Does Having Rheumatoid Arthritis Increase the Dose of Depression Medications? A Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Resources
2.3. Selection of IVs
2.4. Statistical Analyses
2.5. Sensitivity Analysis
3. Results
3.1. Selection of IVs
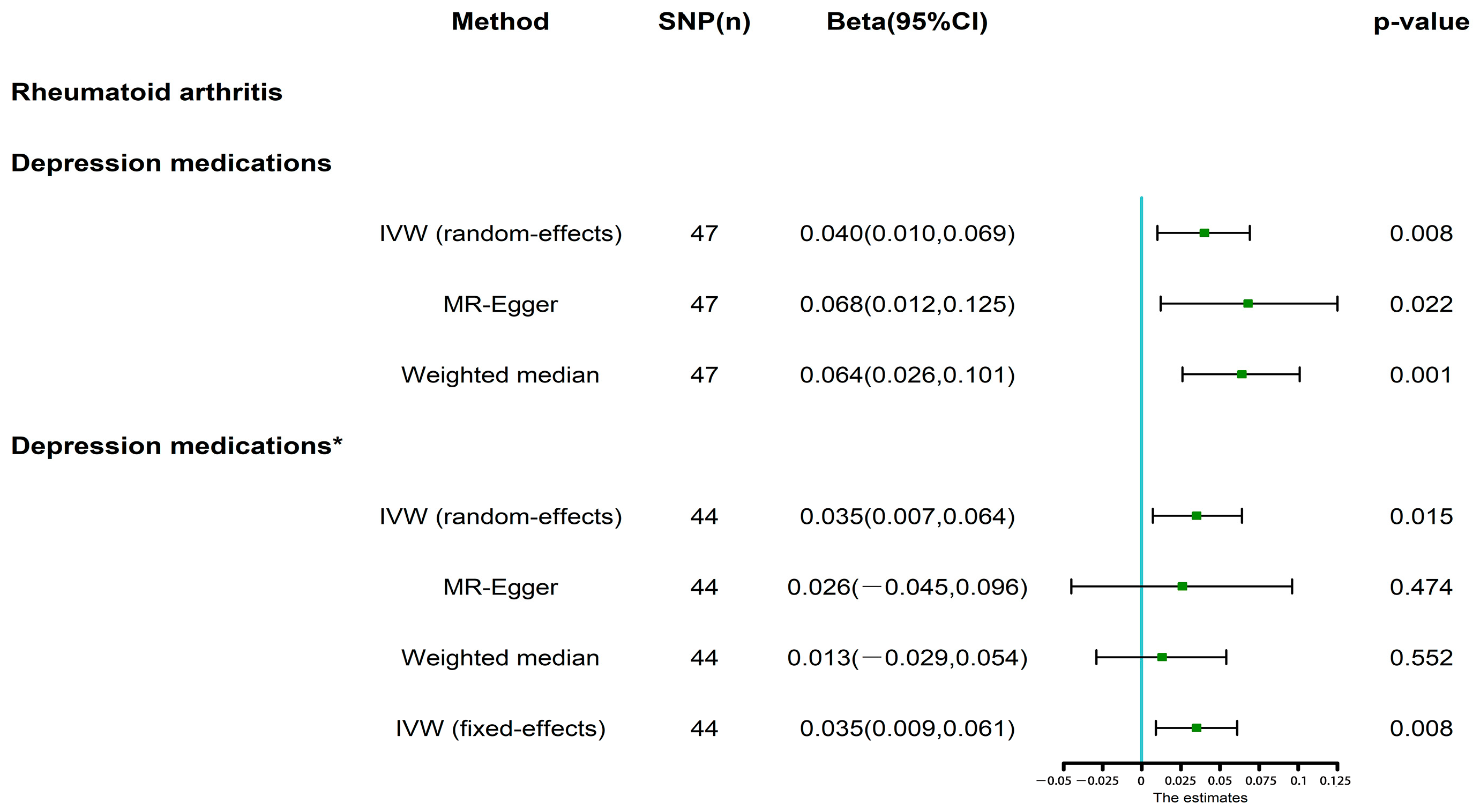
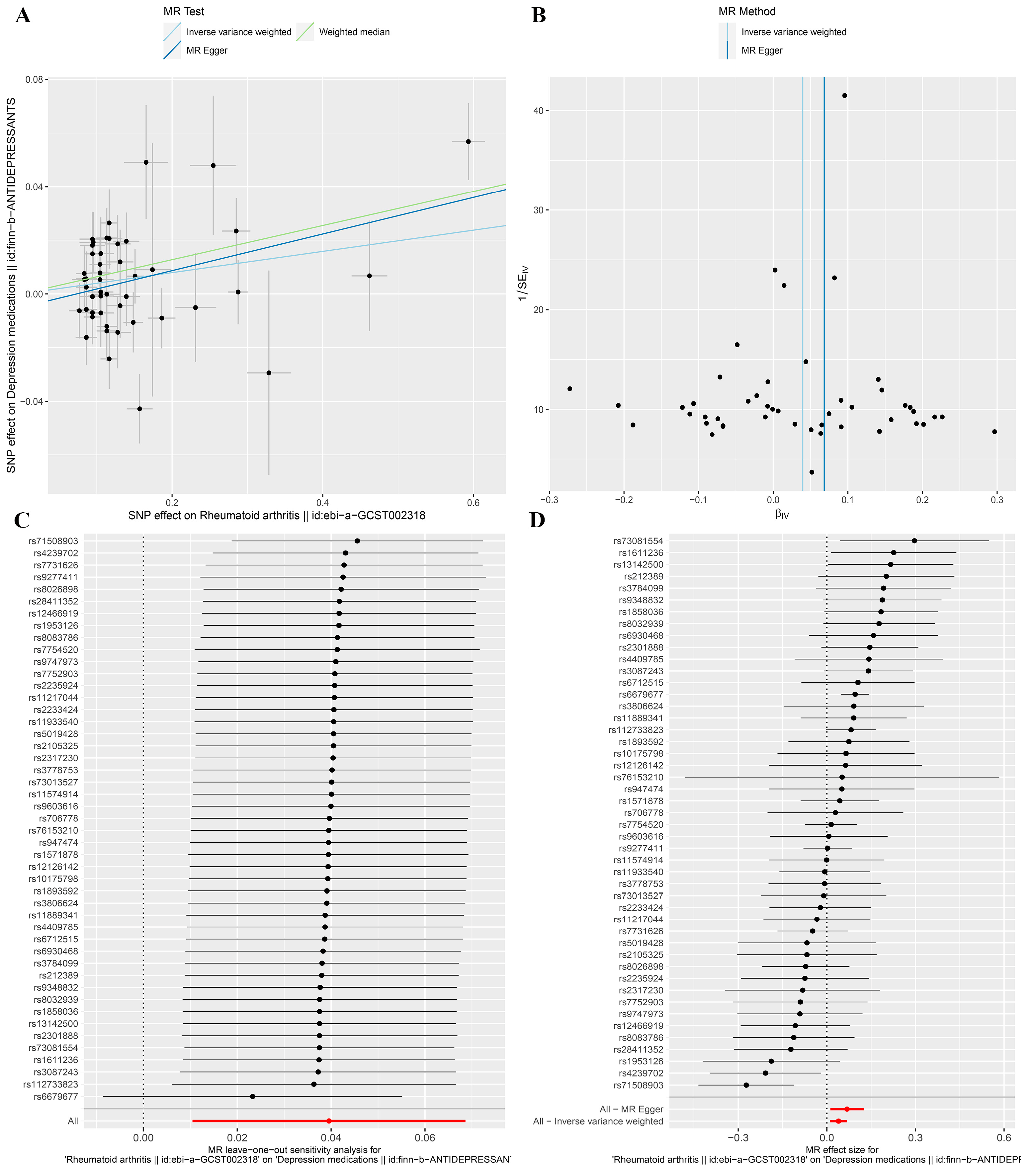
3.2. MR Analysis
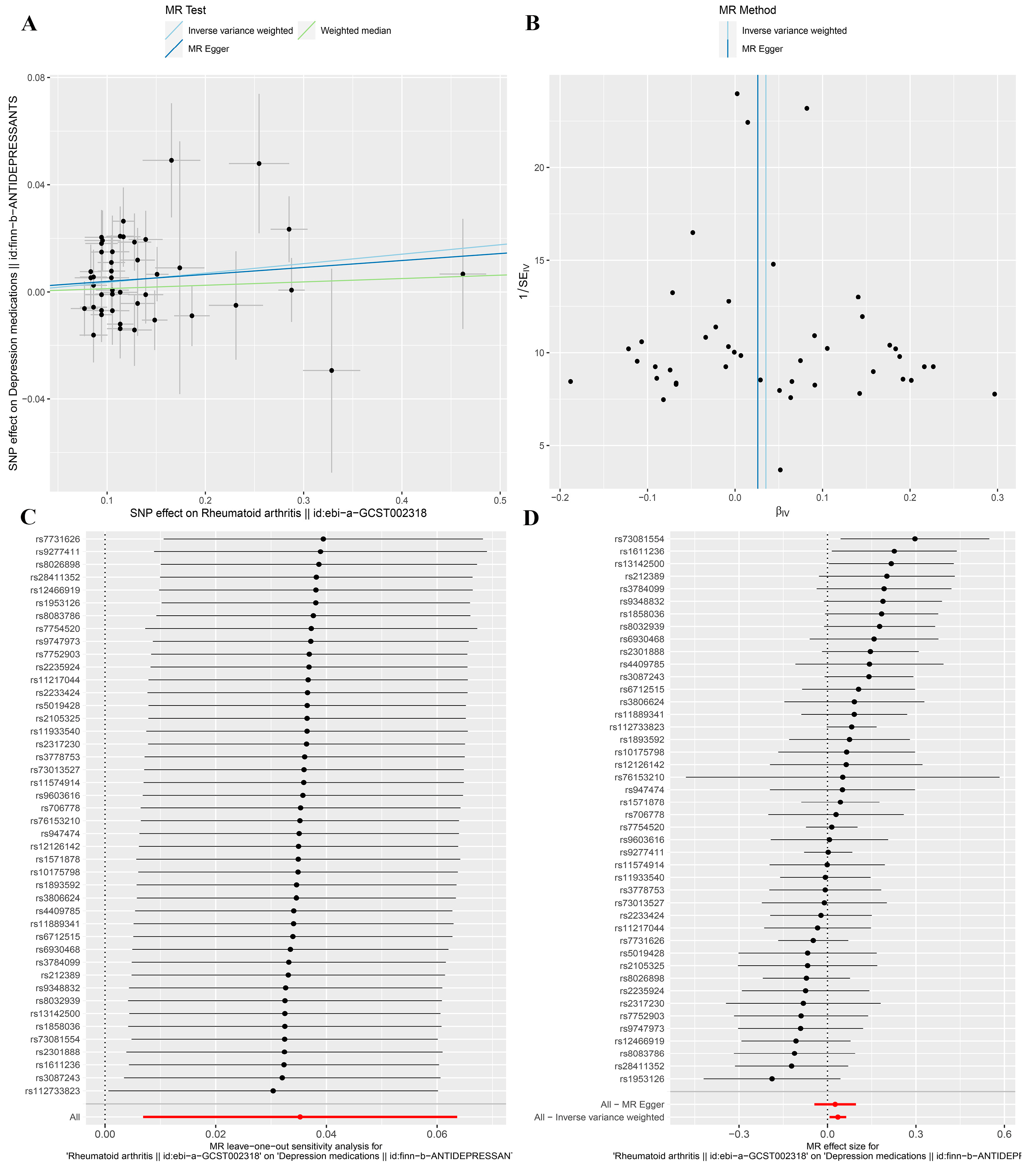
3.3. MR Analysis after Removing Outliers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Vandoolaeghe, E.; Ranjan, R.; Bosmans, E.; Bergmans, R.; Desnyder, R. Increased serum interleukin-1-receptor-antagonist concentrations in major depression. J. Affect. Disord. 1995, 36, 29–36. [Google Scholar] [CrossRef]
- Young, J.J.; Bruno, D.; Pomara, N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014, 169, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Schildkraut, J.J.; Draskoczy, P.R.; Gershon, E.S.; Reich, P.; Grab, E.L. Catecholamine metabolism in affective disorders. IV. Preliminary studies of norepinephrine metabolism in depressed patients treated with amitriptyline. J. Psychiatr Res. 1972, 9, 173–185. [Google Scholar] [CrossRef]
- Diniz, C.; Casarotto, P.C.; Resstel, L.; Joca, S.R.L. Beyond good and evil: A putative continuum-sorting hypothesis for the functional role of proBDNF/BDNF-propeptide/mBDNF in antidepressant treatment. Neurosci. Biobehav. Rev. 2018, 90, 70–83. [Google Scholar] [CrossRef]
- Yu, H.; Lv, D.; Shen, M.; Zhang, Y.; Zhou, D.; Chen, Z.; Wang, C. BDNF mediates the protective effects of scopolamine in reserpine-induced depression-like behaviors via up-regulation of 5-HTT and TPH1. Psychiatry Res. 2019, 271, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, cytokines and brain abnormalities in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 722–729. [Google Scholar] [CrossRef]
- Kverno, K.S.; Mangano, E. Treatment-Resistant Depression: Approaches to Treatment. J. Psychosoc. Nurs. Ment. Health Serv. 2021, 59, 7–11. [Google Scholar] [CrossRef]
