotherapy in Unresectable KRAS-Positive Hepatoid Lung Adenocarcinoma: A Case Report and Literature Review. OncoTargets Ther. 2020, 13, 4359–4364. [Google Scholar] [CrossRef]
- El Khoury, A.; El Khoury, M.; De Luca, R. Immunotherapeutic approach to a case of advanced hepatoid adenocarcinoma of the lung. Memo Mag. Eur. Med. Oncol. 2019, 12, 272–277. [Google Scholar] [CrossRef]
- Gavrancic, T.; Park, Y.-H.A. A Novel Approach Using Sorafenib in Alpha Fetoprotein–Producing Hepatoid Adenocarcinoma of the Lung. J. Natl. Compr. Cancer Netw. 2015, 13, 387–391. [Google Scholar] [CrossRef][Green Version]
- Hayashi, Y.; Takanashi, Y.; Ohsawa, H.; Ishii, H.; Nakatani, Y. Hepatoid adenocarcinoma in the lung. Lung Cancer 2002, 38, 211–214. [Google Scholar] [CrossRef]
- Hiroshima, K.; Iyoda, A.; Toyozaki, T.; Haga, Y.; Baba, M.; Fujisawa, T.; Ishikura, H.; Ohwada, H. Alpha-fetoprotein-producing lung carcinoma: Report of three cases. Pathol. Int. 2002, 52, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lagos, G.G.; Feldman, J.L.; Saqi, A.; Shu, C.A. Hepatoid Adenocarcinoma of the Lung Responsive to Frontline Combination Chemotherapy With Immunotherapy: Case Report. JTO Clin. Res. Rep. 2020, 2, 100130. [Google Scholar] [CrossRef]
- Mokrim, M.; Belbaraka, R.; Allaoui, M.; Kairaouani, M.; Mahassini, N.; Tahri, A.; Errihani, H. Hepatoid Adenocarcinoma of the Lung: A Case Report and Literature Review. J. Gastrointest. Cancer 2011, 43 (Suppl. S36), 125–127. [Google Scholar] [CrossRef] [PubMed]
- Motooka, Y.; Yoshimoto, K.; Semba, T.; Ikeda, K.; Mori, T.; Honda, Y.; Iyama, K.-I.; Suzuki, M. Pulmonary hepatoid adenocarcinoma: Report of a case. Surg. Case Rep. 2016, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.-Q.; Yin, F.-Y.; Li, G.-X.; Chu, J.-G. Hepatoid adenocarcinoma of the lung. Qjm Int. J. Med. 2016, 109, 619–620. [Google Scholar] [CrossRef]
- Seddon, J.E.; Jayarao, M.; Donahue, J.E.; Toms, S.A. Brain metastasis secondary to hepatoid adenocarcinoma of the lung. Interdiscip. Neurosurg. 2021, 24, 101085. [Google Scholar] [CrossRef]
- Shaib, W.; Sharma, R.; Mosunjac, M.; Farris, A.; El Rayes, B. Hepatoid Adenocarcinoma of the Lung: A Case Report and Review of the Literature. J. Gastrointest. Cancer 2014, 45, 99–102. [Google Scholar] [CrossRef]
- Shi, Y.-F.; Lu, J.-G.; Yang, Q.-M.; Duan, J.; Lei, Y.-M.; Zhao, W.; Liu, Y.-Q. Primary hepatoid adenocarcinoma of the lung in Yungui Plateau, China: A case report. World J. Clin. Cases 2019, 7, 1711–1716. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Zhang, J.; Liu, Y. Clinicopathological features and genomic profiles of hepatoid adenocarcinoma of the lung: Report of four cases. Pathol. Res. Pratic. 2021, 229, 153652. [Google Scholar] [CrossRef]