- Gonda, X.; Petschner, P.; Eszlari, N.; Baksa, D.; Edes, A.; Antal, P.; Juhasz, G.; Bagdy, G. Genetic variants in major depressive disorder: From pathophysiology to therapy. Pharmacol. Ther. 2019, 194, 22–43. [Google Scholar] [CrossRef]
- Smolen, J.; Aletaha, D.; Barton, A.; Burmester, G.; Emery, P.; Firestein, G.; Kavanaugh, A.; McInnes, I.; Solomon, D.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18002. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef] [PubMed]
- Sayah, A.; English, J.C., 3rd. Rheumatoid arthritis: A review of the cutaneous manifestations. J. Am. Acad. Dermatol. 2005, 53, 191–209, quiz 210–192. [Google Scholar] [CrossRef]
- Alunno, A.; Gerli, R.; Giacomelli, R.; Carubbi, F. Clinical, Epidemiological, and Histopathological Features of Respiratory Involvement in Rheumatoid Arthritis. Biomed. Res. Int. 2017, 2017, 7915340. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Ryan, S. Psychological effects of living with rheumatoid arthritis. Nurs. Stand. 2014, 29, 52–59. [Google Scholar] [CrossRef]
- Figus, F.A.; Piga, M.; Azzolin, I.; McConnell, R.; Iagnocco, A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun. Rev. 2021, 20, 102776. [Google Scholar] [CrossRef]
- Hassan, A.A.; Nasr, M.H.; Mohamed, A.L.; Kamal, A.M.; Elmoghazy, A.D. Psychological affection in rheumatoid arthritis patients in relation to disease activity. Medicine 2019, 98, e15373. [Google Scholar] [CrossRef]
- Baerwald, C.; Manger, B.; Hueber, A. Depression as comorbidity of rheumatoid arthritis. Z. Rheumatol. 2019, 78, 243–248. [Google Scholar] [CrossRef]
- Qiu, S.; Li, M.; Jin, S.; Lu, H.; Hu, Y. Rheumatoid Arthritis and Cardio-Cerebrovascular Disease: A Mendelian Randomization Study. Front. Genet. 2021, 12, 745224. [Google Scholar] [CrossRef]
- Li, C.; Ou, R.; Shang, H. Rheumatoid arthritis decreases risk for Parkinson’s disease: A Mendelian randomization study. NPJ Park. Dis. 2021, 7, 17. [Google Scholar] [CrossRef]
- Bae, S.C.; Lee, Y.H. Causal association between rheumatoid arthritis and a decreased risk of Alzheimer’s disease: A Mendelian randomization study. Z. Rheumatol. 2019, 78, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.W.; Chen, C.Y.; Stein, M.B.; Klimentidis, Y.C.; Wang, M.J.; Koenen, K.C.; Smoller, J.W. Assessment of Bidirectional Relationships Between Physical Activity and Depression Among Adults: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry 2019, 76, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Kappelmann, N.; Ye, Z.; Lamers, F.; Moser, S.; Jones, P.B.; Burgess, S.; Penninx, B.; Khandaker, G.M. Association of inflammation with depression and anxiety: Evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol. Psychiatry 2021, 26, 7393–7402. [Google Scholar] [CrossRef]
- Okada, Y.; Wu, D.; Trynka, G.; Raj, T.; Terao, C.; Ikari, K.; Kochi, Y.; Ohmura, K.; Suzuki, A.; Yoshida, S.; et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 2014, 506, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Rosoff, D.B.; Davey Smith, G.; Mehta, N.; Clarke, T.-K.; Lohoff, F.W. Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: A multivariable Mendelian randomization study. PLoS Med. 2020, 17, e1003410. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ye, D.; Yang, H.; Song, J.; Sun, X.; Mao, Y.; He, Z. Two-Sample Mendelian Randomization Analysis Investigates Causal Associations Between Gut Microbial Genera and Inflammatory Bowel Disease, and Specificity Causal Associations in Ulcerative Colitis or Crohn’s Disease. Front. Immunol. 2022, 13, 921546. [Google Scholar] [CrossRef]
- Chen, L.; Sun, X.; Wang, Z.; Lu, Y.; Chen, M.; He, Y.; Xu, H.; Zheng, L. The impact of plasma vitamin C levels on the risk of cardiovascular diseases and Alzheimer’s disease: A Mendelian randomization study. Clin. Nutr. 2021, 40, 5327–5334. [Google Scholar] [CrossRef]
- Chen, H.; Mi, S.; Zhu, J.; Jin, W.; Li, Y.; Wang, T.; Li, Y.; Fan, C. No Causal Association Between Adiponectin and the Risk of Rheumatoid Arthritis: A Mendelian Randomization Study. Front. Genet. 2021, 12, 670282. [Google Scholar] [CrossRef]