- Sun, J.N.; Zhang, B.L.; Li, L.K.; Yu, H.Y.; Wang, B. Hepatoid adenocarcinoma of the lung without production of α-fetoprotein: A case report and review of the literature. Oncol. Lett. 2016, 12, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, L.M.; Glatz, K.; Mhawech, P.; Vasei, M.; Lehmann, F.S.; Vecchione, R.; Tornillo, L. Hepatoid Adenocarcinoma With Liver Metastasis Mimicking Hepatocellular Carcinoma: An Immunohistochemical and Molecular Study of Eight Cases. Am. J. Surg. Pathol. 2003, 27, 1302–1312. [Google Scholar] [CrossRef]
- Valle, L.; Thomas, J.; Kim, C.; Szabo, E.; Brown, G.T.; Citrin, D.; Rajan, A. Hepatoid adenocarcinoma of the lung metastasizing to the tonsil. Mol. Clin. Oncol. 2017, 6, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, G.; Wu, G.; Chen, Z.; Sun, Z.; Zheng, P.; Huang, Y.; Wang, F.; Yang, X. Hepatoid Adenocarcinoma of the Lung Metastasizing to the Gingiva. OncoTargets Ther. 2019, 12, 8765–8768. [Google Scholar] [CrossRef]
- Yang, K.; Jiang, H.; Li, Q. Primary pulmonary hepatoid adenocarcinoma. Medicine 2019, 98, e15053. [Google Scholar] [CrossRef] [PubMed]
- Zhuansun, Y.; Bian, L.; Zhao, Z.; Du, Y.; Chen, R.; Lin, L.; Li, J. Clinical characteristics of Hepatoid adenocarcinoma of the lung: Four case reports and literature review. Cancer Treat. Res. Commun. 2021, 29, 100474. [Google Scholar] [CrossRef]
- Xu, S.-Z.; Zhang, X.-C.; Jiang, Q.; Chen, M.; He, M.-Y.; Shen, P. Alpha-fetoprotein-producing hepatoid adenocarcinoma of the lung responsive to sorafenib after multiline treatment: A case report. World J. Clin. Cases 2022, 10, 10236–10243. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Guan, X.; Bao, G.; Liang, J.; Li, T.; Zhong, X. Whole-exome sequencing and bioinformatics analysis of a case of non-alpha-fetoprotein-elevated lung hepatoid adenocarcinoma. Front. Pharmacol. 2022, 13, 945038. [Google Scholar] [CrossRef]
- Remon, J.; Soria, J.-C.; Peters, S. Early and locally advanced non-small-cell lung cancer: An update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef]
- Eramo, A.; Lotti, F.; Sette, G.; Pilozzi, E.; Biffoni, M.; Di Virgilio, A.; Conticello, C.; Ruco, L.; Peschle, C.; De Maria, R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2007, 15, 504–514. [Google Scholar] [CrossRef]