- Yin, K.-J.; Huang, J.-X.; Wang, P.; Yang, X.-K.; Tao, S.-S.; Li, H.-M.; Ni, J.; Pan, H.-F. No Genetic Causal Association Between Periodontitis and Arthritis: A Bidirectional Two-Sample Mendelian Randomization Analysis. Front. Immunol. 2022, 13, 808832. [Google Scholar] [CrossRef]
- Cui, Z.; Hou, G.; Meng, X.; Feng, H.; He, B.; Tian, Y. Bidirectional Causal Associations Between Inflammatory Bowel Disease and Ankylosing Spondylitis: A Two-Sample Mendelian Randomization Analysis. Front. Genet. 2020, 11, 587876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cai, Y.; Liang, J.; Zhang, J.; Jing, Z.; Lv, L.; Zhang, R.; Song, J.; Dang, X.; Song, Q. Causal relationships between rheumatism and dyslipidemia: A two-sample Mendelian randomization study. Front. Endocrinol. 2022, 13, 961505. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Kong, M.; Li, X.; Wei, D.; Zhu, X.; Hong, Z.; Ni, M.; Wang, Y.; Dong, A. Systemic Lupus Erythematosus and Cardiovascular Disease: A Mendelian Randomization Study. Front. Immunol. 2022, 13, 908831. [Google Scholar] [CrossRef]
- Gao, R.-C.; Sang, N.; Jia, C.-Z.; Zhang, M.-Y.; Li, B.-H.; Wei, M.; Wu, G.-C. Association Between Sleep Traits and Rheumatoid Arthritis: A Mendelian Randomization Study. Front. Public Health 2022, 10, 940161. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.-J.; Li, J.-R.; Zhu, Y.-C.; Shen, H. Migraine and Ischemic Stroke: A Mendelian Randomization Study. Neurol. Ther. 2022, 11, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Chen, L.; Yang, H.; Li, H.; He, C.; Yang, L.; Lv, G. Insights into modifiable risk factors of cholelithiasis: A Mendelian randomization study. Hepatology 2022, 75, 785–796. [Google Scholar] [CrossRef]
- Murrough, J.W.; Yaqubi, S.; Sayed, S.; Charney, D.S. Emerging drugs for the treatment of anxiety. Expert Opin. Emerg. Drugs 2015, 20, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Kapczinski, F.; Lima, M.S.; Souza, J.S.; Schmitt, R. Antidepressants for generalized anxiety disorder. Cochrane Database Syst. Rev. 2003, 3, CD003592. [Google Scholar]
- Marrie, R.A.; Hitchon, C.A.; Walld, R.; Patten, S.B.; Bolton, J.M.; Sareen, J.; Walker, J.R.; Singer, A.; Lix, L.M.; El-Gabalawy, R.; et al. Increased Burden of Psychiatric Disorders in Rheumatoid Arthritis. Arthritis Care Res. 2018, 70, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Guo, H.R.; Lin, M.C.; Livneh, H.; Lai, N.S.; Tsai, T.Y. Bidirectional associations between rheumatoid arthritis and depression: A nationwide longitudinal study. Sci. Rep. 2016, 6, 20647. [Google Scholar] [CrossRef]
- Jamshidi, A.R.; Banihashemi, A.T.; Paragomi, P.; Hasanzadeh, M.; Barghamdi, M.; Ghoroghi, S. Anxiety and depression in rheumatoid arthritis: An epidemiologic survey and investigation of clinical correlates in Iranian population. Rheumatol. Int. 2016, 36, 1119–1125. [Google Scholar] [CrossRef]
- Margaretten, M.; Barton, J.; Julian, L.; Katz, P.; Trupin, L.; Tonner, C.; Graf, J.; Imboden, J.; Yelin, E. Socioeconomic determinants of disability and depression in patients with rheumatoid arthritis. Arthritis Care Res. 2011, 63, 240–246. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Y.; Zhang, Q.; Fu, T.; Yin, R.; Guo, G.; Li, L.; Gu, Z. The correlations of socioeconomic status, disease activity, quality of life, and depression/anxiety in Chinese patients with rheumatoid arthritis. Psychol. Health Med. 2017, 22, 28–36. [Google Scholar] [CrossRef]
- Fang, S.; Huang, S.; Tian, F.; Han, Y.; Yang, K.; Zhang, Q. Assessment of bidirectional relationships between depression and rheumatoid arthritis among adults: A two-sample Mendelian randomization study. Clin. Rheumatol. 2022. [Google Scholar] [CrossRef]
- Nerurkar, L.; Siebert, S.; McInnes, I.B.; Cavanagh, J. Rheumatoid arthritis and depression: An inflammatory perspective. Lancet Psychiatry 2019, 6, 164–173. [Google Scholar] [CrossRef]