- Prabavathy, D.; Swarnalatha, Y.; Ramadoss, N. Lung cancer stem cells—Origin, characteristics and therapy. Stem Cell Investig. 2018, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.G.; Son, S.-M.; Lee, H.-C.; Han, H.S.; Lee, K.H.; Kim, D.; Kim, E.-G.; Lee, O.-J. Histologic Changes in Non–Small Cell Lung Cancer under Various Treatments: A Comparison of Histology and Mutation Status in Serial Samples. Cancer Res. Treat. 2022, 54, 737–743. [Google Scholar] [CrossRef]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Hirsh, V.; Zhang, L.; de Marinis, F.; Yang, J.C.; Wakelee, H.A.; Seto, T.; Wu, Y.-L.; Novello, S.; Juhász, E.; et al. Monotherapy Administration of Sorafenib in Patients with Non–Small Cell Lung Cancer (MISSION) Trial: A Phase III, Multicenter, Placebo-Controlled Trial of Sorafenib in Patients with Relapsed or Refractory Predominantly Nonsquamous Non-Small-Cell Lung Cancer after 2 or 3 Previous Treatment Regimens. J. Thorac. Oncol. 2015, 10, 1745–1753. [Google Scholar] [CrossRef] [PubMed]

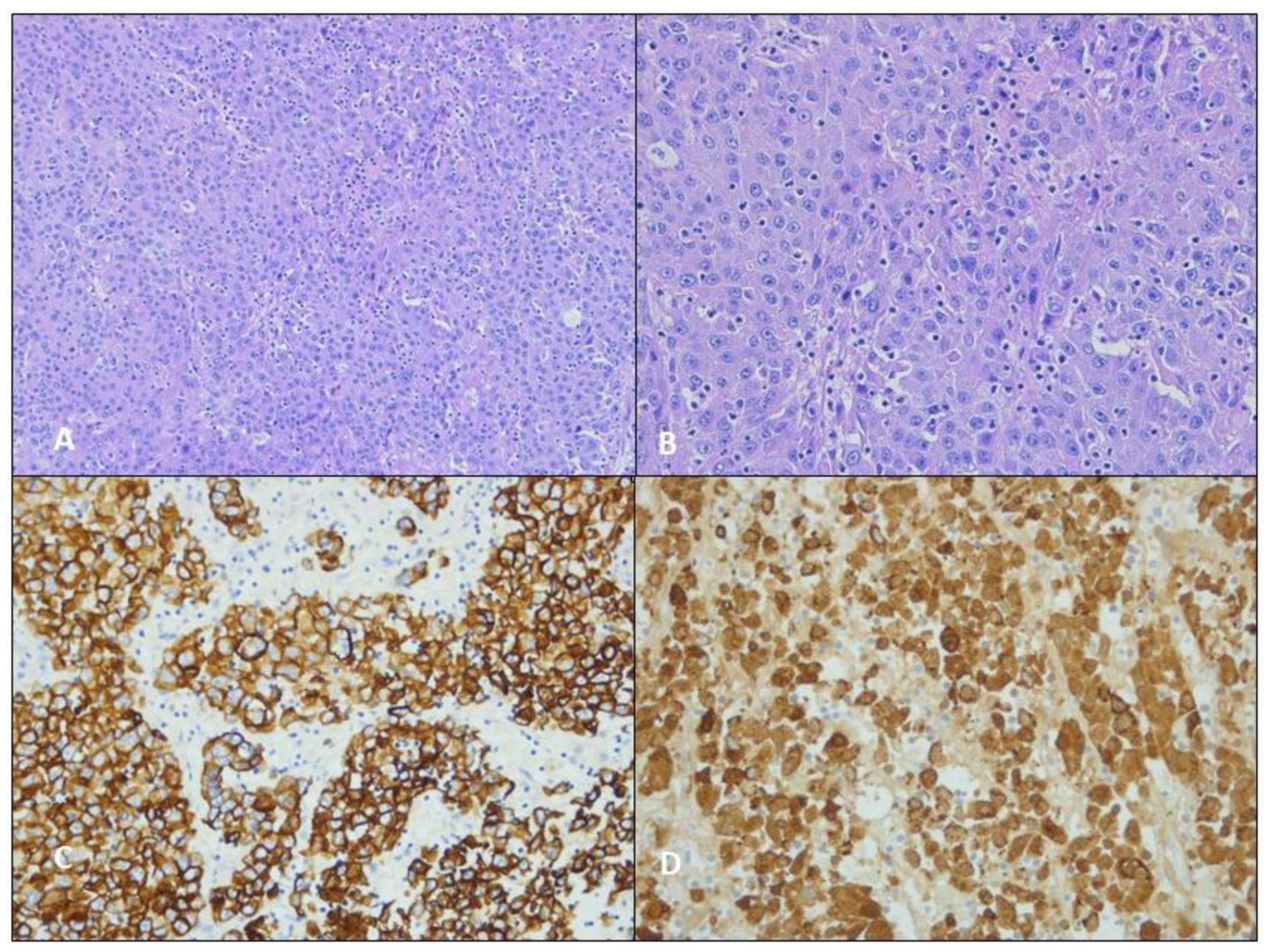
| ANALYSES | Case 1 | Case 2 | Case 3 | ANALYSES | Case 1 | Case 2 | Case 3 |
|---|---|---|---|---|---|---|---|
| MNF116 | -/+ | + | + | EGFR | wt | wt | wt |
| p40 | - | - | - | ALK | wt | wt | wt |
| Syn | - | - | - | ROS | wt | wt | wt |
| CgA | - | - | - | RET | wt | wt | wt |
| Ki67 | 40% | 80% | 60% | MET | wt | wt | wt |
| TTF-1 | - | - | - | K-RAS | wt | wt | wt |
| BRG1 | + | + | + | BRAF | wt | wt | wt |
| CD34 | - | - | - | Pan-TRK | NA | NA | wt |
| ER | - | - | NA | ||||
| PR | - | - | NA | PD-L1 | 3% | - | 90% |
| HepPar-1 | + | + | + |
| Variables | Results |
|---|---|
| Age (years) mean ± DS (range); median | 59.7 ± 8.5 (33–79); 61 |
| Sex (M/F) n(%) | 57 (86.4)/9 (13.6) |
| Smokers n(%) | 44 (66.7) |
| Alpha-fetoprotein (ng/mL) mean ± DS (range); median | 13,543 ± 36,097 (<20–203,320); 902 |
| Thoracic pain at diagnosis n(%) | 25 (36.8) |
| Neoplasm surface (cm2) | 38.1 ± 29.0 (4.4–132); 31.4 |
| Side (right/left) n(%) | 38 (64.4)/21 (35.6) |
| Lung field n(%) | |
| Upper lobe | 41 (68.4) |
| Mid lobe | 2 (3.3) |
| Lower lobe | 12 (20.0) |
| Others (parahilar and paratracheal) | 5 (8.3) |
| Stage n(%) | |
| I | 5 (8.8) |
| II | 5 (8.8) |
| III | 23 (40.4) |
| IV | 24 (42.1) |
| Lymph nodes involvement (yes/no) n(%) | 40 (72.7)/15 (27.3) |
| Metastases involvement (yes/no) n(%) | 23 (41.8)/32 (58.2) |
| Surgery (yes/no) n(%) | 25 (50.0)/25 (50.0) |
| Lobectomy | 21 (84.0) |
| Pneumonectomy | 2 (8.0) |
| Wedge resection | 2 (8.0) |
| Chemotherapy n(%) | |
| Chemotherapy only | 26 (52.0) |
| Adjuvant | 6 (12.0) |
| Chemotherapy protocol n(%) | |
| Double drug scheme | 16 (50.0) |
| Triple drug scheme | 6 (18.8) |
| Platinum n(%) | |
| Cisplatinum | 10 (47.6) |
| Carboplatinum | 9 (42.9) |
| Oxaliplatinum | 2 (9.5) |
| Second drug n(%) | |
| Docetaxel | 3 (13.6) |
| Etoposide | 1 (4.5) |
| Gemcitabine | 1 (4.5) |
| Paclitaxel | 10 (45.5) |
| Pemetrexed | 5 (22.7) |
| Uracil | 1 (4.5) |
| Vinorelbine | 1 (4.5) |
| Other first-line drugs n(%) | |
| Anlotinib | 1 (9.1) |
| Bevacizumab | 2 (18.2) |
| Crizotinib | 1 (9.1) |
| Durvalumab | 1 (9.1) |
| Erlotinib | 1 (9.1) |
| Pembrolizumab | 4 (36.4) |
| Sorafenib | 1 (9.1) |
| Author | Year | Treatment | Surgery | Platinum-Based Scheme | Other Drugs | Savage Therapy or Other Regimens | Follow-Up (Time in Months) | Status |
|---|---|---|---|---|---|---|---|---|
| Al-Najjar et al. [13] | 2015 | CT | Cisplatinum, Docetaxel | 12 | Dead | |||
| Anusha G. et al. [26] | 2021 | CT | Carboplatinum, Pemetrexed | NA | NA | |||
| Ayub A. et al. [18] | 2019 | Surg | Lobectomy | 6 | Dead | |||
| Basse V. et al. [27] | 2018 | CT | Durvalumab | NA | NA | |||
| Chandan VS et al. [28] | 2016 | Surg | Lobectomy | NA | NA | |||
| Surg | Lobectomy | NA | NA | |||||
| Che YQ. et al. [29] | 2014 | CT | Cisplatinum, Paclitaxel | Nedplatin | 20 | Dead | ||
| Chen HF. et al. [30] | 2019 | Surg | Lobectomy | Cisplatinum, Pemetrexed | Osimertinib | 29 | Alive | |
| Chen L. et al. [31] | 2020 | CT | Oxaliplatinum, Docetaxel | Pemetrexed, Oxaliplatinum, Bevacizumab | 53 | Dead | ||
| El Khoury A. et al. [32] | 2019 | CT | Cisplatinum, Etoposide | Pembrolizumab | Docetaxel, Nedaplatin | 14 | Alive | |
| Gavrancic T et al. [33] | 2015 | CT | Carboplatinum, Paclitaxel | Sorafenib | Vinorelbine, Sorafenib | 11 | Dead | |
| Haninger DM et al. [19] | 2014 | Surg | Wedge | 37 | Alive | |||
| Surg | Wedge | 10 | Dead | |||||
| Hayashi Y. et al. [34] | 2002 | Surg | Lobectomy | 32 | Alive | |||
| Hiroshima K. et al. [35] | 2002 | Surg | Lobectomy | 12 | Dead | |||
| Surg | Sleeve lobectomy | Pneumonectomy | 5 | Dead | ||||
| Hou Z et al. [15] | 2021 | CT | Cisplatinum, Pemetrexed | Anlotinib, Sorafenib | 13 | Dead | ||
| Khozin S. et al. [21] | 2012 | CT | Crizotinib | 6 | Alive | |||
| Kuan K. et al. [20] | 2019 | Surg | Lobectomy | 4 | Dead | |||
| Lagos G.G. et al. [36] | 2021 | CT | Carboplatinum, Paclitaxel | Pembrolizumab | 7 | Alive | ||
| Li J. et al. [7] | 2019 | CT | Anlotinib | 6 | Dead | |||
| Mokrim M. et al. [37] | 2012 | CT | Cisplatinum, Vinorelbine | 7 | Alive | |||
| Motooka Y. et al. [38] | 2016 | Surg | Lobectomy | 51 | Alive | |||
| Papatsimpas et al. [16] | 2012 | CT | Carboplatinum, Paclitaxel | Bevacizumab | Erlotinib | 6 | Dead | |
| Qian GQ. et al. [39] | 2016 | CT | Erlotinib | 1 | Dead | |||
| Seddon J.E. et al. [40] | 2021 | CT | Carboplatinum, Pemetrexed | Pembrolizumab | Carboplatinum, Paclitaxel, RT | 17 | Dead | |
| Shaib W. et al. [41] | 2014 | CT | Cisplatinum, Docetaxel | 48 | Alive | |||
| Shi YF. et al. [42] | 2019 | Surg | Lobectomy | Carboplatinum, Paclitaxel | 14 | Dead | ||
| Sun H. et al. [43] | 2022 | Surg | Lobectomy | 36 | Alive | |||