- Fakra, E.; Marotte, H. Rheumatoid arthritis and depression. Jt. Bone Spine 2021, 88, 105200. [Google Scholar] [CrossRef]
- Vallerand, I.A.; Patten, S.B.; Barnabe, C. Depression and the risk of rheumatoid arthritis. Curr. Opin. Rheumatol. 2019, 31, 279–284. [Google Scholar] [CrossRef]
- Hilderink, P.H.; Burger, H.; Deeg, D.J.; Beekman, A.T.; Oude Voshaar, R.C. The temporal relation between pain and depression: Results from the longitudinal aging study Amsterdam. Psychosom. Med. 2012, 74, 945–951. [Google Scholar] [CrossRef]
- Gerrits, M.; van Oppen, P.; van Marwijk, H.W.J.; Penninx, B.; van der Horst, H.E. Pain and the onset of depressive and anxiety disorders. Pain 2014, 155, 53–59. [Google Scholar] [CrossRef]
- Euesden, J.; Matcham, F.; Hotopf, M.; Steer, S.; Cope, A.P.; Lewis, C.M.; Scott, I.C. The Relationship Between Mental Health, Disease Severity, and Genetic Risk for Depression in Early Rheumatoid Arthritis. Psychosom. Med. 2017, 79, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.C.; Fu, E.H.; Chua, A.N.; Cheak, A.A.; Mak, A. Clinical and psychosocial factors associated with depression and anxiety in Singaporean patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2011, 14, 37–47. [Google Scholar] [CrossRef]
- Covic, T.; Adamson, B.; Spencer, D.; Howe, G. A biopsychosocial model of pain and depression in rheumatoid arthritis: A 12-month longitudinal study. Rheumatology 2003, 42, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Cheon, Y.H.; Lee, S.G.; Kim, M.; Kim, H.O.; Sun Suh, Y.; Park, K.S.; Kim, R.B.; Yang, H.S.; Kim, J.M.; Son, C.N.; et al. The association of disease activity, pro-inflammatory cytokines, and neurotrophic factors with depression in patients with rheumatoid arthritis. Brain Behav. Immun. 2018, 73, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Richards, B.L.; Whittle, S.L.; Buchbinder, R. Antidepressants for pain management in rheumatoid arthritis. Cochrane Database Syst. Rev. 2011, 11, CD008920. [Google Scholar]
- Silva Almodóvar, A.; Nguyen, D.; Nahata, M.C. Evidence Needed for Efficacy of Antidepressant Medications Among Patients with Rheumatoid Arthritis. Ann. Pharmacother. 2022, 56, 1065–1075. [Google Scholar] [CrossRef]

| Traits | GWAS ID | Sample Size | Number of SNPs | PMID | Author |
|---|---|---|---|---|---|
| Rheumatoid arthritis | ebi-a-GCST002318 | 57,284 | 9,700,598 | 24390342 | Okada et al., 2014 [26] |
| Depression medications | finn-b-ANTIDEPRESSANTS | 118,669 | 16,379,737 | FinnGen consortium |
| MR-PRESSO | IVW Estimates | MR-Egger Pleiotropy Test | |||
|---|---|---|---|---|---|
| Rheumatoid Arthritis | Global p-Value | Cochran’s Q | p-Value | MR-Egger Intercept | p-Value |
| Depression medications | 0.002 | 77.72 | 0.002 | −0.005 | 0.251 |
| Depression medications * | 0.179 | 51.35 | 0.179 | 0.001 | 0.779 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Xie, J.; Yang, M.; Yu, H.; Hou, W.; Xu, K.; Wang, J.; Xu, P. Does Having Rheumatoid Arthritis Increase the Dose of Depression Medications? A Mendelian Randomization Study. J. Clin. Med. 2023, 12, 1405. https://doi.org/10.3390/jcm12041405
Wan X, Xie J, Yang M, Yu H, Hou W, Xu K, Wang J, Xu P. Does Having Rheumatoid Arthritis Increase the Dose of Depression Medications? A Mendelian Randomization Study. Journal of Clinical Medicine. 2023; 12(4):1405. https://doi.org/10.3390/jcm12041405
Chicago/Turabian StyleWan, Xianjie, Jiale Xie, Mingyi Yang, Hui Yu, Weikun Hou, Ke Xu, Jiachen Wang, and Peng Xu. 2023. "Does Having Rheumatoid Arthritis Increase the Dose of Depression Medications? A Mendelian Randomization Study" Journal of Clinical Medicine 12, no. 4: 1405. https://doi.org/10.3390/jcm12041405
APA StyleWan, X., Xie, J., Yang, M., Yu, H., Hou, W., Xu, K., Wang, J., & Xu, P. (2023). Does Having Rheumatoid Arthritis Increase the Dose of Depression Medications? A Mendelian Randomization Study. Journal of Clinical Medicine, 12(4), 1405. https://doi.org/10.3390/jcm12041405