| Surg | Lobectomy | 26 | Alive | |||||
| Surg | Lobectomy | 18 | Dead | |||||
| Surg | Lobectomy | Oxaliplatinum, Uracil | NA | NA | ||||
| Sun J.N. et al. [44] | 2016 | Surg | Pneumonectomy | 23 | Alive | |||
| Terracciano et al. [45] | 2003 | Surg | Lobectomy | 2 | Dead | |||
| Tonyali O. et al. [5] | 2020 | Surg | Pneumonectomy | Carboplatinum, Paclitaxel | Nivolumab | 14 | Dead | |
| Valle L. et al. [46] | 2017 | CT | Cisplatinum, Pemetrexed | 55 | Dead | |||
| Wang C. et al. [47] | 2019 | CT | Bevacizumab | 9 | Dead | |||
| Yang K. et al. [48] | 2019 | Surg | Lobectomy | 18 | Dead | |||
| Zhuansun Y. et al. [49] | 2021 | CT | Non platinum, Paclitaxel | Bevacizumab | 9 | Dead | ||
| Surg | Lobectomy | Immunotherapy (Notspecified) | 22 | Dead | ||||
| Chen, Ding et al. [10] | 2022 | Surg | Lobectomy | Cisplatinum, Paclitaxel | 13 | Dead | ||
| Zhen, Xiao-Chen et al. [50] | 2022 | CT | Carboplatinum, Paclitaxel | Pembrolizumab | Oxaliplatinum, Gemcitabine | 13 | Alive | |
| Yao, Guan. et al. [51] | 2022 | Surg | Lobectomy | 6 | Dead | |||
| OUR CASES | 2022 | Surg | Lobectomy | Carboplatinum, Paclitaxel | 5 | Alive | ||
| CT | Cisplatinum, Gemcitabine | Atezolizumab | 15 | Dead | ||||
| Surg | Lobectomy | Atezolizumab | 5 | Alive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonis, A.; Dell’Amore, A.; Verzeletti, V.; Melan, L.; Zambello, G.; Nardocci, C.; Comacchio, G.M.; Pezzuto, F.; Calabrese, F.; Rea, F. Hepatoid Adenocarcinoma of the Lung: A Review of the Most Updated Literature and a Presentation of Three Cases. J. Clin. Med. 2023, 12, 1411. https://doi.org/10.3390/jcm12041411
Bonis A, Dell’Amore A, Verzeletti V, Melan L, Zambello G, Nardocci C, Comacchio GM, Pezzuto F, Calabrese F, Rea F. Hepatoid Adenocarcinoma of the Lung: A Review of the Most Updated Literature and a Presentation of Three Cases. Journal of Clinical Medicine. 2023; 12(4):1411. https://doi.org/10.3390/jcm12041411
Chicago/Turabian StyleBonis, Alessandro, Andrea Dell’Amore, Vincenzo Verzeletti, Luca Melan, Giovanni Zambello, Chiara Nardocci, Giovanni Maria Comacchio, Federica Pezzuto, Fiorella Calabrese, and Federico Rea. 2023. "Hepatoid Adenocarcinoma of the Lung: A Review of the Most Updated Literature and a Presentation of Three Cases" Journal of Clinical Medicine 12, no. 4: 1411. https://doi.org/10.3390/jcm12041411
APA StyleBonis, A., Dell’Amore, A., Verzeletti, V., Melan, L., Zambello, G., Nardocci, C., Comacchio, G. M., Pezzuto, F., Calabrese, F., & Rea, F. (2023). Hepatoid Adenocarcinoma of the Lung: A Review of the Most Updated Literature and a Presentation of Three Cases. Journal of Clinical Medicine, 12(4), 1411. https://doi.org/10.3390/jcm12041411







